Characterization of the 2-ketogluconate utilization operon in Pseudomonas aeruginosa PAO1
Abstract
The Pseudomonas aeruginosa protein PtxS negatively regulates its own synthesis by binding to the upstream region of its gene. We have recently identified a 14 bp palindromic sequence within the ptxS upstream region as the PtxS operator site (OP1). In this study, we searched the P. aeruginosa genomic sequence to determine whether this 14 bp sequence exists in other regions of the P. aeruginosa chromosome. Another PtxS operator site (OP2) was located 47 bp downstream of ptxS. DNA gel shift experiments confirmed that PtxS specifically binds to a 520 bp fragment that carries OP2. The DNA segment 3′ of OP2 contains four open reading frames (ORF1–ORF4), which code for 29, 32, 48 and 35 kDa proteins respectively. The molecular weight of the products of ORFs 2 and 3 were confirmed by T7 expression experiments. Computer analyses suggest that ORF2 encodes an ATP-dependent kinase; ORF3, a transporter; and ORF4, a dehydrogenase. The predicted product of ORF1 showed no homology to previously identified proteins and contains all the conserved amino acids within the aldose 1-epimerase protein motif. Examination of the ptxs–ORF1 intergenic region (using promoter fusion experiments) showed that no potential promoter exists. An isogenic mutant defective in ORF1 was constructed in the P. aeruginosa strain PAO1. In contrast to its parent strain, the mutant failed to grow on a minimal medium in which 2-ketogluconate was the sole carbon source. Similarly, a previously constructed ptxS isogenic mutant of PAO1 did not grow in a minimal medium containing 2-ketogluconate as the sole carbon source. Furthermore, a plasmid carrying a fragment that contains ptxS and ORFs 1–4 complemented the defect of the previously described P. aeruginosa 2-ketogluconate-negative mutant. In the presence of 10 mM 2-ketogluconate, the in vitro binding of PtxS to a DNA fragment that carries either OP1 or OP2 was inhibited. These results suggest that: (i) ptxS together with the other four ORFs constitute the 2-ketogluconate utilization operon (kgu) in P. aeruginosa. Therefore, ORFs 1–4 were designated kguE, kguK, kguT and kguD respectively. (ii) PtxS regulates the expression of the kgu operon by binding to two operators (OP1 and OP2) within the operon; and (iii) 2-ketogluconate is the molecular inducer of the kgu operon or the molecular effector of PtxS.
Introduction
Pseudomonas aeruginosa is a Gram-negative pathogen that causes a variety of infections, including wound infections, nosocomial infections and lung infections (Woods, 1976; Bodey et al., 1983). The ability of P. aeruginosa to cause these infections results from the production of several extracellular virulence factors (Woods and Iglewski, 1983). Among these different virulence factors, exotoxin A is considered the most toxic. Exotoxin A is an ADP-ribosyl transferase enzyme that catalyses the transfer of the ADP-ribosyl moiety of NAD+ onto elongation factor 2 of eukaryotic cells, causing cessation of the protein synthesis process and subsequent cell death (Iglewski and Kabat, 1975). Exotoxin A production by P. aeruginosa is a complicated process that involves several regulatory genes (Wick et al., 1990; Vasil and Ochsner, 1999).
We have described previously one of the exotoxin A-positive regulatory genes, ptxR (Hamood et al., 1996). When carried on a plasmid, ptxR causes a four- to fivefold increase in exotoxin A synthesis in P. aeruginosa (Hamood et al., 1996). The enhancement in exotoxin A synthesis by ptxR appears to occur at the transcriptional level (Hamood et al., 1996). Available evidence suggests that ptxR enhances toxA transcription through the previously described toxA transcriptional regulator, regA (Hamood et al., 1996; Colmer and Hamood, 1999). We have also identified another gene, ptxS, which codes for a protein that belongs to the GalR–LacI family of transcriptional repressors and appears to interfere with the enhancement of exotoxin A synthesis by ptxR (Colmer and Hamood, 1998). ptxS, which is located 5′ of ptxR, is transcribed in the opposite orientation to ptxR from the other strand of DNA.
PtxS shares some of the characteristic features of the GalR–LacI repressors. For example, similar to other repressors (such as GalS, PurR and CytR), PtxS autoregulates its synthesis by binding to its own upstream region (Swanson et al., 1999). DNase I footprinting analysis showed that PtxS binds to a 20 bp fragment within the ptxS upstream region that contains a 14 bp palindromic sequence, which may represent the PtxS binding site (B. L. Swanson and A. N. Hamood, manuscript in preparation; Swanson et al., 1999). In addition, ptxS expression (as determined by the level of β-galactosidase activity produced by a ptxS–lacZ fusion) in the P. aeruginosa isogenic mutant PAO1::ptxS was four- to fivefold higher than that in PAO1 (Swanson et al., 1999). However, unlike many of the GalR repressors that are known to bind to specific effector molecules (carbohydrate, nucleotides or amino acids) (Hammer-Jesperson, 1983; Bouchez and Tourneur, 1991; Vyas et al., 1991), no specific effector molecules have been identified to which PtxS binds. In this study, we tried to determine whether the PtxS binding site exists in other regions of the P. aeruginosa chromosome. We have identified a PtxS binding site immediately 3′ of ptxS. This sequence is located within the upstream region of an operon that appears to be involved in the utilization of 2-ketogluconate.
Results
Identification of a second PtxS binding site
We have shown previously that PtxS binds to a 20 bp region within the ptxS upstream region (Swanson et al., 1999). Within this 20 bp fragment, a 14 bp palindrome exists (TGAAACCGGTTTCA), which may represent the PtxS operator site (B. L. Swanson and A. N. Hamood, manuscript in preparation; Swanson et al., 1999). In this study, we tried to determine whether the palindromic sequence exists in other regions of the P. aeruginosa chromosome and if PtxS specifically binds to this sequence. The presence of such a sequence within the upstream region of other genes may indicate that the expression of these genes is regulated by PtxS. We have searched the complete nucleotide sequence of the P. aeruginosa strain PAO1 (at http://www.pseudomonas.com) for potential PtxS binding sites. The 14 bp palindrome was identified at two locations: one within the ptxS upstream region as determined previously (Swanson et al., 1999); and the other sequence immediately 3′ of ptxS (on the same DNA strand). Initial DNA binding experiments were conducted to determine whether the DNA fragment that carries this palindromic sequence specifically binds to PtxS. Analysis of the region downstream of ptxS revealed that the potential PtxS binding site is carried on a 520 bp KpnI–HindIII fragment (data not shown). The 520 bp fragment was obtained from plasmid pAH50 that we had constructed previously during the isolation and characterization of ptxR and ptxS (Hamood et al., 1996). The fragment was end-labelled using T4 polynucleotide kinase, as described previously (Swanson et al., 1999), and incubated in a typical DNA binding reaction with the cell lysate of Escherichia coli K38/pJAC17 (in which PtxS was overproduced). Incubation of the 520 bp fragment with the lysate of K38/pJAC17 produced a gel shift band (Fig. 1A, lane 5). The specificity of this binding band was confirmed by competition experiments, in which the gel shift band was eliminated upon the addition of excess unlabelled 520 bp fragment (Fig. 1A, lane 6). In another competition experiment, the binding band was not eliminated upon the addition of excess unlabelled non-specific DNA (Fig. 1B). A gel shift band that migrated at the same distance was detected when the 520 bp fragment was incubated with the lysate of the P. aeruginosa strain PAO1 (Fig. 1A, lane 2). No gel shift band was detected when the 520 bp fragment was incubated with the lysate of the ptxS isogenic mutant PAO ptxS::Ω (Fig. 1A, lane 3). To confirm these results further, we localized the binding activity to a 50 bp fragment 3′of ptxS. This fragment, which contains the 14 bp palindromic sequence, was synthesized by polymerase chain reaction (PCR). A specific gel shift band was detected when the 50 bp fragment was incubated with the lysates of K38/pJAC17 and PAO1, but not PAOptxS::Ω (data not shown). These results indicate that PtxS specifically binds to this palindromic sequence. We will refer to the PtxS operator site within the ptxS upstream region as OP1 and to the newly identified operator site as OP2.
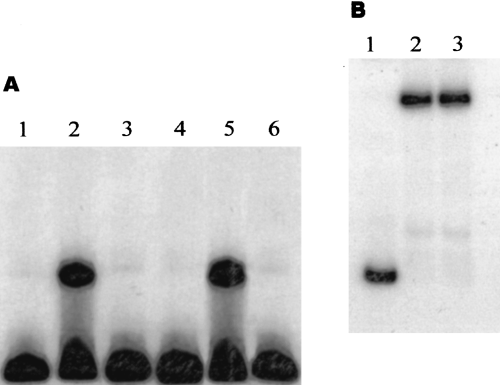
A. PtxS binding to the labelled 520 KpnI–HindIII fragment, which is located 3′ of ptxS and carries OP2. The probe was incubated with 10 µg of the lysate of PAO1 and the ptxS isogenic mutant PAO ptxS::Ω and 1 µg of the lysate of the E. coli strain K38/pJAC17 (in which PtxS is overproduced). The specificity of this binding was determined by a gel shift competitive experiment in which an excess (20×) of unlabelled 520 bp fragment was added to the binding reaction. Lanes: 1, 520 bp probe alone; 2, 520 bp probe + the lysate of PAO1; 3, 520 bp probe + the lysate of PAO ptxS::Ω; 4, 520 bp probe + the lysate of K38/pT7-5 (negative control); 5, 520 bp probe + the lysate of K38/pJAC17; 6. 520 bp probe + the lysate of K38/pJAC17 + 20× unlabelled 520 bp fragment.
B. Competitive gel shift experiments using an excess of non-specific unlabelled competitive DNA (1.8 kb toxA internal BamHI fragment). Lanes: 1, 520 bp probe alone; 2, 520 bp probe + the lysate of K38/pJAC17; 3, 520 bp probe + the lysate of K38/pJAC17 + 20× unlabelled 1.8 kb toxA internal BamHI fragment.
Identification of a potential 2-ketogluconate utilization operon
OP2 may be located within the upstream region of a gene(s) that is regulated by PtxS. To examine such a possibility, we searched the available P. aeruginosa genomic sequence for a potential open reading frame(s) (ORFs) in the region 3′ of ptxS. We have identified four potential ORFs that are transcribed in the same direction (Table 1) (Fig. 2). There were no clear transcription initiation or transcription termination signals between these genes.
| ORF | Molecular weight of the product (no. of amino acids) | Proposed function of gene product | Similar gene product(% identity)a |
|---|---|---|---|
| ORF1 (kguE)b | 28.9 kDa (261)c | Epimerased | – |
| ORF2 (kguK)b | 32.6 kDa (316)c | 2-keto-gluconokinase | B. subtilis 2-dehydro-3-deoxygluconokinase (52%) |
| ORF3 (kguT)b | 48.1 kDa (436)c | 2-ketogluconate transporter | Agrobacterium vitis putative tartrate transporter (32%) |
| ORF4 (kguD)b | 35.6 kDa (328)c | 2-ketogluconate-dehydrogenase | E. coli putative 2-hyroxyacid dehydrogenase (57%) |
- a . Proteins sequences exhibiting similarities to the products of ORFs 1–4 were identified using blastp of the Wisconsin GCG program. Similarity and identity percentages were calculated using the GCG bestfit program.
- b . The proposed names of the genes are indicated in parentheses.
- c . The number of amino acids in the predicted product is indicated in parentheses.
- d . The predicted protein contains all the amino acids within the aldose 1-epimerase motif.
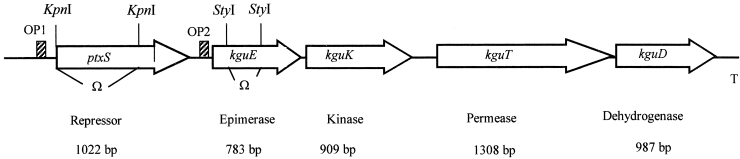
The physical map of the putative kgu operon. The size (in bp) of each open reading frame is indicated. The positions of the ptxS internal KpnI fragment and the kguE internal StyI fragment, which were deleted and replaced by the 2 kb Ω fragment during the construction of the ptxS and kguE isogenic mutants are indicated. OP1 and OP2 indicate the position of the PtxS operator site (the 14 bp palindromic sequence). T indicates the position of the strong rho-independent transcription termination signal.
ORF1, ORF2, ORF3 and ORF4
The 783 bp ORF1, which is located 72 bp downstream of ptxS and has a G+C content of 67%, encodes a 261-amino-acid protein with a predicted molecular weight of 28.9 kDa (Table 1; Fig. 2). Although the amino acid sequence of this predicted protein showed no significant similarity to other proteins in the database, it contains all the conserved amino acids within the aldose 1-epimerase proteins motif (fy) p… (ilv)… (ilv).y. (ilv) (at position 219–231) (Bouffard et al., 1994; Blattner et al., 1997; data not shown). A putative Shine–Dalgarno (SD) sequence was identified 12 bp from the predicted ATG start codon (data not shown). However, based on comparisons with known E. coli promoters, no −10 or −35 sites were found upstream of this start codon. ORF2 is located 28 bp 3′ of ORF1 and has 912 nucleotides and a 67.8% G+C content (Table 1). A putative SD sequence was identified 12 bp from the predicted ATG start codon (data not shown). The 32.6 kDa protein encoded by ORF2 showed a high degree of homology to several carbohydrate kinases. The most significant homology was detected with the 2-keto-3-deoxygluconate kinase of Bacillus subtilis, KdgK (46% identity and 59% similarity; Table 1). The protein belongs to the PfkB family of carbohydrate kinases (Wu et al., 1991). Both signature 1 and 2 of this family were detected at the amino- and carboxy-terminus regions of the predicted protein (Fig. 3). In addition, the protein contains a putative ATP-binding motif (amino acids 256 to 277) (Fig. 3). ORF3 is located 62 bp downstream of ORF2. It contains 1308 nucleotides, a 66.3% G+C content and a potential SD sequence 12 bp upstream of the ATG start codon (Table 1). Although potential −10 and −35 sites that resemble those of the E. coliσ70 were identified, these sequences overlap the SD site and are less likely to function in the transcription of ORF3. Hydropathy analysis of the 48 kDa predicted protein encoded by ORF3 showed several hydrophobic regions, which suggests that the protein is an integral membrane protein (data not shown). In addition, the amino acid sequence of the protein showed similarity to several transporter proteins (data not shown). The highest degree of similarity was to the putative tartrate transporter of Agrobacterium vitis (50% similarity and 32% identity). The protein is likely to belong to the anion:cation symporter (ACS) family of transporters (Pao et al., 1998). It contains all but one of the amino acids within the conserved G(X)2E(X)4P(X)8W(X)P(X)2ER motif (data not shown; Pao et al., 1998). ORF4 is located 18 bp 3′ of ORF3. It consists of 987 nucleotides, has a 70.8% G+C content and a potential SD sequence 10 bp upstream of the ATG start codon (Table 1). The amino acid sequence of the 35.59 kDa predicted protein encoded by ORF4 showed a high degree of homology to several known dehydrogenases (data not shown). The most significant resemblance (57% identity and 68% similarity) was to the E. coli putative 2-hydroxyacid dehydrogenase, YiaE (Fig. 4). The protein contains most of the conserved amino acids within the 2-hydroxyacid dehydrogenase signal (amino acids 157 to 185 and 207 to 229; Fig. 4). In addition, other potential motifs (such as the ATP/GTP and cyclic nucleotide-binding motifs) were identified (Fig. 4).
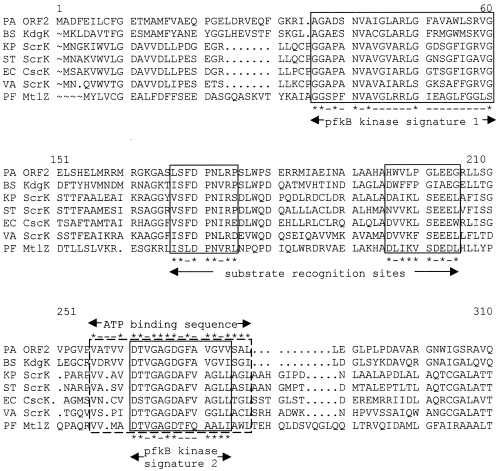
Multiple sequence alignment of the protein encoded by KguK and other bacterial fructokinases. The amino acid sequences are from Bacillus subtilis 2-keto-3-deoxygluconate kinase (BS KdgK, accession number P5084), Klebsiella pneumonia sucrose fructokinase (KP ScrK, accession number P26420), Salmonella typhimurium sucrose fructokinase (ST ScrK, accession number P26984), E. coli fructokinase (EC Csck, accession number P4071), V. alginolyticus sucrose fructokinase (VA ScrK, accession number P22824) and P. fluorescens mannitol fructokinase (PF Mt1Z, accession number O30496). Consensus motifs are boxed. Asterisks identify the conserved residues in each motif.
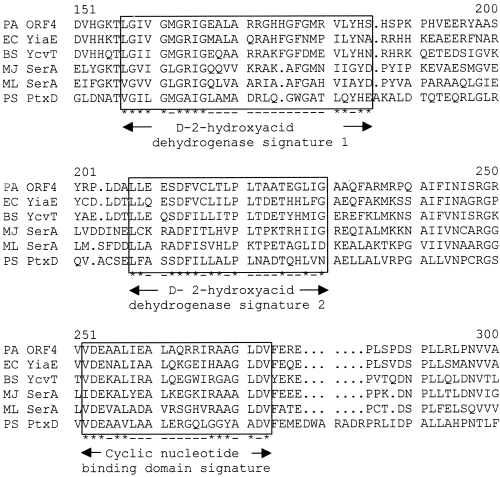
Multiple sequence alignment of the product of KguD with other bacterial dehydrogenases. The amino acid sequences are from E. coli putative 2-hydroxyacid dehydrogenase (EC YiaE, accession number P37666), B. subtilis glycerate dehydrogenase (BS YcvT, accession number H70032), Methanococcus jannaschii D-3-phosphoglycerate dehydrogenase (MJ SerA, accession number Q58424), Mycobacterium leprae D-3-phosphoglycerate dehydrogenase (ML SerA, accession number O33116), P. stutzeri putative dehydrogenase (PS PtxD, accession number AF061070). Consensus motifs are boxed. Asterisks identify the conserved residues in each motif.
Expression of ORF1 and ORF2 in E. coli
To determine the translational products encoded by the potential ORFs, we expressed the genes from the T7 promoter and selectively labelled their products using the T7 expression system (Tabor and Richardson, 1985). The DNA fragment that carries an intact ptxS, ORF1, ORF2 and ORF3 was obtained from the original cosmid (pAH50) that contains ptxS and ptxR (Hamood et al., 1996). Based on the available nucleotide sequences of the three ORFs, we isolated a 5.7 kb BamHI fragment that carries these ORFs from pAH50 and cloned it into the BamHI site of the pT7-6 expression vector generating pBS50. The orientation of the genes carried on the fragment was confirmed using available restriction sites. The cloned genes were expressed from the T7 promoter, and their products were selectively radiolabelled with [35S]-methionine as described previously (Tabor and Richardson, 1985). The E. coli K-38 strain containing the plasmid vector pT7-6 was used as a negative control. As shown in Fig. 5, lane 2, three bands that represent proteins encoded by the ORFs carried on the BamHI fragment were detected. The band at approximately 37 kDa most probably represents PtxS (based on previous analyses) (Colmer and Hamood, 1998). The 32 kDa and 29 kDa polypeptides most probably represent the products encoded by ORF2 and ORF1 respectively (based on the comparison with the size of the predicted proteins; Table 1). No polypeptide was detected that represents the predicted 48 kDa product of ORF3. The reason for our failure to detect this product is not known at this time.

SDS–PAGE analysis of the translational products of ORFs 1 and 2 using the T7 expression system. The 5.8 kb BamHI fragment, which carries intact ptxS, ORF1, ORF2 and ORF3, was cloned in the BamHI site of the T7-6 expression vector generating plasmid pBS50. Expression experiments were performed as described previously (Tabor and Richardson, 1985; Hamood et al., 1996). The 48 kDa product of ORF3 was not detected. Lanes: 1, E. coli strain K38/pT7-6 (negative control); and 2, K38/pBS50. The sizes of the molecular mass standards are shown on the left of the autoradiogram.
Involvement of ORFs 1–4 in 2-ketogluconate utilization in P. aeruginosa
The above results suggest that ORFs 1–4 may constitute a potential operon that is involved in carbohydrate metabolism in P. aeruginosa. To examine this possibility, the ability of an isogenic mutant in this operon to grow in minimal medium containing either glucose or other glucose metabolites as a sole carbon source was examined. An isogenic mutant that carries a deletion within ORF1 was constructed in the P. aeruginosa strain PAO1 as described previously (Stibitz et al., 1986; Colmer and Hamood, 1998). In this mutant (PAO1kgu::Ω), a 276 bp StyI internal fragment of ORF1 was deleted and replaced by the 2 kb omega fragment, which carries transcription termination and translation stop codons in both orientations (Prentik and Kirsch, 1984; Fig. 2). The construction of the mutant was confirmed by Southern blot hybridization experiments (data not shown). If ORFs 1–4 constitute an operon, the mutation in ORF1 should have a polar effect on the transcription of the other genes within the potential operon. Both PAO1 and PAO1kgu::Ω were grown in minimal media containing a 10 mM concentration of glucose, 2-ketogluconate, gluconate, glucose-6-phosphate or 6-phosphogluconate (products of the P. aeruginosa glucolysis cycle) as a sole carbon source. In comparison with PAO1, PAO1kgu::Ω grew in all media tested except the one containing 2-ketogluconate as the sole carbon source (Table 2).
| Carbon source(10 mM)a | Strain | ||||
|---|---|---|---|---|---|
| PAO1 | PAO1ptxS::Ω | PAO1kgu::Ω | PRP621/pLAFR1b | PRP621/pAH50c | |
| Glucose | + | + | + | + | + |
| Gluconate | + | + | + | + | + |
| 2-keto-gluconate | + | − | − | − | + |
| Mannitol | + | + | + | + | + |
| Fructose | + | + | + | + | + |
| Succinate | + | + | + | + | + |
| Glycerol | + | + | + | + | + |
| Citrate | + | + | + | + | + |
- a . Cells were grown in M-9 minimal medium containing the specific carbon source at 32°C for 48 h.
- b . The PRP621 strain was produced by the non-specific mutagenesis of PAO1. The strain cannot grow in a minimal medium that contains 2-ketogluconate as the sole carbon source ( Temple et al., 1998).
- c . Plasmid pAH50 carries a 20 kb fragment of the PAO1 chromosome. The 20 kb fragment contains ptxR, ptxS and kguE, kguK, kguT and kguD. Plasmid pLAFR1 is a cloning vector (negative control).
Further complementation experiments, using the previously described P. aeruginosa 2-ketogluconate-negative mutant PRP621 (Temple et al., 1998), were conducted to confirm that ORFs 1–4 are involved in 2-ketogluconate utilization. This mutant, which mapped at minutes 46–48 of the P. aeruginosa chromosome, does not grow in a minimal medium in which 2-ketogluconate is the sole carbon source (Temple et al., 1998; P. Phibbs, personal communication). Thus, plasmid pAH50 (which carries ptxS and ORFs 1–4) was introduced into the PRP621 strain. In comparison with PRP621, which carries a cloning vector (pLAFR1), PRP621/pAH50 grew in M-9 minimal medium supplemented with 2-ketogluconate as the sole carbon source (Table 2). These results suggest that ORFs 1–4 constitute a potential operon that is involved in the metabolization of 2-ketogluconate in P. aeruginosa. We propose to call this operon the kgu operon.
Depending on the growth conditions, P. aeruginosa metabolizes glucose to the intermediate 6-phosphogluconate by three possible pathways (Temple et al., 1998). Under anaerobic and oxygen-limited conditions, glucose is actively transported into the periplasm by a specific glucose binding protein and then converted into 6-phosphogluconate (Hunt and Phibbs, 1983; Temple et al., 1998). Under aerobic conditions, glucose is first oxidized in the periplasm into gluconate or 2-ketogluconate (Roberts et al., 1973; Temple et al., 1998). The gluconate is imported into the periplasm by a specific permease (encoded by gnuT gene) and assimilated into 6-phosphogluconate by a glucokinase (encoded by the gnuK gene) (Temple et al., 1998). Temple et al. (1998) suggested previously that 2-ketogluconate is actively transported across the cytoplasmic membrane of P. aeruginosa by an active transport system and converted into 6-phosphogluconate by an ATP-dependent kinase (KguK) and an NAD(P)H-dependent reductase (KguR). Although the products of ORFs 1–4 have not been biochemically characterized, their amino acid homology to other previously characterized proteins (Table 1) suggests that they are required for different aspects of 2-ketogluconate utilization. For example, ORF1 may code for an aldose-epimerase. In addition, similar to gnuT, ORF3 may code for the 2-ketogluconate permease. Furthermore, ORFs 2 and 4 may code for KguK and KguR respectively. Therefore, we propose the names kguE, kguK, kguT and kguD(dehydrogenase) for ORFS 1–4 respectively (Fig. 2). The location of these genes (and their products) in the 2-ketogluconate utilization pathway is illustrated in Fig. 6.
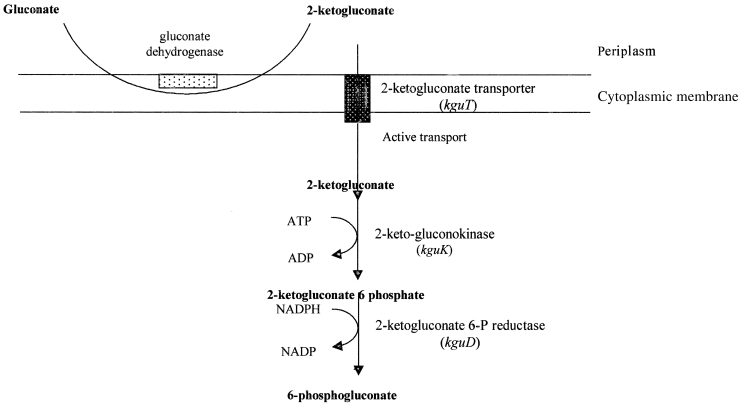
A schematic diagram of the 2-ketogluconate utilization pathway in P. aeruginosa. The genes (within the kgu operon) that code for the different enzymes are indicated. The location of the potential epimerase (that is encoded by kguE) within the pathway is not known at this time.
Identification of the PtxS molecular effector (kgu molecular inducer)
Binding of PtxS to the upstream region of ORF1 suggests that the putative kgu operon is regulated by PtxS (Fig. 1). Similar to other GalR–LacI proteins, this binding may be modulated by an effector molecule (one of the metabolites of the 2-ketogluconate utilization pathway). Therefore, to identify the PtxS molecular effector, the effect of different metabolites on the in vitro binding of PtxS to the 520 bp KpnI–HindIII fragment, which carries OP2, was examined. In a typical protein–DNA binding reaction, the lysate of the E. coli strain K38/pJAC17 was incubated with the 520 bp fragment in the presence of 10 mM glucose, 2-ketogluconate, gluconate, glucose-6-phosphate or 6-phosphogluconate. As shown in Fig. 7A, the binding of PtxS to the 520 bp fragment was inhibited in the presence of 2-ketogluconate. None of the other sugars tested caused any detectable change (Fig. 7A).
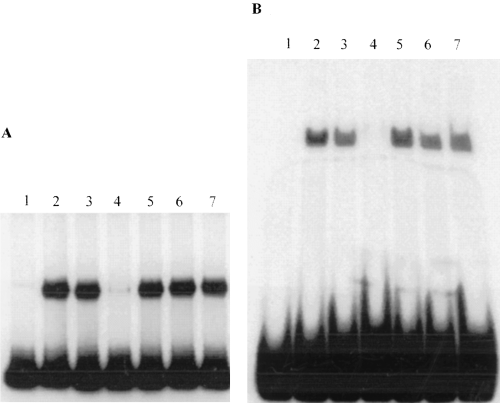
Gel shift experiments to determine the effect of different sugars on the binding of PtxS to the two different probes that carry OP1 and OP2.
A. The 520 bp probe (which carries OP2) was incubated with 1 µg of the lysate of the E. coli strain K38/pJAC17 (in which PtxS is overproduced) in the presence of 10 mM of the indicated sugar. Lanes: 1, 520 bp probe alone; 2, 520 bp probe + K38/pJAC17 lysate; 3, 520 bp probe + K38/pJAC17 lysate + glucose; 4, 520 bp probe + K38/pJAC17 lysate + 2-ketogluconate; 5, 520 bp probe + K38/pJAC17 lysate + d-gluconic acid; 6, 520 bp probe + K38/pJAC17 lysate + d-glucose-6-phosphate; 7, 520 bp probe + K38/pJAC17 lysate + 6-phosphogluconate.
B. The 52 bp fragment (which carries OP1) was incubated with the lysate of K38/pJAC17 in the presence of 10 mM of the indicated sugar. Lanes: 1, 52 bp probe alone; 2, 52 bp probe + K38/pJAC17 lysate; 3, 52 bp probe + K38/pJAC17 lysate + glucose; 4, 52 bp probe + K38/pJAC17 lysate + 2-ketogluconate; 5, 52 bp probe + K38/pJAC17 lysate + d-gluconic acid; 6, 52 bp probe + K38/pJAC17 lysate + d-glucose-6-phosphate; 7, 52 bp probe + K38/pJAC17 lysate + 6-phosphogluconate.
We also examined the effect of 2-ketogluconate on PtxS binding to OP1. Previous studies have shown that effector molecules interfere with the binding of several of the autoregulated proteins of the GalR–LacI family to the upstream region of their genes (Gerlach et al., 1990; Rolfes and Zalkin, 1990; Weickert and Adhya, 1993a). The lysate of E. coli strain K38/pJAC17 was incubated with the 52 bp fragment of the ptxS upstream region (which carries OP1) in the presence of 10 mM 2-ketogluconate. As shown in Fig. 7B, 2-ketogluconate interfered with the PtxS binding to the 52 bp fragment. Other tested metabolites produced no detectable effect (Fig. 7B). These results suggest that 2-ketogluconate is the molecular inducer that affects both PtxS autoregulation and the regulation of the kgu operon by PtxS.
Examination of Fig. 7 indicates that 10 mM ketogluconate has a more pronounced effect on PtxS binding to OP1 than on its binding to OP2. As all experiments were conducted using the same amount of proteins and DNA probe, it is possible, therefore, that 2-ketogluconate inhibits PtxS binding to OP1 more efficiently than PtxS binding to OP2. To examine such a possibility, we determined the effect of two different concentrations of 2-ketogluconate on PtxS binding to OP1 and OP2. At 5 and 10 mM concentrations of 2-ketogluconate, PtxS binding to OP2 was significantly reduced, whereas PtxS binding to OP1 was eliminated (data not shown). Additional titration experiments were conducted to determine the minimum concentration of 2-ketogluconate that is required to interfere with PtxS binding to OP1. PtxS binding to OP1 was inhibited at 3 mM concentration and reduced at 1 mM concentration of 2-ketogluconate (Fig. 8). However, PtxS binding to OP1 was not affected at either 100 µM or 10 µM 2-ketogluconate (Fig. 8).
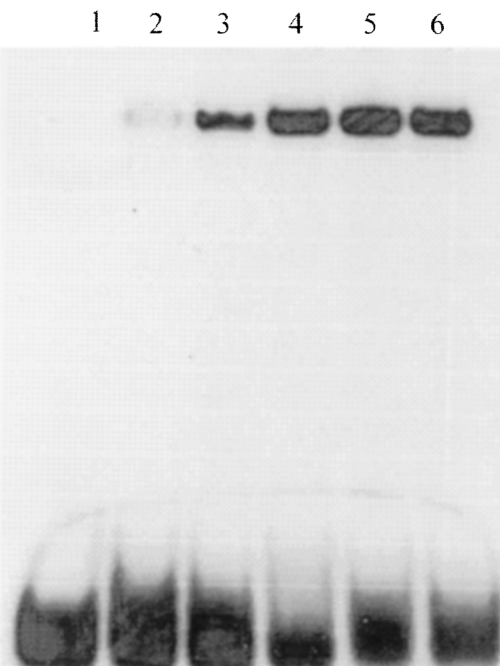
Gel shift experiments to determine the minimum concentration of 2-ketogluconate that is required to interfere with PtxS binding to OP1. The 52 bp fragment (that carries OP1) was incubated with 1 µg of the lysate of K38/pJAC17 in the presence of different concentrations of 2-ketogluconate. Lanes: 1, 52 bp probe alone; 2, 52 bp probe + K38/pJAC17 lysate + 3 mM 2-ketogluconate; 3. 52 bp probe + K38/pJAC17 lysate + 1 mM 2-ketogluconate; 4, 52 bp probe + K38/pJAC17 lysate + 100 µM 2-ketogluconate; 5, 52 bp probe + K38/pJAC17 lysate + 10 µM 2-ketogluconate; and 6, 52 bp probe + K38/pJAC17 lysate (positive control).
Determining whether ptxS is part of the kgu operon
The presence of the14 bp palindromic sequence within the upstream region of kguE (Fig. 2) suggests that the kgu operon is regulated by the adjacent, but separate, ptxS gene. However, it is also possible that ptxS, which is separated from ORF1 by 72 bp and is transcribed in the same direction as ORF1 (Fig. 2), is part of the kgu operon. Thus, to determine whether kguE–kguD constitutes a separate operon, we investigated the presence of a potential promoter within the 520 bp KpnI–BamHI fragment that carries the ptxS–ORF1 intergenic region (Fig. 2). The 520 bp fragment was cloned in the lacZ broad-host-range vector pMP190 (Spaink et al., 1987), and the resulting plasmid (pBS54), in which the lacZ gene is transcribed in the same direction as ORF1, was introduced into PAO1. Cells were grown in LB broth containing 2% glucose (to induce the potential kgu operon). The presence of high levels of glucose in the growth media has been shown to induce the P. aeruginosa 2-ketogluconate pathway (Lessie and Phibbs, 1984). However, the level of β-galactosidase activity produced by pBS54 was similar to that produced by the control strain (PAO1 carrying the cloning vector pMP190; data not shown). This suggests that ptxS together with the other four genes constitute an operon.
To support this possibility further, we examined the ability of the previously constructed ptxS isogenic mutant (PAO1ptxS::Ω) (Colmer and Hamood, 1998) to grow in a minimal medium containing 2-ketogluconate as the sole carbon source. If ptxS is a separate gene, PAO1ptxS::Ω would be capable of using 2-ketogluconate (the negative regulation of the kgu operon is eliminated). Alternatively, if ptxS is part of the kgu operon, the PAO1 ptxS::Ω mutant would not grow in this medium (similar to the kguE deletion mutant described above). The M-9 minimal medium containing 2-ketogluconate as the sole carbon source supported the growth of PAO1 but not PAO1ptxS::Ω (Table 2). These results suggest that ptxS is the first gene in the kgu operon.
Discussion
The results presented in this study suggest that the identified gene cluster represents an operon that is involved in the utilization of 2-ketogluconate in P. aeruginosa and is negatively regulated by PtxS. This suggestion is supported by several findings. First, a P. aeruginosa mutant that carries a mutation in the second gene of the operon (kguE) was not able to grow on a minimal media that contained 2-ketogluconate as the sole carbon source (Table 2). Secondly, the defect of the previously described 2-ketogluconate utilization mutant PRP621 was complemented by a plasmid that carries the intact operon (Table 2). Thirdly, the in vitro binding of PtxS to its operator sites within the upstream regions of ptxS and kguE was inhibited in the presence of 2-ketogluconate (Fig. 7). Fourthly, the predicted functions of the proteins encoded by kguK, kguT and kguD appear to correspond to those of the previously proposed enzymes of the 2-ketogluconate pathway (Fig. 6, Table 1). Although other P. aeruginosa genes may be required for 2-ketogluconate utilization, those genes are less likely to be part of the kgu operon, as we have identified a strong Rho-independent transcriptional termination signal (CGCGCCGGGCCGACGCCCGGCGCG…TTTTT) 4 bp 3′ of kguD (data not shown). We were not able to assign a function for the predicted product of kguE. The predicted product showed no homology to any protein that is involved in carbohydrate utilization. The protein showed some homology (29% identity, 45% similarity) to a hypothetical Mycobacterium tuberculosis protein (data not shown).
With regard to the relationship between PtxS and the kgu operon, our results point out two unique features. First, although our in vitro binding studies suggest that PtxS negatively regulates the expression of the kgu operon (1, 7), ptxS appears to be part of the kgu operon. Both computer analysis and an experimental approach failed to identify either a potential transcriptional start site or a potential promoter within the upstream region of kguE (data not shown). In addition, the PAO1ptxS::Ω isogenic mutant failed to grow in a minimal medium in which 2-ketogluconate is the sole carbon source (Table 2). Secondly, two PtxS operator sites were identified: one within the ptxS upstream region (OP1); and another (OP2) within the upstream region of ORF1 (Fig. 2). Most of the genes that code for GalR–LacI repressors are known to be independently transcribed from their target genes (Weickert and Adhya, 1993b). The operator sites for these proteins are usually located within either the upstream region or the coding sequence of the genes they regulate (Gerlach et al., 1990; Weickert and Adhya, 1993a;Adhya, 1996). Furthermore, the GalR–LacI proteins that are known to be autoregulated possess two operator sites: one within the upstream region of their genes; and one within the upstream region of the target gene (Gerlach et al., 1990; Rolfes and Zalkin, 1990; Weickert and Adhya, 1993a). Hager et al. (1999) recently described a P. aeruginosa gene, gnuR, which is divergently transcribed from the gnuT and gnuK genes and codes for a protein, GnuR, that belongs to the GalR family of repressors. GnuR negatively regulates the expression of gnuT and gnuK. Similar to PtxS, GnuR contains a helix–turn–helix motif (DNA binding motif). In addition, gluconate appears to be the inducer metabolite of the gluconate operon (Hager et al., 1999). Furthermore, there appear to be three GnuR operator sites within the upstream regions of gnuR, gnuK and gnuT (Hager et al., 1999).
The presence of a negative regulatory gene and its target genes in a single transcriptional unit has been reported previously. For example, the sucrose 6-phosphate gene of Streptococcus mutans, scrB, and its negative regulatory gene, scrR, are located within the same operon (scrR is 3′ of scrB) (Gering and Bruckner, 1996). In Bacillus subtilis, the first gene in the kdgRKAT operon (which is involved in the late stages of galacturonic acid utilization) is the negative regulatory gene, kdgR (Pujic et al., 1998). Both ScrR and KdgR belong to the GalR–LacI family of repressors (Gering and Bruckner, 1996; Pujic et al., 1998). Inactivation of either of these genes appears to upregulate the expression of their respective operons. A specific mutation in the scrR of S. mutans elevated the expression of scrB (Gering and Bruckner, 1996). Similarly, the transcription of the kdg operon in the B. subtilis knock-out mutant in kdgR was galacturonate independent (Pujic et al., 1998). In contrast, the inactivation of ptxS rendered the kgu operon non-functional (Table 2). At this time, it is not known whether the OP2 site plays any role in the expression of the kgu operon. Several genes that are regulated by GalR–LacI proteins contain two operator sites (Rolfes and Zalkin, 1990; Weickert and Adhya, 1993a). These genes are thought to be regulated by the DNA looping mechanism, in which binding of the regulatory protein to both operators causes looping of the intervening DNA sequence (which carries the promoter region) (Weickert and Adhya, 1993a). However, based on available evidence and our recent preliminary transcriptional analysis of ptxS, the ptxS promoter is likely to be 5′ of the OP1 site and will not be included in the intervening DNA sequence between the OP1 and OP2 sites. In addition, in the previously described genes, the OP2 site is located within either the upstream region or the coding sequence of the regulated gene (Weickert and Adhya, 1993a). In contrast, the DNA loop between OP1 and OP2 in the kgu operon would contain the entire ptxS structural gene (Fig. 2). It is possible that the efficiency with which PtxS binds to either OP1 or OP2 plays a role in the regulation of the kgu operon by PtxS. As shown in Fig. 7A, a low level of PtxS binding to OP2 was detected even at 10 mM concentrations of 2-ketogluconate. However, PtxS binding to OP1 was eliminated at 5 and 10 mM concentrations of 2-ketogluconate (Fig. 7B). This suggests that PtxS binds more efficiently to OP2 than to OP1. The significance of these differences on the expression of the kgu operon in vivo is yet to be determined.
The results presented in this study confirm our previous analysis, which suggested that the 14 bp palindromic sequence (OP1 and OP2) is the PtxS operator site (B. L. Swanson and A. N. Hamood, submitted). We have shown previously that deletion of, or single basepair changes within, the 14 bp sequence interfered with PtxS binding and significantly enhanced ptxS expression (B. L. Swanson and A. N. Hamood, submitted). The 14 bp sequence appears to be the only operator site for PtxS within the P. aeruginosa chromosome. During our search of the PAO1 genomic sequence, we identified several regions that contained most of the nucleotides within the 14 bp sequence. However, none of these sequences contains the exact 7 bp inverted repeats (data not shown). The two most probable of these sequences contained 12 bp of the 14 bp of the palindrome (they lack the TA nucleotides at the periphery of each half of the repeat). Both sequences are located within the upstream regions of two P. aeruginosa genes: the wpbW locus (which is involved in the synthesis of lipopolysaccharides; Rocchetta et al., 1998) and the parC gene (which codes for the P. aeruginosa topoisomerase IV subunit; Akasaka et al., 1999). However, preliminary DNA gel shift experiments revealed no specific binding between PtxS and a DNA fragment that carries the 12 bp sequence within the upstream region of the wpbW locus (data not shown).
Available evidence suggests that, in P. aeruginosa, PtxS appears to have a direct effect on 2-ketogluconate utilization and an indirect effect on exotoxin A production (through its effect on the toxA positive regulatory gene, ptxR) (1, 7, Tables 1 and 2; Colmer and Hamood, 1998). Whether these effects are dependent on each other is not known at this time. Recent studies have suggested that glucose might influence the expression of certain virulence factors in Gram-negative bacteria (possibly through an increase in the activity of the cAMP–CRP complex; Edwards and Schifferli, 1997; Skorupki and Taylor, 1997). However, it has been shown previously that the presence or absence of repressing carbon sources had no effect on the intracellular level of cAMP in P. aeruginosa (Siegel et al., 1977; Phillips and Mulfinger, 1981; McGregor et al., 1995). In addition, although we have identified a potential CRP binding site within the ptxS–ptxR intergenic region (Colmer and Hamood, 1998; Swanson et al., 1999), this site plays no role in either PtxS binding to the OP1 site or ptxS expression (data not shown). As ptxR is divergently transcribed from ptxS (Colmer and Hamood, 1998), it is possible that the PtxS binding site overlaps with a specific sequence that is required for the efficient transcription of ptxR. Such an effect, which may be influenced by the level of 2-ketogluconate in the medium, is less likely to occur in the initiation of ptxR transcription. The recently identified ptxR transcriptional start sites (T1 and T2) do not overlap with OP1. The distance between OP1 and the ptxR putative T1 and T2 sites are 164 and 313 bp respectively. However, OP1 may overlap with the binding site of a ptxR positive regulatory protein. Preliminary DNA gel shift experiments showed that the ptxS–ptxR intergenic region specifically binds to other P. aeruginosa proteins (other than PtxS; data not shown). At this time, we do not know whether the presence of different levels of 2-ketogluconate (as a sole carbon source) would affect either ptxR expression and/or exotoxin A synthesis.
Experimental procedures
Bacterial strains, plasmids, media and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 3. E. coli strains were grown in Luria–Bertani medium [1% Bacto tryptone (Difco Laboratories), 0.5% yeast extract, 1% NaCl] (Miller, 1972). P. aeruginosa strain PAO1 and its ptxS isogenic mutants were grown in M-9 media (Miller, 1972) with the addition of 10 mM carbon source (Sigma-Aldrich Chemicals) as indicated. A calcium-free solution of 100 mM 2-ketogluconate was prepared as follows: 1.45 g of 2-ketogluconic acid salt (Sigma; H20 content 1.5 mol mol−1) was dissolved in 48 ml of distilled water, heated to 70°C for 10 min and 12 ml of 10.5 M potassium phosphate buffer (pH 7.0) was added. After centrifugation for 15 min at 6.0 g, the resulting 100 mM 2-ketogluconate solution was decanted from the insoluble calcium phosphate and filter sterilized. Cultures were grown at 37°C with vigorous aeration. Antibiotics were used at the following concentrations: ampicillin, 75 µg ml−1 (E. coli); streptomycin, 400 µg ml−1 (P. aeruginosa); rifampin, 80 µg ml−1 (P. aeruginosa).
| Strains/plasmids | Description | Source or reference |
|---|---|---|
| Pseudomonas aeruginosa | ||
| PAO1 | Prototroph | Holloway et al. (1979) |
| PRP621 | PAO1 derivative, kgu-11, hiu-107, ami-151a | Temple et al. (1998) |
| PAO1kguE::Ω | ΔkguE (orf1)::Ω, Smr | This study |
| PAO1ptxS::Ω | ΔptxS::Ω, Smra | Colmer and Hamood (1998) |
| Escherichia coli | ||
| K-38 | HfrC, host for pT7 expression system | Tabor and Richardson (1985) |
| Plasmids | ||
| pLAFR1 | Tcr, Kmr, incP lambda cos+ broad-host-range cloning vector | Friedman et al. (1982) |
| pAH50 | pLAFR1 carrying a 25 kb EcoRI fragment of PAO1 that contains intact ptxS and ORFs 1–4 | This study |
| pJAC17 | pT7-5 carrying a 1487 bp HincII–HindIII fragment that contains intact ptxS | Colmer and Hamood (1998) |
| pUC18 | Cbr/Apr ColE1, general cloning vectora | Yanisch-Perron et al. (1985) |
| pBS47 | pUC18 carrying the 5.7 kb BamHI fragment | This study |
| pT7-6 | Cbr, cloning vector for the T7 expression system | Tabor and Richardson (1985) |
| pBS50 | pT7-6 carrying the 5.7 kb BamHI fragment | This study |
| pMP190 | Smr, Cmr, β-galactosidase transcriptional fusion vectora | Spaink et al. (1987) |
| pBS54 | kgu promoter fusion in pMP190 | This study |
| pBS55 | 2.3 kb SmaI fragment carried in pUC18 | This study |
| pBS56 | pBS55 carrying Ω in the StyI site of ORF1 | This study |
- a .Δ, deletion; Ω, the 2.0 kb SmaI omega fragment; Sm, streptomycin; Ap, ampicillin; Cb, carbenicillin; ColE1, ColEI origin of replication; Cm, chloramphenicol; r, resistant; kgu, 2-ketogluconate utilization.
DNA manipulations
DNA manipulations and other molecular biology techniques were performed essentially as described previously (Ausubel et al., 1988). Plasmids were introduced into P. aeruginosa by electroporation as described previously (Smith and Iglewski, 1989). P. aeruginosa chromosomal DNA was extracted according to the methods of Goldberg and Ohman (1984). PCR amplification of specific DNA fragments was carried out as described previously (White, 1993). Plasmid pBS54 (Table 3) was constructed by cloning the 520 bp fragment that carries the ptxS–ORF1 intergenic region (Fig. 2) in the lacZ broad-host-range vector pMP190 (Spaink et al., 1987). The resulting plasmid (pBS54), in which the lacZ gene is transcribed in the same direction as ORF1, was introduced into PAO1.
Gel shift assay
DNA fragments were obtained by either restriction digestion or PCR. The fragments were labelled with [γ32P]-ATP (Amersham-Pharmacia Biotech) by the end-labelling technique using T4 polynucleotide kinase (Ausubel et al., 1988). The binding experiments were performed as described previously (Swanson et al., 1999) with the addition of 10 mM carbon source to the binding reaction as indicated.
Expression experiments
PtxS was overproduced in the E. coli strain K-38 for use in the gel shift assay by the T7 expression system as described previously (Tabor and Richardson, 1985; Colmer and Hamood, 1998). PtxS and its downstream ORFs were expressed in the T7 expression system with the addition of Tran[35S]-methionine/cysteine (NEN Biochemicals) as indicated to label the expressed proteins selectively.
Nucleotide sequence analysis and protein sequence comparisons
The nucleotide sequence was analysed using the dna strider program. The protein sequence comparisons were performed using the GCG software package (Devereux et al., 1984). The Kyte and Doolittle (1982) hydropathy plot analysis was used to examine the amino acid sequence of the predicted proteins for potential hydrophobic regions. DNA sequences downstream of ptxS were obtained by searching the chromosomal database at the P. aeruginosa genome project website (http://www.pseudomonasgenome.com).
β-Galactosidase assay
A 1 ml pellet of the cell cultures was washed and resuspended in 100 µl of distilled water. The cells were disrupted by sonication, and the levels of β-galactosidase activity were determined as described previously (Miller, 1972).
Construction of a kgu isogenic mutant in P. aeruginosa strain PAO1
An isogenic mutant of the P. aeruginosa strain PAO1 that carries a deletion in ORF1 was constructed by the gene replacement technique described previously (Stibitz et al., 1986; Hamood et al., 1996). Plasmid pBS47, which is a pUC18 recombinant plasmid that carries a 5.7 kb BamHI fragment of the PAO1 chromosome, was used for these experiments. The 5.7 kb BamHI fragment contains ptxS, ORF1, ORF2, ORF3 and part of ORF4 (Fig. 2). A 2.3 kb SmaI fragment, which carries ORFs 1 and 2, was isolated from pBS47 and cloned into the SmaI site of pUC18 generating plasmid pBS55. Plasmid pBS55 was digested with StyI, and a 276 bp ORF1 internal fragment was deleted. The 5′ protruding ends were converted to blunt ends, and the 2.0 kb SmaI Ω fragment was inserted into the deleted region, generating plasmid pBS56. The Ω fragment carries the T4 transcriptional termination signal and translational stop codon in both orientations (Prentik and Kirsch, 1984). Plasmid pBS56 was introduced into PAO1 by electroporation, and transformants were recovered on LB plates containing Sm. The resulting Smr colonies were then screened for Smr, Cbs colonies. These clones represent cells in which a double cross-over event occurred, resulting in the loss of the cloning vector and the wild-type copy of ORF1 and the retention of the Ω-interrupted copy of ORF1. This insertion should create a polar effect and obliterate the transcription of the entire 2-ketogluconate utilization operon. Construction of the isogenic mutant was confirmed by Southern blot hybridization experiments as described previously (Ausubel et al., 1988).
Acknowledgements
The authors thank Jane A. Colmer for her help in several experiments and her software expertise. This work was supported by grants AI-333-86 (to A.H.) and AI-15940 (to M.V.) from the National Institutes of Health. Britta Swanson was supported in part by a fellowship from the Cystic Fibrosis Foundation.




