Oxygenated mycolic acids are necessary for virulence of Mycobacterium tuberculosis in mice
Abstract
Members of the Mycobacterium tuberculosis group synthesize a family of long-chain fatty acids, mycolic acids, which are located in the cell envelope. These include the non-oxygenated α-mycolic acid and the oxygenated keto- and methoxymycolic acids. The function in bacterial virulence, if any, of these various types of mycolic acids is unknown. We have constructed a mutant strain of M. tuberculosis with an inactivated hma (cmaA, mma4) gene; this mutant strain no longer synthesizes oxygenated mycolic acids, has profound alterations in its envelope permeability and is attenuated in mice.
Introduction
Mycolic acids, high molecular weight (C60–C90) α-alkyl, β-hydroxy fatty acids are produced by all species of mycobacteria and constitute 40–60% by weight of the complex cell envelope (Goren and Brennan, 1979; Minnikin, 1982). This envelope consists of a plasma membrane, a cell wall and an outer capsule (Daffé and Draper, 1998). Mycolic acids are arranged in a bilayer with the cell wall lipids, forming a permeability barrier of extremely low fluidity, distinct from the plasma membrane (Liu et al., 1995). According to the current model (Nikaido et al., 1993; Daffé and Draper, 1998), the inner leaflet is made up of the highly structured mycolic acids arranged perpendicular to the cell wall and covalently linked to arabinogalactan, and the outer leaflet is made up of other lipids. In addition to α-mycolic acid, which may contain double bonds and cyclopropane rings and which are synthesized by all mycobacterial species (Dafféet al., 1983), most species also produce oxygenated mycolic acids. Alterations in the proportion and structures of mycolic acids have been shown to produce significant changes in the fluidity of this inner layer resulting in changes in permeability (George et al., 1995; Jackson et al., 1999).
All members of the Mycobacterium tuberculosis complex (Mycobacterium tuberculosis, Mycobacterium africanum, Mycobacterium bovis and Mycobacterium microti) are pathogenic and synthesize the same combination of mycolic acids, i.e. α-mycolic acids, ketomycolic and methoxymycolic acids (Fig. 1), except the attenuated vaccine strain, M. bovis BCG Pasteur, which only produces α-mycolic and ketomycolic acids. A gene cluster from the M. tuberculosis complex has been described, coding for four highly similar methyl transferases, which when introduced into Mycobacterium smegmatis on a multicopy plasmid causes the synthesis of ketomycolic and methoxymycolic acids, in addition to a novel mycolic acid, hydroxymycolic acid (Dubnau et al., 1997) (Yuan and Barry, 1996). None of these are normally made in this mycobacterial species. We postulated that the methyl transferase coded by cmaA (mma4), methylates a double bond in α-mycolic acid (or in a precursor molecule) which can then be hydrated, resulting in the insertion of a hydroxy group adjacent to a methylated carbon, thus producing hydroxymycolic acid (Dubnau et al., 1997). In this model, hydroxymycolic acid is the substrate for an oxidase which inserts the keto function into mycolic acids. Such an enzyme is present in members of the M. tuberculosis complex, because they naturally produce ketomycolic acid. M. smegmatis must contain an equivalent enzyme activity, because introduction of only the cmaA (mma4) gene into M. smegmatis is sufficient for the formation of ketomycolic acid as well as hydroxymycolic acid (Dubnau et al., 1997). Yuan and Barry, (1996) also detected hydroxymycolic acid in M. smegmatis carrying the same gene cluster from M. tuberculosis and although they did not detect ketomycolic acid, they also considered that hydroxymycolate is a common intermediate for both ketomycolic and methoxymycolic acid and they also postulated that mma4 (cmaA) is required for the synthesis of this intermediate. cmaB (mma3) encodes the protein responsible for inserting the methoxy function into mycolic acid and it was shown that the sequence differences between cmaB (mma3) in M. bovis BCG Pasteur and M. tuberculosis H37Rv account for the lack of methoxymycolic acid in the former strain (Dubnau et al., 1998; Yuan et al., 1998). cmaC (mma2) and cmaD (mma1) which are also in the gene cluster, are responsible for cyclopropanation of the oxygenated mycolic acids (Yuan and Barry, 1996; Dubnau et al., 1997).
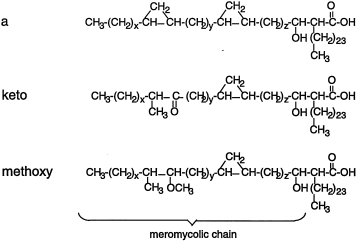
Structure of mycolic acids of M. tuberculosis complex
To establish firmly the role of the cmaA gene in the biosynthesis of oxygenated mycolic acids, to determine whether these mycolates contribute to the permeability of the cell wall and to assess their role in virulence, we constructed a mutant of M. tuberculosis H37Rv with a disrupted cmaA (mma4) gene. Because of the results, described below, we propose that this gene, originally called cmaA (for cyclopropanation of mycolic acids) (Dubnau et al., 1997) or mma4 (for methoxy mycolic acids) (Yuan and Barry, 1996), be renamed hma (hydroxymycolic acid).
Results
Construction of inactivated hma gene in M. tuberculosis
A 1.77 kb hygromycin-R (Lydiate et al., 1989) cassette was inserted into the unique SacI site of hma in the cma gene cluster from BCG (Dubnau et al., 1997) (Fig. 2). This construct was electroporated into M. tuberculosis H37Rv, with selection for hygromycin resistance, followed by selection for sucrose resistance (Suc-R), which would result from a double cross-over (Fig. 2A). One of the Hyg-R Suc-R clones was shown by Southern hybridization (Fig. 2B) to have the expected disruption in the hma gene. We refer to this strain as hma::hyg. The cmaB (mma3) gene of this mutant strain could have originated from either the BCG gene cluster which was used to construct the inactivated hma or from the host M. tuberculosis chromosome. If the gene had originated from BCG, the methoxymycolate synthetase would be defective (Dubnau et al., 1998; Yuan et al., 1998), but if it came from the host M. tuberculosis chromosome, it would result in an active gene product. In fact, the sequence of cmaB (mma3) from the hma::hyg mutant strain was determined and was the same as the sequence of M. tuberculosis H37Rv (data not shown).
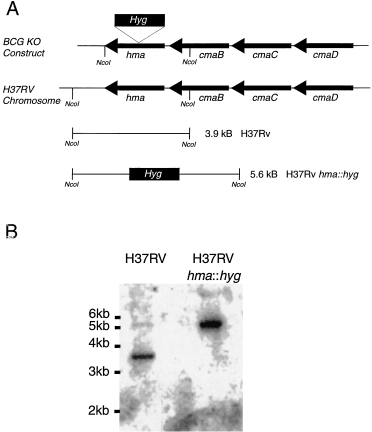
Inactivation of the chromosomal hma gene of M. tuberculosis by allelic replacement with hma containing a hyg cassette.
A. The hyg cassette (1.77 kb) was inserted into hma of M. bovis BCG in a plasmid containing the cma gene cluster (Dubnau et al., 1997). The resulting 8.3 kb EcoRI DNA fragment was subcloned into pSM111 (constructed in the laboratory of I. Smith), a vector containing the sacB gene from B. subtilis which confers sucrose sensitivity to mycobacteria (Pelicic et al., 1996) and which cannot replicate in mycobacteria. Although the M. tuberculosis gene sequences are nearly identical (Yuan and Barry, 1996), hma of M. bovis BCG lies on a 1.6 kb NcoI fragment, whereas hma of M. tuberculosis lies on a 3.9 kb NcoI fragment. Insertion of the hyg cassette into the unique SacI site of M. bovis BCG hma increases the size of the NcoI fragment to 3.37 kb. A plasmid containing this construct was used to select the M. tuberculosis recombinant strain (hma::hyg) and the inactivated gene should be on a 5.6 kb NcoI fragment.
B. Southern hybridization of NcoI-digested DNA from M. tuberculosis H37Rv and from the M. tuberculosis H37Rv Hyg-R Suc-R recombinant using as a probe, a 32P-labelled DNA fragment containing hma from M. bovis BCG.
The mutant strain grew with approximately the same doubling time as the isogenic parent when grown in rolling cultures and was equally sensitive to various antibiotics, including isoniazid, rifampicin, ciprofloxacin and ampicillin (data not shown).
Lipid composition of hma::hyg mutant strain with its parent M. tuberculosis H37Rv
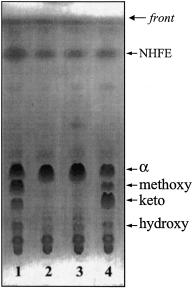
To address the question of the effects of the disruption of the hma gene on the cell envelope, we compared the lipid composition of M. tuberculosis H37Rv and the isogenic mutant strain. Lipids extracted by organic solvents from M. tuberculosis H37Rv or the hma::hyg strain represented 20 ± 3% and 21 ± 1% of the bacterial dry weights respectively. No obvious difference was seen between the two strains in terms of major lipids including sulphatides, triacyl glycerol, glycerol mycolate, trehalose dimycolate, trehalose monomycolate and phospholipids (Brennan, 1988; Daffé and Draper, 1998). Analysis of the saponification products of the crude lipid extracts showed that the content of C12–C30 fatty methyl esters of the mutant is similar to that of M. tuberculosis H37Rv (Dafféet al., 1983). However, although the total amount of mycolates found in the extractable lipid fraction and in the cell wall of the two strains are similar, there is a drastic difference in the mycolate pattern between the mutant and the wild-type strains. The parent strain makes three types of mycolates (Dafféet al., 1983), α-mycolate, methoxymycolates and ketomycolates, but only α-mycolates were synthesized by the mutant (Fig. 3). We conclude that a functional hma gene is necessary for the synthesis of both methoxy and ketomycolates as previously proposed (Dubnau et al., 1997; Yuan et al., 1998). These mycolates are believed to be synthesized from a common precursor, hydroxymycolate, first identified in a strain of M. smegmatis transformed by the hma-containing gene cluster (Yuan and Barry, 1996; (Dubnau et al., 1997) and recently characterized in members of the M. tuberculosis complex as a minor compound (Quémard et al., 1997). Fractionation of the saponification products from M. tuberculosis H37Rv and the hma::hyg mutant strain confirmed the presence of the hydroxymycolate in M. tuberculosis H37Rv but none was found in the strain with the disrupted hma gene (Fig. 3). These results show that the hma gene product is required for the production of all oxygenated mycolates in M. tuberculosis H37Rv. It codes for an enzyme which performs the key step in biosynthesis of all oxygenated mycolic acids.
Chenodeoxycholate and glycerol uptake by M. tuberculosis H37Rv and the hma::hyg mutant strain. Glycerol (A) and chenodeoxycholate (B) uptake by M. tuberculosis H37Rv (filled symbols) and hma::hyg (open squares).

Thin-layer chromatography of the fatty acid methyl esters from strains of M. tuberculosis H37Rv. M. tuberculosis H37Rv (lane 1), hma::hyg (lane 2), hma::hyg (mma3 on a multicopy plasmid) (lane 3), hma::hyg (hma on a multicopy plasmid) (lane 4). Developing solvent: petroleum ether–diethyl ether [9:1 (v/v), 3 runs] Visualizion by treatment with molybdophosphoric acid [10% (w/v) in ethanol] and charring. NHFE, non-hydroxylated fatty acid methyl esters.
Complementation of hma::hyg mutant strain
The hma::hyg mutant strain was complemented by introducing a replicative plasmid [pMV261 (Stover et al., 1991)] containing the wild-type hma gene cloned downstream from the hsp60 promoter. The presence of the hma gene from M. tuberculosis H37Rv was sufficient to complement the defect in the hma::hyg mutant for the production of keto-, methoxy- and hydroxymycolates (Fig. 3). As expected, the mutant strain carrying cmaB (mma3) cloned into the same vector had no effect on the mycolic acid profile (Fig. 3). We conclude that the activity of the cmaB (mma3) gene product as well as that of the enzyme which inserts the keto-function both require the Hma protein, whose activity results in the formation of hydroxymycolic acid, the common substrate and it is for this reason that we renamed the gene hma. The fact that the mutant strain was complemented fully by hma on a multicopy plasmid, confirms that the other genes necessary for formation of keto- and methoxymycolic acid were functional in the mutant strain which was defective only in the production of the Hma protein.
Permeability of hma::hyg mutant strain and its parent, M. tuberculosis H37Rv
The mycolate layer contributes to the characteristically hydrophobic barrier of the mycobacterial envelope. This layer, which is of abnormal thickness and unusually low fluidity (Jarlier and Nikaido, 1990; Jarlier and Nikaido, 1994), results in the low diffusion of lipophilic molecules such as chenodeoxycholate (Liu et al., 1996; Yuan et al., 1997; Jackson et al., 1999). The mycobacterial cell envelope also possesses a very low permeability to polar molecules such as glycerol which, although it is a hydrophilic molecule, has been recently shown to penetrate the mycobacterial cell wall through lipid domains (Jackson et al., 1999). In order to determine whether the absence of oxygenated mycolic acids affected this barrier, we compared the permeability of the wild-type strain of M. tuberculosis H37Rv and the hma::hyg mutant strain with the hydrophobic chenodeoxycholate and the hydrophilic glycerol, commonly used as a substrate for mycobacterial growth. The mutant strain showed a very low initial rate of uptake and accumulation of both compounds compared with the isogenic M. tuberculosis H37Rv (Fig. 4). Furthermore, the mutant strain showed a much greater resistance to H2O2 than its parent (Fig. 5). This resistance to H2O2, a small molecule which probably diffuses passively through the envelope, can be explained by the increased rigidity of the cell wall in the mutant strain.
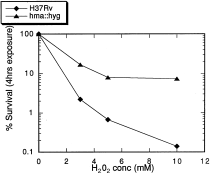
Sensitivity to H2O2 of M. tuberculosis H37Rv and the hma::hyg mutant strain. Exponentially growing broth (Jacobs et al., 1991) cultures were exposed to H2O2 for 4 h and then assayed for cfu.
Attenuation phenotype of the hma::hyg mutant strain
The hma::hyg mutant strain, its isogenic parent, M. tuberculosis H37Rv and the mutant strain carrying the complementing hma gene on a multicopy plasmid were used to infect the THP-1 cell line which differentiates to a macrophage state in the presence of phorbol esters. These strains all grew at about the same rate during the infection (Fig. 6). In contrast to these findings, the strain with the hma::hyg mutation was significantly attenuated for growth in a mouse model of infection using aerosolization, in all the organs tested (Fig. 7). Growth attenuation of the hma::hyg mutant strain, compared with wild type, was also observed when the intravenous route of infection was employed (data not shown). The mutated strain grows more slowly in the lungs, spleen and liver and is cleared more rapidly from the liver than the wild-type strain; this attenuated phenotype is fully complemented by the presence of hma on a multicopy plasmid.
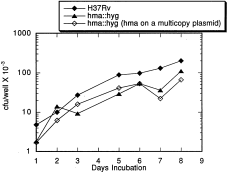
Growth of M. tuberculosis H37Rv and various mutant strains in cultures of the monocytic cell line, THP-1. THP-1 cells (ATCC TIB-202) were induced to the macrophage-like state by 24 h treatment with 40 nM PMA (12-O-tetradecanoylphorbol-13-acetate) (Tsuchiya et al., 1982). Exponentially growing cultures of M. tuberculosis strains were diluted to about 5 × 104cfu ml−1 in tissue culture growth medium just prior to infections. Intracellular bacteria were assayed for cfu per well at the indicated times.
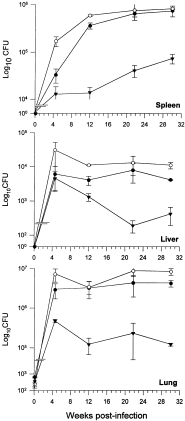
Attenuation of M. tuberculosis H37Rv hma::hyg in the mouse model of infection. Female C57BL/6 mice were infected with ≈ 500 cfu of the various strains of M. tuberculosis H37Rv: closed circles, wild type; closed triangles, hma::hyg mutant; open circles, hma::hyg mutant complemented with the wild-type gene. At various time intervals post-infection, mice were assessed for bacterial burden in the spleen, liver and lungs. Three mice per group were studied at each time interval. Error bars represent standard deviation.
Discussion
By constructing a mutant strain of M. tuberculosis H37Rv with a disrupted hma (formerly mma4 or cmaA) gene, we have demonstrated that biosynthesis of keto-, methoxy- and hydroxymycolic acids depends upon the activity of hma. This gene codes for an enzyme which performs the key step in biosynthesis of all oxygenated mycolic acids. The mutant strain shows decreased permeability to chenodeoxycholate, a hydrophobic substrate and glycerol, a hydrophilic substrate. This result was consistent with the lack of oxygenated mycolates in the mutant strain, because these molecules are expected to confer a marked fluidity to the cell wall (Minnikin, 1982).
Yuan et al. (1998) showed that a strain of M. tuberculosis carrying a plasmid with mma3 (coding for a methyl transferase required for the formation of methoxymycolic acid) overproduces methoxymycolic acid. This strain was deficient in ketomycolic acid. Surprisingly, the uptake of glucose, but not chenodeoxycholate or glycine, was decreased in the methoxymycolate overproducing strain and it was unable to grow in THP-1 cells. These results contrast with those presented here, because the hma::hyg strain, producing no oxygenated mycolic acids was able to grow in THP-1 cells. The authors concluded that the lack of ketomycolic acid was responsible for the phenotype. However, the alterations in cell wall permeability and inability to grow in macrophages could have been due to the overproduction of methoxymycolic acid and not to the lack of ketomycolic acid.
The cell wall is considered to be a major factor in the virulence of pathogens, especially that of mycobacteria, with such an unusually high lipid content. Two recent reports (Camacho et al., 1999; Cox et al., 1999) show that inactivation of genes involved in synthesis or transport of a cell wall associated lipid (phthiocerol dimycocerosate) results in attenuation in the mouse model. Mycolates are known to be the major and hallmark lipids of the mycobacterial cell wall. Inactivation of fbpC, a gene coding for one of the three mycoloyl transferases (Belisle et al., 1997), resulted in a significant decrease in the level of all cell wall-linked mycolate (40%) and in an increase in the permeability of the cell envelope, but the strain was not attenuated in macrophages (Jackson et al., 1999). Similarly, the disruption of fbpB, also coding for mycoloyl transferase, caused no attenuation of the mutant strain in human and mouse macrophage like cell lines (Armitage et al., 2000). Although the mycolate content of the mutant has not been compared with that of the parent strain, it is likely that its cell wall mycolate content has been affected. This assumption is partly based on the fact that FbpA, FbpB and FbpC have been shown to possess a mycoloyl transferase activity in vitro (Belisle et al., 1997). Furthermore, Corynebacterium glutamicum, a bacterium phylogenetically close to mycobacteria, contains a gene, csp1, that codes for a secreted protein, PS1, whose N-terminal portion is similar to Fbp (Joliff et al., 1992); PS1 has been recently shown to transfer corynomycoloyl residues both onto trehalose monomycolate and onto the cell wall arabinogalactan (Puech et al., 2000). Inactivation of the csp1 gene also resulted in a 50% decrease of the cell wall-linked corynomycolate content and an increase of the permeability of the cell envelope of C. glutamicum (Puech et al., 2000), similar to that observed for the fcpC-disrupted mutant of M. tuberculosis (Jackson et al., 1999). Interestingly, complementation of the csp1-inactivated mutant by any of the fbP genes of M. tuberculosis led to the transfer of mycoloyl residues onto the cell wall and restored the permeability of the cell envelope (Puech et al., 2000). In contrast to the decrease of the cell wall-linked mycolate content and the concomittant increase of the permeability of the cell envelope of the fbpC-disrupted mutant (Jackson et al., 1999), inactivation of the hma gene resulted in a decreased permeability of the cell envelope of the hma::hyg mutant strain but with no decrease of its cell wall associated mycolate content. Altogether, these data demonstrate that cell wall mycolates are involved in the outer permeability barrier of mycobacteria and that the fluidity of this barrier depends on the types of mycolates produced by the mycobacterial strain.
The attenuation of the hma::hyg mutant strain in mice deserves consideration. Early physiological experiments on M. tuberculosis showed that the bacteria isolated directly from the mouse lung did not respire when incubated with carbohydrates or glycolytic intermediates, but did when incubated with fatty acids (Segal and Bloch, 1956). In contrast, bacteria from broth cultures were able to respire with all substrates used. The fact that cytochromes from bacteria grown in mouse lungs are in a reduced form and that oxidative phosphorylation is not observed in these bacteria led early workers to propose that M. tuberculosis growing in lung tissue has adapted to a facultative anaerobic mode of metabolism (reviewed in Segal, 1984) using fatty acids as a major substrate. We propose that the attenuation of the hma::hyg mutant strain of M. tuberculosis in the mouse model is due to decreased permeability of its cell wall. This loss of permeability could decrease the availability of specific nutrients normally utilized by M. tuberculosis during growth in tissues of the mouse. Our results can be explained by the hypothesis that M. tuberculosis uses different substrates when growing in mouse tissues than it does in artificial media and that the permeability of the cell wall to these substrates may be a necessary component of virulence. The fact that the mutant strain grows more slowly in mouse lungs than the wild type but after 5 weeks is maintained at a steady-state level, like the wild-type strain, supports the idea of a nutritional defect for growth in mice in the mutant strain rather than an increased sensitivity to bactericidal mechanisms elaborated by the cellular immunity system. In that case, one would expect that after activation of this system, there would be a decrease in viable count of the mutant strain. In this respect, it should be mentioned that an increased dependence on nutrients in broth culture has been recently described for a strain with a disruption in fbPA (Armitage et al., 2000), another gene coding for mycoloyl transferase. However, the reason for this defect has not been addressed.
To our knowledge, the hma::hyg mutant strain described above is the first report of M. tuberculosis with a single mutation resulting in a strain with altered mycolic acid composition. The fact that it is attenuated in mice suggests that the product of hma is important for the pathogenicity of the tubercle bacillus in this model. Like proteins involved in the biosynthesis of other specific cell envelope components of M. tuberculosis, Hma represents a good target for novel antimycobacterial drug development.
Experimental procedures
Isolation, fractionation and analysis of lipids
Lipids were extracted from wet cells for 4 days with CHCl3/CH3OH [1:2, (v/v)] at room temperature with continuous stirring; the bacterial residue was re-extracted five times with CHCl3/CH3OH (2:1, v/v) and the organic phases were pooled and concentrated (Quémard et al., 1997; Jackson et al., 1999). The crude lipid extracts were partitioned between the aqueous and the organic phases arising from a mixture of CHCl3/CH3OH/H2O [8:4:2, (v/v/v)]; the lower organic phases were collected, evaporated to dryness to yield the crude lipid extracts from each strain and comparatively examined by thin-layer chromatography (TLC) on silica gel-coated plates (G-60, 0.25 mm thickness, Merck) developed with petroleum ether–diethyl ether [98:2; 9:1 or 7:3, (v/v)], CHCl3/CH3OH [9:1, (v/v)] or CHCl3/CH3OH/H2O [30:8:1 or 65:25:4, (v/v/v)]. In order to separate mycolate-containing lipids from other compounds, cold CH3OH was added to the diethyl ether solution of crude lipids (10–50 mg ml−1) and the mixture was left at 4°C for 1 h and then centrifuged (4000 g, 15 min). The pellets, which consisted primarily of mycolate-containing molecules, were recovered, dried under vacuum, dissolved in CHCl3 and applied to a silica gel 60 column (particle size 0.063–0.200 mm, Merck) equilibrated in CHCl3. The column was irrigated stepwise with increasing concentrations of CH3OH in CHCl3. Fractions were collected in bulk. Glycoconjugates were revealed by spraying plates with 0.2% anthrone in concentrated H2SO4, followed by heating at 110°C. The Dittmer–Lester reagent (Dittmer and Lester, 1964) was used for visualizing phosphorus-containing substances. Fractionations were monitored by TLC on silica gel-coated plates as described above and fractions containing the same lipid compounds were pooled and rechromatographed to yield purified substances (as judged from the absence of contaminant by TLC), which were analysed by infrared using a Perkin-Elmer FTIR 1600 spectrophotometer.
Delipidated cells from 4-week-old bacilli, lipid extracts and the various fractions eluted from the silica gel 60 column were dried under vacuum prior to weighing and saponified; the fatty acids were methylated and analysed as described (Dafféet al., 1983). Quantification of mycolic acids resulted from the saponification of at least 300 mg (dry weight) of delipidated cells from M. tuberculosis H37Rv and the hma::hyg mutant in the same growth phase. Three sequential determinations from separate preparations of delipidated cells were performed. Mycolic methyl esters were analysed by TLC using petroleum ether–diethyl ether [9 : 1, (v/v)] four runs, or dichoromethane as developing solvents (Dafféet al., 1983). Mass spectrometry analysis of mycolates was carried out as described (Dubnau et al., 1997).
Permeability assays
Exponentially grown cells from M. tuberculosis H37Rv and the hma::hyg mutant were first labelled for 16 h with [5,6-3H]-uracil (2 × 10−5 M, 1.85 TBq mmol−1) to quantify the biomass present in aliquots used in accumulation assays. Cells were collected by centrifugation and washed with 10 mM HEPES (pH 7.2). Aliquots of labelled cells were counted, dried and weighed in order to correlate 3H-labelling with cell dry weight. Accumulation assays were performed under continuous agitation. [14C]-glycerol (6.5 × 10–6 M, 5.66 GBq mmol−1, purchased from Amersham) or [14C]-chenodeoxycholate (2 × 10–5 M, 1.8 GBq mmol−1, purchased from DuPont NEN) was added to a 1 ml mixture of HEPES containing ≈ 40 mg of 3H-labelled 3-week-old M. tuberculosis H37Rv and hma::hyg cells. Aliquots (0.1 ml) were taken up at different time intervals and added on the top of an eppendorf centrifuge tube containing 0.25 ml oil [silicon–paraffin oil 1:0.2, (v/v)]. Cells were separated from the accumulation medium by centrifugation (13 000 g, 1 min). Centrifuged tubes were frozen on dry ice and the pellets were dropped into counting flasks by cutting the cone bottom. Then, the scintillation solution (Aqualuma) was added and the vials were sonicated for 30 min in a water bath to disperse cells. Countings were performed using a Packard Tricarb 1900 TR equipped with a 3H/14C program.
Growth in THP-1 cells
THP-1 (ATCC TIB-202) is a monocytic human cell line that can be induced with phorbol esters to a macrophage-like state (Tsuchiya et al., 1982). THP-1 cells were grown in RPMI 1640 supplemented with 10% FCS, 0.45% glucose, 0.15% sodium pyruvate, 0.05 mM 2-mercaptoethanol and 4 mM l-glutamine. Cultures were maintained at 1–5 × 105 cells ml−1 and the cells were induced to the macrophage-like state by 24 h treatment with 40 nM PMA (12-O-tetradecanoylphorbol-13-acetate) (Tsuchiya et al., 1982), plating them in 96-well plates. Exponentially growing cultures of M. tuberculosis strains were diluted to about 5 × 104 cfu ml−1 in tissue culture growth medium just prior to infections. Infections were carried out in triplicate (at a multiplicity of infection of 0.1–0.5 cfu per macrophage) and after about 3 h the supernatants were removed, the cells washed twice with 0.1 ml of phosphate-buffered saline (PBS) and fresh media was added. Intracellular bacteria were assayed for cfu well−1 by removing the supernatant, lysing the macrophages with 0.05% SDS and plating at various dilutions on 7H10 plates supplemented with ADN (Jacobs et al., 1991). Extracellular bacteria were enumerated by plating the supernatant; the numbers were always less than 1% of the intracellular bacteria.
Aerosol infection of mice
Female C57BL/6 mice, 8- to 10-week-old, were infected with ≈ 500 cfu of M. tuberculosis H37Rv, the mutant hma::hyg strain, and the hma::hyg strain with the complementing hma plasmid, via the respiratory route, using the ‘nose-only’ aerosolization apparatus (In-TOX Products) as previously described (Tsenova et al., 1997). Aerosols carrying M. tuberculosis were generated from a bacterial suspension consisting of 1 × 107 cfu ml–1 of PBS (pH 7.4) with 0.05% Tween. Mice were exposed to the aerosols for 20 min and three mice were used for each experimental point. At various times after infection, tissue bacterial burden was assessed as previously described (Chan et al., 1995).
Acknowledgements
This work was supported by NIH grant AI44856, awarded to I.S. and grant no. 99N92/0621 from the Direction des Relations Internationales du CNRS (Coopération internationale CNRS/NSF) awarded to M.D. We also thank D. Dubnau for his encouragement, advice, interest and tons of jokes.




