The G-protein FlhF has a role in polar flagellar placement and general stress response induction in Pseudomonas putida
Abstract
The flhF gene of Pseudomonas putida, which encodes a GTP-binding protein, is part of the flagellar–motility–chemotaxis operon. Its disruption leads to a random flagellar arrangement in the mutant (MK107) and loss of directional motility in contrast to the wild type, which has polar flagella. The return of a normal flhF allele restores polar flagella and normal motility to MK107; its overexpression triples the flagellar number but does not restore directional motility. As FlhF is homologous to the receptor protein of the signal recognition particle (SRP) pathway of membrane protein translocation, this pathway may have a role in polar flagellar placement in P. putida. MK107 is also compromised in the development of the starvation-induced general stress resistance (SGSR) and effective synthesis of several starvation and exponential phase proteins. While somewhat increased protein secretion in MK107 may contribute to its SGSR impairment, the altered protein synthesis pattern also appears to have a role.
Introduction
Partial or complete starvation is the dominant experience of bacteria in nature, and it profoundly influences their characteristics. For example, starvation activates the general stress response (SGSR) that makes starving bacteria markedly more resistant to killing by a variety of stresses and antimicrobial agents, express unique biochemical activities and show enhanced virulence (Hengge-Aronis, 1996; Matin et al., 1999 and references therein).
We previously isolated a transposon mutant of Pseudomonas putida (MK107) that was impaired in starvation (stationary phase) survival (Kim et al., 1995). The gene affected in MK107 is σ54 regulated and expressed in the exponential phase, but exhibits a marked increase in transcription in the stationary phase. We now show that the gene affected in MK107 is flhF, which codes for a GTP-binding (G)-protein. As flhF has been implicated in flagellar biogenesis (Carpenter et al., 1992), we were led to investigate motility in MK107, in addition to the role of this gene in starvation-related phenomena. We found that flhF determines, directly or indirectly, the flagellar placement at the pole of P. putida, that its overproduction increases flagellar number per cell and that its disruption compromises SGSR and effective synthesis of several exponential and starvation phase proteins.
Results
The flhF gene is part of a large operon
To determine the identity of the gene affected in MK107, the wild-type (MK1; Table 1) genomic DNA corresponding to the transposon insertion region of MK107 was sequenced (Experimental procedures). Figure 1, which is derived from our own data (submitted to the GenBank database under accession no. AF183382), that of Ditty et al. (1998) and of The Institute of Genomic Research (TlGR; K. E. Nelson, personal communication), indicates that the affected gene, flhF, resides in a large operon, which is concerned with flagellar synthesis, chemotaxis and motility. No obvious transcriptional terminator was seen within the region shown.
| Strains | ||
|---|---|---|
| Escherichia coli | ||
| DH5α | supE44 ΔlacU169 (φ80lacZdM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Hanahan (1983) |
| XL-1 blue MR | ΔmcrA183 ΔmcrCBhsdSMRmrr173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac | Stratagene |
| B834(DE3) | F-ompT hsdSB(rB-mB-) gal dcm met (DE3) | Novagen |
| CC118 λpir | Δ(ara-leu)araDΔlacX74 galE galK phoA20 thi-1 rpsE rpoB | Herrero et al. (1990) |
| argE(Am) recA1 λpir phage lysogen; RifT | ||
| AMS 3000 | DH5α containing pSP6; Apr | This study |
| Pseudomonas putida | ||
| MK1 | Derivative of ATCC12633; Rifr | Kim et al. (1995) |
| MK107 | Tn5 mutant of MK1; Kmr, Rifr | Kim et al. (1995) |
| MK200 | MK107 with flhF in pMMB67EH; Rifr, Kmr, Cbr | This study |
| MK201 | MK107 with pMMB67EH; Rifr, Kmr, Cbr | This study |
| MK202 | Tn5 mutant of P. putida MK107 with single copy insertion | This study |
| of the 9.5 kb EcoRI fragment from pSP9; Rifr, Kmr, Smr, Spr | ||
| Plasmids | ||
| pUC 18/19 | Cloning vector; Ampr | Yanisch-Perron et al. (1985) |
| Bluescript SK+ | Cloning vector; Ampr | Stratagene |
| SuperCos | Cosmid vector; Ampr, Kmr | Stratagene |
| pET28a+ | Translation vector with T7 lac promoter and His-Tag sequence; Kmr | Novagen |
| pUC18NotI | pUC18 derivative with NotI–EcoRI–SalI–HindIII–NotI as multicloning site; Apr | Herrero et al. (1990) |
| pUT mini Tn5/Sm | Tn5-based delivery plasmid with Smr; Apr, Smr | Herrero et al. (1990) |
| pMMB67EH | tac expression cloning vector with multicloning site of pUC; Apr | Stratagene |
| pMK103 | pMMB67EH containing 14.3 kb PstI MK107 genomic fragment; Apr, Kmr | Kim et al. (1995) |
| pSP1 | pBluescriptSK+ containing the 5.4 kb HindIII/EcoRI fragment of pMK103; Apr | This study |
| pSP2 | SuperCos1 with ≈ 40 kb MK1 genomic DNA with flhF and contiguous genes; Apr, Kmr | This study |
| pSP3 | pUC18 containing 9.5 kb EcoRI fragment from pSP2; Apr | This study |
| pSP4 | pBluescriptSK+ containing the flhF gene; Apr | This study |
| pSP5 | pMMB67EH containing the flhF gene; Apr | This study |
| pSP6 | pET28a+ containing the flhF gene; Kmr | This study |
| pSP9 | p18NotI containing 9.5 kb EcoRI fragment from pSP3; Apr | This study |
| pSP10 | pUT mini-Tn5 containing 9.5 kb NotI fragment from pSP9 in Tn5/Sm; Apr, Smr | This study |
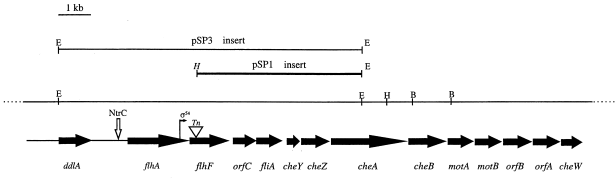
Partial map of the P. putida operon containing the flhF gene. Arrows show the ORFs and transcription direction. The inverted triangle marks the transposon-insertion site in MK107. The EcoRI/HindIII region (pSP1 insert; Table 1) was used as hybridization probe (see Experimental procedures). The vertical right-pointing arrow within the flhA coding region shows the σ54 promoter location (Kim et al., 1995); the location of the NtrC binding site (Ow et al., 1983) is also shown. The region from the 3′ end of flhA to the 5′ end of motA was sequenced both by us and by Ditty et al. (1998); the ORFs downstream of motA are reproduced from Ditty et al. (1998) with permission. Sequence upstream of flhA was obtained from TIGR (K. E. Nelson, personal communication). The EcoRI fragment used in complementing MK107 (pSP3 insert; Table 1) is also shown.
The transposon had inserted after the 42nd N-terminal nucleotide of the flhF gene. Based on the predicted amino acid sequence, flhF codes for a 437-amino-acid protein (48 kDa molecular weight), which is hydrophilic, devoid of an N-terminal signal sequence, a helix–turn–helix motif, as well as transmembrane regions. The FlhF protein contains the three consensus sequences typical of GTP-binding proteins and is homologous to the signal recognition particle (SRP) pathway family of proteins (Dever et al., 1987; Carpenter et al., 1992; Wolin, 1994; De Gier et al., 1997). Among the Escherichia coli SRP proteins, FlhF resembles the receptor protein, FtsY, more than the signal recognition particle protein, Ffh. It has an overall 22% identity and 22% similarity to FtsY, with 32% identity and 50% similarity in the GTP-binding C-terminus region (data not shown).
The flhF transposon does not have a polar effect
Given that the flhF gene is part of a large operon, it seemed prudent to ensure that the phenotype of MK107 results solely from the disruption of this gene. Reverse transcriptase–polymerase chain reaction (RT–PCR) and Western analyses were used to determine whether open reading frames (ORFs) downstream of the MK107 transposon-insertion site were expressed. RT–PCR products showed expression of all the ORFs whose transcription we looked for (C-terminus region of flhF, orfC and fliA;Fig. 1) in both MK107 and MK1; Western analysis confirmed that FliA was expressed to the same extent in both strains (data not shown).
MK107 shows altered motility and flagellar arrangement, which are restored by a wild-type flhF allele
As flhF is located in an operon concerned with cell motility and has been implicated in flagellar biogenesis, we investigated the motility of MK107. It failed to spread on motility plates, in contrast to the wild type (Fig. 2A and B, insets), and phase-contrast microscopy revealed that, although actively motile, it lacked directional movement. Electron micrographs showed that, unlike the wild-type polar location, the flagella in MK107 were randomly distributed on the cell surface (Fig. 2A and B); the cell shown in Fig. 2B has flagella only on one side, but other cells (Fig. 2E) showed a random distribution. MK107 also possessed on average more flagella per cell than MK1: 3.2 ± 0.5 versus 2 ± 0.3.

Spread size of colonies on motility agar plates (top right corner insets) and electron micrographs of the flagellar arrangement of different P. putida strains: (A) MK1; (B) MK107; (C) MK202; (D) MK200 (Table 1); (E) MK107 with another flagellar arrangement. The bar represents 1 µm. Magnifications were 13 000 × in (A–D) and 22 000 × in (E).
That the flhF gene was responsible for the altered motility and flagellar distribution of MK107 is confirmed by the fact that motility agar-spreading, directional motility, as well as polar flagellar location were restored in MK202 (i.e. strain MK107 containing a random insert of a single copy of the wild-type flhF allele on its chromosome; Table 1; Fig. 2C and inset). The average flagellar number also decreased in this strain to resemble the wild type, i.e. 2.6 ± 1 flagella per cell.
FlhF overproduction results in a large number of polar flagella
To determine the effect of overproduction of normal FlhF in strain MK107, we cloned the flhF gene in the pMMB67EH plasmid under the control of the tac promoter, constructing pSP5 and strain MK200 (i.e. MK107 containing pSP5; Table 1). Not only did MK200 regain polar flagella, it showed a marked increase in flagella per cell: 12 ± 2 (Fig. 2D). pMMB67EH, from which pSP5 is derived, generates five to eight copies per cell, and Western analysis showed an ≈ sixfold increase in FlhF levels in MK200 compared with MK201 (i.e. MK107 containing the pMMB67EH without the flhF gene; Table 1; data not shown). The results confirm that the FlhF protein directly or indirectly determines the polar flagellar location and show, in addition, that this protein is the limiting factor in determining the number of flagella per cell. Strain MK200 appeared to regain some directional motility, as judged by phase-contrast microscopy. Nevertheless, it did not spread on motility agar plates (Fig. 2D, inset). The control strain MK201 showed a flagellar arrangement and spread pattern similar to that of MK107 data not shown.
MK200 shows greatly enhanced protein secretion
The flagellar channel has recently been implicated in cellular protein secretion (Young et al., 1999). As the flagellar content and arrangement differs in the above P. putida strains, and FlhF overproduction upregulates flagellar number, these strains could differ in cellular protein secretion. MK1 secreted the least amount and number of proteins (Fig. 3). The secretion was increased somewhat in MK107, while MK202 resembled the wild type in this respect. MK200 showed vastly increased secretion both quantitatively and qualitatively. A T-broth control (Experimental procedures) indicated that the protein bands in lanes 1–4 were not derived from the medium. There was no evidence of cell lysis in any of the cultures, and viable counts continued to increase throughout incubation. This notion is further supported by the fact that Western blots failed to detect the cytoplasmic protein σs in the culture supernatants.

Silver stain of SDS–PAGE gel of proteins secreted by MK1 (lane 1), MK107 (lane 2), MK202 (lane 3) and MK200 (lane 4) after 12 h incubation. Lane 5 shows the band produced by T-broth that was treated in the same way as the other samples (Experimental procedures). Molecular weight markers are included. See text for a description of the controls.
Western blots did reveal the presence of the periplasmic protein, β-lactamase, in the culture supernatant of MK200, but not in that of MK201, suggesting that the increased flagellar number enhanced the outer membrane leakiness in the former strain. As the amount of FlhF and flagellar number and arrangement in MK201 is similar to that in MK107, it can be assumed that the latter strain is also not leaky to a periplasmic protein such as β-lactamase. While the secretion of β-lactamase by MK200 implies outer membrane leakiness in the increased protein secretion by this strain (Fig. 3), it remains possible that the increased activity of the type III secretion pathway also has a role; this pathway is made up of homologues of components of the flagellar apparatus (Macnab, 1996).
MK107 is unable to develop SGSR, which is restored by a wild-type flhF allele
As stated above, the flhF gene was shown to have a role in starvation survival of P. putida. Starvation genes fall into two classes: those concerned with starvation survival alone (Fraley et al., 1998); and those that confer SGSR and affect a broad spectrum of starvation protein synthesis (Lange and Hengge-Aronis, 1991; McCann et al., 1991). We therefore determined whether flhF disruption affected SGSR. Starved MK 107 cells were less resistant to heat and H2O2, as well as to ethanol compared with MK1 (Table 2). However, there was no difference in the resistance to these stresses of exponential phase cells of the two strains (data not shown). That the disruption of flhF had a role in the compromised SGSR of MK107 was confirmed by the fact that the strain MK201 (Table 1) resembled the wild type in SGSR (Table 2). MK200 (which overproduces FlhF) possessed greater SGSR (10–20%) than MK107, but less than that of MK1 (data not shown).
| Percentage viability remaining after exposure to: | |||
|---|---|---|---|
| Strain | Heata | Oxidationb | Ethanolc |
| MK1 | 100 | 100 | 100 |
| MK107 | 8 | 43 | 28 |
| MK202 | 95 | 92 | 90 |
- a . Starved cells exposed to 49°C for 20 min.
- b . Starved cells exposed to 2 mM H 2O2 for 30 min.
- c . Starved cells exposed to 12% ethanol for 30 min.
MK107 is impaired in starvation protein synthesis
As SGSR development requires starvation protein synthesis (Matin et al., 1999), the above results suggest that flhF mutation interferes with this synthesis. A comparison of exponential and stationary phase two-dimensional gel maps (Experimental procedures) showed that the synthesis of at least 50 starvation proteins is altered in MK107; selected regions of the gels (Fig. 4A and B) illustrate the point. Some 30 polypeptides in these regions have higher levels in the starved wild-type cells than in the mutant [e.g. 1–5, 10–12 cluster, 15–20 (Fig. 4A), and 1–6, 8, 10, 13 (Fig. 4B)], although the reverse is true for other polypeptides [e.g. spots 7, and 8 (Fig. 4A) and spot 9 (Fig. 4B)]. The top part of each figure is the polypeptide synthesis map of exponential phase MK1 cells in the corresponding region of the gel; the exponential phase polypeptide synthesis pattern of MK107 cells in this region was very similar.
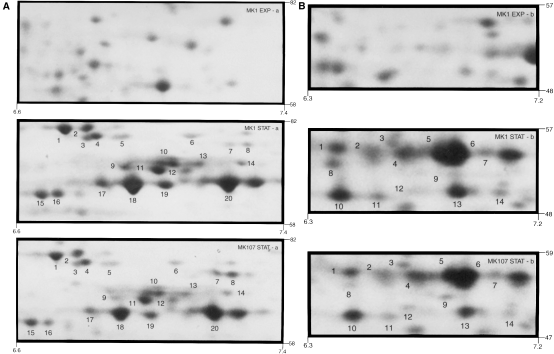
A and B. Two digitally enlarged parts of two-dimensional gel maps of polypeptides synthesized by MK1 and MK107 in starvation phase. The top picture in each case provides the polypeptide map of the corresponding region of exponential phase MK1; this pattern was very similar in MK107. The molecular weight and pH values of the chosen region are shown.
However, in other regions of the gels, the exponential phase MK1 and MK107 protein synthesis pattern differed markedly (Fig. 5A and B), affecting over 70 polypeptides in the regions shown. Most of the numbered polypeptides are higher in MK1 than in MK107 in the region shown in Fig. 5A, while the opposite was the case with several polypeptides shown in Fig. 5B; overall, most of the affected polypeptides showed a higher synthesis rate in the wild type. As stated above, the flhF gene is expressed at a significant level in the exponential phase cells; these results show that it has a direct or indirect effect on the synthesis of exponential phase proteins. Restoration of the flhF wild-type allele to MK107 tended to restore the wild-type protein synthesis pattern (data not shown).
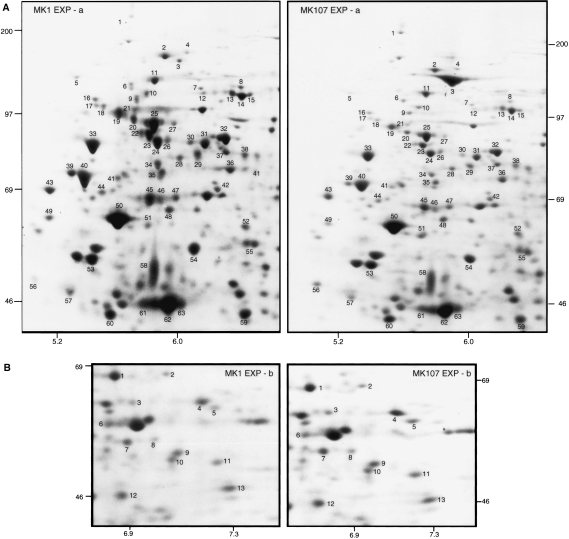
A and B. Digitally enlarged parts of two-dimensional gel maps of polypeptides synthesized by exponential phase MK1 and MK107 cells. The molecular weight and pH values of the selected regions are shown.
σs levels increase to a lesser extent in starving MK107 than in MK1
Increase in σs concentration is responsible for the enhanced synthesis of starvation proteins and SGSR development in several bacteria, including P. putida (Ramos-Gonzalez and Molin, 1998; Matin et al., 1999). σs levels were comparable during exponential growth in MK1 and MK107. At the onset of starvation, the levels increased in both, but less in MK107, the difference becoming pronounced after 1 h of starvation (Fig. 6). This difference persisted for the rest of the experiment, i.e. up to 5 h of starvation (data not shown).

σs levels in MK1 (solid bars) and MK107 (open bars) during growth and starvation. OD660 measurements were used to follow growth and transition in starvation phase: (●) MK1; (○), MK107. Insets show Western blots of σs (≈ 40 kDa molecular weight). The experiment was performed numerous times; the variation for each time point in independent experiments was within 5%.
Discussion
We have identified the gene that we previously implicated in the starvation survival of P. putida. It is flhF, which encodes a G-protein and resides in an operon concerned with flagellar synthesis, chemotaxis and motility. We show here that mutation in this gene has a consequence in addition to starvation-related general resistance; namely, that it leads to loss of directional motility and random flagellar arrangement in P. putida, which is normally directionally motile and has polar flagella.
The most common flagellar arrangement in bacteria is either peritrichous or polar. Little is known about what determines either arrangement or what brings a flagellum to its ultimate location. Flagellar assembly is thought to begin with the placement of the FliF protein on the inner side of the cytoplasmic membrane to form the MS ring (Jenal and Shapiro, 1996; Macnab, 1996). Once this ring is positioned, the flagellar biogenesis proceeds with the formation of the L and P rings, the hook and the filament. Thus, it is the positioning of the MS ring that determines flagellar location, and the question therefore is what determines this positioning. To our knowledge, only one previous study impinged on the genetic basis of flagellar placement. Richardson et al. (1990) isolated a mutant of Vibrio cholerae, which, like P. putida, is polarly flagellated, that possessed lateral appendages. But these appendages lacked the flagellar core and represented merely the membranous sheaths. The mutant was non-motile, and the identity of the gene responsible for this effect was not determined. In contrast, MK107 contains complete flagella that remain functional, as MK107 is motile – that it lacks directional motility is probably because it is not endowed with the naturally peritrichous bacterium's mechanism to co-ordinate randomly distributed flagella.
Our finding, therefore, is the first instance in which a known gene is linked to the placement of functional flagella at a specific location. But, while we clearly establish that flhF is this gene, how the FlhF protein consummates this role is not clear. A plausible scenario is based on the fact that FlhF is homologous to the SRP pathway protein family, which translocates membrane proteins in a wide variety of organisms (Wolin, 1994; Rapoport et al., 1996; De Gier et al., 1997). In the SRP system, the signal recognition particle/4.5S RNA complex, in its GTP-bound form, attaches to the N-terminal domain of the newly formed protein as it comes off the ribosome, and then docks with its receptor on the membrane, resulting in the insertion of the protein into the membrane. FlhF is homologous to both the signal recognition particles and the membrane receptors of this pathway and could act in either capacity. If the former, the MK107 phenotype would result from its inability to find its proper location on the membrane; if acting in the latter capacity, the mutated FlhF may be unable to recognize its proper receptor, delivering FliF to random locations on the membrane, possibly by recruiting illegitimate receptors. FliF appears to be a good candidate for transport by the SRP pathway, in being a membrane protein with a hydrophobic N-terminal region and a large periplasmic domain (Newitt et al., 1999), and in not being a substrate for the general secretory pathway (Jenal and Shapiro, 1996). Regardless of these speculations, it is clear that the dramatic alteration of flagellar location in MK107 provides a powerful system for exploring the mechanistic basis of flagellar location in P. putida.
It is not clear why FlhF overexpression (in MK200) causes hyperflagellation. The hierarchical arrangement of flagellar genes in P. putida is not known (Montie, 1998). In the enterics, the flagellar structural components are encoded by class 2 and 3 operons (Macnab, 1996). If this is also true of P. putida, it would mean that increased levels of FlhF can upregulate the class 2 and 3 operon expression and, hence, the flagellar components.
MK107 was shown previously to be impaired in starvation survival, and we show here that it is also impaired in SGSR. MK107 secretes somewhat more proteins than MK1 (Fig. 3), and it is possible that its increased stress sensitivity in the stationary phase results from the energy drain imposed by this secretion. However, MK200, which secretes vastly more protein than MK107, shows greater SGSR than MK107, which suggests that additional factors have a role. MK107 is impaired in exponential and stationary phase protein synthesis, and this may be a major reason for its increased stress sensitivity. This notion is strengthened by the finding that MK107 does not increase its σs levels in stationary phase to the same extent as the wild type: this sigma factor has been implicated in the synthesis of proteins involved in bacterial stress resistance, including that of P. putida (Matin et al., 1999). Whether the impaired protein synthesis in MK107 is the direct result of flhF mutation or an indirect consequence of flagellar dislocation remains to be explored.
Experimental procedures
Bacterial strains, plasmids, and growth media
All strains and plasmids used in this study are listed in Table 1. Luria–Bertani (LB), tryptone and M9 minimal media were prepared as described previously (Sambrook et al., 1989). The following antibiotics were used at the indicated concentrations (in µg ml−1): for E. coli: ampicillin (100), kanamycin (30), chloramphenicol (20); for P. putida: kanamycin (50), rifampicin (150), carbenicillin (4000), streptomycin (400), spectinomycin (50). IPTG (1 mM) was added to media when needed for the induction of the tac promoter.
Localization of the MK107 transposon-insertion site homologue in the wild-type MK1
The transposon-insertion site in MK107 was cloned previously as a 14.3 kb genomic insert in pMK103 (Kim et al., 1995). A 5.4 kb EcoRI/HindIII region of this insert was cloned to yield pSP1 (Table 1; Fig. 1) and labelled with digoxygenin (DIG; Boehringer Mannheim) to probe a SuperCos1 chromosomal DNA MK1 cosmid library, prepared according to the Stratagene protocol. Cosmid DNA from the positive colonies was digested with EcoRI enzyme. Four of the digests each generated a 9.5 kb fragment, which hybridized with the probe; one of these (from pSP2; Table 1) was cloned in pUC18 (generating pSP3) and sequenced. Also sequenced was the EcoRI/HindIII fragment of the genomic insert in pSP1.
Single-copy complementation of the mutated gene in MK107
Random insertion of the 9.5 kb EcoRI genomic fragment of pSP3 [containing the flhF gene and contiguous sequence (Fig. 1; see Results)] into the MK107 chromosome was attained as described previously (DeLorenzo et al., 1990). The fragment was first flanked with NotI sites by cloning in p18NotI (yielding pSP9) and then cloned in pUT/mini-Tn5, resulting in pSP10 (Table 1). pSP10 was transformed into MK107 via E. coli CC118 λpir strain, as described previously (Kim et al., 1995). Exconjugants were selected on LB plates containing rifampicin, kanamycin, streptomycin and spectinomycin. In several of these, plate-spreading motility was restored. MK202, which exhibited a marked restoration, was selected for further study; Southern hybridization confirmed random insertion of the EcoRI fragment into the MK107 chromosome. Some of the flhF flanking sequences were included in the complementing fragment to ensure expression in vivo. It has been shown that these influence its transcription; moreover, the upstream NtrC binding site (Fig. 1) could regulate its σ54 promoter (Kim et al., 1995).
Multicopy complementation of the flhF gene
The flhF gene was cloned using PCR, with pSP3 as template; the primers were complementary to appropriate sequences (accession no. AF183382) and were designed to flank the PCR product with EcoRI and HindIII restriction sites. The PCR product was cloned into pMMB67EH via pSP4. This yielded pSP5, which was introduced into MK107, generating strain MK200. Sequencing ensured that the PCR procedure did not introduce mutations. The flhF gene in pSP5 is controlled by the tac promoter. The control strain (MK201) consisted of MK107 containing the empty plasmid pMMB67EH.
Stress tests
These were carried out essentially as described previously (Groat et al., 1986; Jenkins et al., 1988). Briefly, 24 h-starved cell suspensions (≈ 5 × 108 cells ml−1) were used. For heat challenge, the culture (2 ml) was placed in a preheated 49°C heat block; samples were removed at various intervals and processed for viability determination by spreading serial dilutions on LB plates. For ethanol (12%) and oxidative (2 mM H2O2) shocks, the cell suspension was mixed with the indicated agent, and viability was determined periodically.
DNA extraction and sequencing
Total, plasmid and cosmid DNA were isolated as described (Birnboim and Doly, 1979; Del Sal et al., 1988). Large-scale preparations were made using a Qiagen midi kit. DNA bands from agarose gels were isolated by QiaexII gel extraction kit. Sequencing was performed after generating nested deletions, subcloning of restriction fragments or using ordered primers. DNA strider and blast programs were used to identify the open reading frames (ORFs) and the Tfsitescan-dynamicPlus server to analyse regulatory sequences. The helix–turn–helix motif was searched as described by Dodd and Egan (1990).
RNA extraction and RT–PCR
Total RNA was prepared using the Qiagen RNeasy Total RNA kit. RT–PCR reactions were performed using the Promega Access RT–PCR system. Total RNA (1 µg) was digested with RNase-free DNase to remove DNA quantitatively. The following primers (50 pmol) were used: for flhF (forward) 5′-atcggcgcccaggagcag-3′; (reverse) 5′-agctactttgcacacgcttcatggtc-3; for orfC: (forward) 5′-agcttggccgcagggtca-3′; (reverse) 5′-gaggttgtcgccgatttc-3′; for fliA: (forward) 5′-aatatgacgccagcaaag-3′; (reverse) 5′-cgcgcaggttcgacatgg-3′. Negative controls omitted the AMV-reverse transcriptase from the reaction mixture. The PCR products were separated in 2% agarose/TAE gel and visualized by UV transillumination after ethidium bromide staining.
Motility assay
Motility plates were made from half-strength LB solidified with 0.4% Bacto agar. Inoculum from well-isolated colonies was stabbed in the centre of the plates and incubated overnight at 30°C. Similar results were obtained when 5 µl (A660 of 2.4) of stationary phase cultures were spotted.
Electron microscopy
Formvar carbon-coated copper grids were placed on drops of diluted (A660 of 0.5) late exponential phase culture and incubated for 3 min to allow cell adherence. After three washings in distilled water, the grids were negatively stained (2 min) in 1% uranyl acetate and washed again. To quantify flagella/cell, 200 cells of each strain were examined; flagella were counted only in well-isolated cells: 30 MK1, 73 MK107, 30 MK202 and 28 MK200. A Philips CM12 transmission electron microscope operating at 80 kV was used.
Analysis of secreted proteins
Cells were grown overnight in tryptone broth and pelleted by centrifugation (6000 × g for 20 min). The supernatants were filtered through 0.22 µm filters, and the volumes were normalized to correspond to an A660 of 2 and treated with 10% trichloroacetic acid (TCA) to precipitate the proteins. After washing in acetone, the precipitate was dissolved in SDS sample buffer. Equal volumes were heated (95°C for 10 min), run on a 12% SDS–PAGE and visualized by silver staining (Blum et al., 1987). The control sample consisted of culture medium treated in the same way. For determining σs and β-lactamase proteins, the SDS–PAGE gels were immunoblotted and analysed using appropriate polyclonal antibodies; extracts of appropriate cells served as positive controls.
FlhF overproduction, purification and preparation of a polyclonal anti-FlhF antiserum
For FlhF overproduction, a DNA fragment containing the flhF gene was cloned into pET28a+ (Novagen), yielding pSP6 (Table 1), which was transformed into E. coli DH5α, generating AMS3000. The overproduced FlhF in strain AMS3000 formed inclusion bodies after induction with IPTG (37°C for 3 h). FlhF was purified according to the Novagen protocol and submitted for antibody production (Josman Laboratories) as described previously (Zgurskaya et al., 1997).
Immunoblotting
Protein (10 µg) of lysed cells was boiled and subjected to 12% SDS–PAGE with subsequent electroblotting and immunodetection, using nitrocellulose membranes (Bio-Rad) and anti-FlhF, -RpoS, -FliA or -β-lactamase polyclonal antiserum. The polyclonal antibody used against RpoS also reacts with an unknown protein of ≈ 55 kDa molecular weight that remains unchanged during growth or phase transitions; this served as an internal control (Zgurskaya et al., 1997). The membranes were developed with the ECL reagent system (Amersham protocol).
Two-dimensional gel electrophoresis
Two-dimensional gel analysis was performed by Kendrick Laboratories according to the method of O’Farrell (1975), essentially as described previously (Groat et al., 1986). Cells were labelled by 15 min incubation with a 40 µCi ml−1 culture of 35S-‘Met protein express labelling mix’ (specific activity 1175 Ci mmol−1; NEN Life Science). After a 1 min chase with cold 1 mM methionine + cysteine, the cells were washed twice in prewarmed M9 medium, lysed and treated with DNase and RNase. An aliquot was added to BSA carrier solution and ice-cold 50% TCA, centrifuged and washed with 10% TCA. The pellet was dissolved in Soluene 350 (Kendrick Laboratories) and counted. Samples of 107 d.p.m. were loaded on the gels for each culture. Mid-exponential (A660 of 1.0; 0.3% glucose–M9 medium) or starving-phase cells were used; the latter were prepared by suspending mid-exponential phase cells in prewarmed glucose-free M9 medium and incubating for 40 min at 30°C (Givskov et al., 1994). Isoelectric focusing used 2.0% pH 4–8 ampholines (Hoefer Scientific Instruments) and 9600 V-h. After equilibration of the tube gels in SDS sample buffer, SDS slab gel electrophoresis (4 h at 12.5 mA/gel) was performed. Appropriate molecular weight markers were used. The gels were dried and autoradiographed using Kodak X-OMAT AR film. Autoradiographs were acquired on an AGFA flatbed scanner (ARCUS II) at 1.25-fold of the original size (300 dots per inch) through Adobe PhotoShop, version 5.5. Boxes and circles were placed on the scans using Adobe Illustrator version 8.0. Selected boxes were enlarged fourfold. Background was adjusted to equal levels in all scans.
Acknowledgements
This work was supported by grant DE-FG03-97ER-62494-A002 to A.M., and NIH training grant NRSA 5 T32 AI07328-10 to S.P. C.H.P. was supported by a postdoctoral grant from the Korea Research Foundation. We thank Nafisa Ghori and Ingela Blom for help in electron microscopy, and cloning and sequencing respectively. Thanks are also due to K. Postle for providing β-lactamase and K. Hughes for FliA antibodies, K. E. Nelson of TIGR for providing the specified P. putida sequence (Fig. 1) before its placement on the TIGR website, and to C. Harwood for permission to reproduce part of their sequence.




