Role of Lon and ClpX in the post-translational regulation of a sigma subunit of RNA polymerase required for cellular differentiation in Bacillus subtilis
Abstract
The RNA polymerase sigma subunit, σH (Spo0H) of Bacillus subtilis, is essential for the transcription of genes that function in sporulation and genetic competence. Although spo0H is transcriptionally regulated by the key regulatory device that controls sporulation initiation, the Spo0 phosphorelay, there is considerable evidence implicating a mechanism of post-translational control that governs the activity and concentration of σH. Post-translational control of spo0H is responsible for the reduced expression of genes requiring σH under conditions of low environmental pH. It is also responsible for heightened σH activity upon relief of acid stress and during nutritional depletion. In this study, the ATP-dependent proteases LonA and B and the regulatory ATPase ClpX were found to function in the post-translational control of σH. Mutations in lonA and lonB result in elevated σH protein concentrations in low-pH cultures. However, this is not sufficient to increase σH-dependent transcription. Activation of σH-dependent transcription upon raising medium pH and in cells undergoing sporulation requires clpX, as shown by measuring the expression of lacZ fusions that require σH for transcription and by complementation of a clpX null mutation. A hypothesis is presented that low environmental pH results in the Lon-dependent degradation of σH, but the activity of σH in sporulating cells and in cultures at neutral pH is stimulated by a ClpX-dependent mechanism in response to nutritional stress.
Introduction
Bacteria often encounter harmful environmental changes that include nutritional depletion as well as extremes of temperature, pH, oxygen availability and osmolarity. The soil, spore-forming bacterium, Bacillus subtilis, can sense, process and respond to signals derived from environmental changes that exert stress on the cell (Kunst et al., 1994; Grossman, 1995; Hecker et al., 1996). Among these responses are the development of a motile state (Ordal et al., 1993), the establishment of genetic competence, a physiological state whereby the cell is endowed with the capacity to acquire DNA from its environment (Dubnau, 1993), the production of antibiotics (Zuber et al., 1993), the production and secretion of extracellular degradative enzymes for acquiring nutrition from complex organic material (Kunst et al., 1994; Pero and Sloma, 1993) and a shift from aerobic to anaerobic metabolism in response to oxygen limitation (Nakano and Zuber, 1998). Sporulation, a process of cellular differentiation that gives rise to a highly resistant cell type called the endospore (Errington, 1993; Stragier and Losick, 1996), is initiated if other measures fail to alleviate nutritional stress. Complex regulatory networks operate that allow B. subtilis to choose the most appropriate response to environmental change (Kunst et al., 1994; Grossman, 1995; Hecker et al., 1996; Hecker and Volker, 1998). How B. subtilis responds to change is profoundly affected by the population density of the local environment (Grossman and Losick, 1988; Grossman, 1995; Perego and Hoch, 1996; Lazazzera and Grossman, 1998). Associated with the regulatory network that controls the cell's response to environmental change are regulatory factors found in all bacteria, including RNA polymerase (RNAP) sigma subunits that confer template specificity to RNAP holoenzyme (Helmann, 1991; Dubnau et al., 1994; Kunst et al., 1994; Grossman, 1995; Haldenwang, 1995; Hoch, 1995).
The initiation of sporulation is characterized by the activation of regulatory mechanisms that govern sporulation gene expression. One of these mechanisms involves the RNAP sigma subunit, σH (Carter and Moran, 1986; Dubnau et al., 1988), encoded by the spo0H gene. The σH subunit is required for the transcription not only of genes that function in sporulation but also of genes required for competence development and TCA cycle activity (Carter et al., 1988; Jaacks et al., 1989; Tatti et al., 1989; Predich et al., 1992; Asai et al., 1995; Solomon et al., 1995). The process of cytokinesis following the formation of the sporulation septum requires σH (Levin and Losick, 1996), as does the transcription of the gene encoding σF (Wu et al., 1989; 1991; Stragier and Losick, 1996), which directs the transcription of genes that are expressed in the forespore compartment. The key regulator of sporulation initiation, Spo0A (Hoch, 1993; 1995), is encoded by a gene possessing two promoters, one of which is used by the σH form of RNAP (Siranosian and Grossman, 1994; Chibazakura et al., 1995). Maximal transcription of the spo0A gene from this promoter is required for sporulation initiation. In the development of the competent state, σH directs the transcription of the phrC gene, encoding one of the peptide pheromones that mediates the cell density-dependent control of competence development (Carter et al., 1990; Solomon et al., 1995; 1996).
The activity of σH is detected in high-cell-density cultures undergoing starvation
Maximal activity of σH depends on Spo0A, which indirectly stimulates spo0H transcription by repressing the negative regulator of spo0H, abrB (Weir et al., 1991; Strauch, 1995; Fujita and Sadaie, 1998a) (Fig. 1). Spo0A-phosphate accumulates in cells of stationary phase cultures at high cell density by virtue of the signal transduction system known as the Spo0 phosphorelay (Burbulys et al., 1991). The phosphorelay is a multistep phosphoryl transfer system that is acted upon by regulatory signals at each step of its reaction sequence. The regulatory signals are derived from conditions, both environmental and metabolic, that promote sporulation. The integration of these signals results in the increase in Spo0A-phosphate concentration (Burbulys et al., 1991; Hoch, 1993; Ireton et al., 1993; Grossman, 1995). Spo0A-phosphate represses abrB, thereby stimulating spo0H expression. Together, σH and Spo0A-phosphate activate the transcription of genes necessary for the first morphological steps of sporulation. The two regulatory factors affect each other through a positive autoregulatory circuit (Grossman, 1995; Fujita and Sadaie, 1998a).
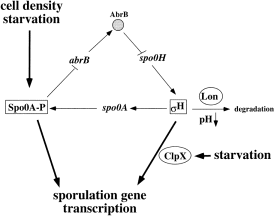
. Schematic diagram illustrating the regulation of spo0H and its product, σH. Conditions of high cell density and starvation stimulate the production of active Spo0A-phosphate. Spo0A represses abrB, thereby releasing spo0H transcription from AbrB-dependent repression. The product of spo0H increases in concentration and stimulates the transcription of spo0A, thus completing an autoregulatory loop. In conjunction with Spo0A-phosphate, σH stimulates the transcription of sporulation genes. The activity of σH is dependent on ClpX, whereas σH stability is controlled by Lon.
The transcriptional control of spo0H alone does not completely account for the post-exponential induction of σH-dependent transcription. The σH protein is subject to post-translational control (Healy et al., 1991; Frisby and Zuber, 1994; Cosby and Zuber, 1997), including the temperature-dependent degradation of σH by a mechanism requiring ClpC (Nanamiya et al., 1998). Previous studies have shown that the concentration and activity of σH were negatively affected by low environmental pH resulting from the accumulation of acidic glycolytic end-products (acetate and lactate) in nutrient broth sporulation medium containing high concentrations of glucose and glutamine (DSM-GG) (Cosby and Zuber, 1997). The consequences of low pH could include the reduction of cytoplasmic pH caused by the influx of protonated acidic compounds. Ionization of the weak acids upon entry into the cytoplasm might result in the lowering of intracellular pH as well as an increase in anion concentration (Luli and Strohl, 1996). Acetic acid has been observed to reduce oxygen uptake and ATP generation in B. subtilis cells by over 70%. Reduction in PMF and amino acid uptake are also effects attributable to acetic acid (Sheu and Freese, 1972; Sheu et al., 1972; 1975). The reduction in the growth rate in late exponential phase in the DSM-GG cultures is probably due to acid stress rather than to starvation. This conclusion is based on the observation that, after the addition of a pH stabilizer, such as Tris-HCl or MOPS, there is a resumption of growth along with an increase in cell number and a higher final optical density (OD) (Cosby and Zuber, 1997; Cosby et al., 1998). Thus, starvation is not encountered until after pH stress is relieved, suggesting that the absence of nutritional stress along with the effects of acidic pH, together in DSM-GG cultures, would cause the suppression of σH concentration and activity. The effect of low pH on gene expression appears to be unique to the σH form of polymerase, as there is little effect of pH on the expression of σA-dependent gene expression (Cosby and Zuber, 1997). Mutations that render glucose utilization defective or cause an increase in medium pH also result in an increase in σH-dependent gene expression and enhanced sporulation frequency (Frisby and Zuber, 1994; Cosby and Zuber, 1997; W. M. Cosby and P. Zuber, unpublished results). Adjustment of medium pH to neutrality results in an increase in σH protein levels and σH activity, without significantly affecting the transcription of the spo0H gene (see Results ). Amino acid starvation and the stringent response are also factors that are thought to influence σH activity (Healy et al., 1991; Drzewiecki et al., 1998).
In this study, we have investigated the nature of σH post-translational control and show that the reduction in σH concentration in low-pH culture results from the presence of the ATP-dependent protease, Lon. We also present evidence that the induction of σH activity in a medium that promotes sporulation or after pH adjustment of a glucose/glutamine medium is dependent on the regulatory ATPase of the Clp family of proteins, ClpX.
Results
Effect of low medium pH and σH concentration
As reported previously, DSM-GG cultures show reduced expression of lacZ fusions made to the genes whose transcription requires the σH form of RNAP. This expression is reduced at low pH but increases when the cultures are treated with Tris-HCl. We had observed that the concentration of σH in cells of DSM-GG cultures at low pH was significantly lower than in cells of neutral pH cultures (Cosby and Zuber, 1997; Fig. 2A).
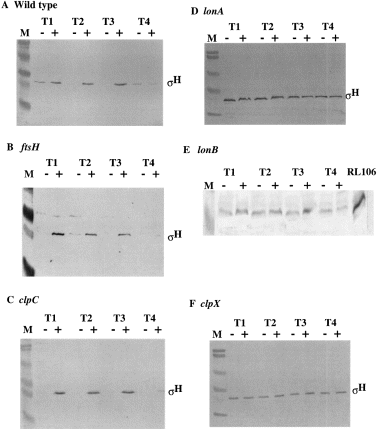
. Western analysis of σH levels in wild-type and protease- deficient mutant strains. Parallel cultures of strains were grown in DSM-GG. At or near T0 (OD595 = 1.7–2.0), Tris-HCl was added to a concentration of 25 mM to raise the pH to neutrality. Whole-cell extracts of cells collected at T1, T2, T3 and T4 (1, 2, 3 and 4 h after T0, which marks the end of exponential growth) were applied to SDS–polyacrylamide gels, and the proteins were resolved by electrophoresis. The − and + denote omission and addition of Tris-HCl buffer respectively. M refers to molecular weight markers. The band containing σH protein is indicated, as are strains from which extracts were obtained. A. Strain JH642. B. ORB3092. C. LAB2611. D. LAB2875. E. ORB3001; the lane marked RL106 shows the band of σH protein produced in a strain that carries a plasmid-amplified spo0H gene. This is included as a marker. F. LAB2876.
Mutations in lonA, lonB and clpX result in elevated σH concentration in low-pH cultures
The reduction in σH concentration at low pH is not the result of reduced spo0H transcription (Frisby and Zuber, 1994; Cosby and Zuber, 1997). Therefore, the involvement of proteolytic regulation of σH was investigated. Several proteases have been implicated in the regulation of sigma subunits in prokaryotes (Herman et al., 1995; Hofmeister et al., 1995; Gottesman, 1996; Pratt and Silhavy, 1996; Schweder et al., 1996; Zhou and Gottesman, 1998). Several, such as Lon, FtsH and the Clp family of proteins, have homologues in B. subtilis (Riethdorf et al., 1994; Gerth et al., 1996; Deuerling et al., 1997; Lysenko et al., 1997; Gerth et al., 1998; Hecker and Volker, 1998). We obtained and constructed mutant strains bearing insertions and deletions of several genes encoding proteases or their regulatory subunits. These include clpC, clpX, clpP, ftsH, lonA and lonB. Predicting that one or more of these would function in the proteolytic regulation of σH, strains bearing mutations in each were grown to late exponential growth and stationary phase in DSM-GG with and without pH adjustment. Samples were collected late in log phase and into stationary phase, and the level of σH was determined by Western blot analysis (Fig. 2). The σH concentration in wild-type cells (JH642) showed little detectable protein in the samples from the low-pH culture, but was significantly higher in the cells of cultures treated with Tris-HCl buffer. The same pattern of σH concentration was observed in the ftsH and clpC mutants (Fig. 2B and C). However, the lonA, lonB and clpX mutants showed levels of σH in the cells of the DSM-GG cultures at low pH indistinguishable from those detected in cells of the Tris-HCl-treated cultures (Fig. 2D–F).
The increase in σH concentration observed in lon and clpX mutants could have been the result of heightened spo0H expression (transcription or translation). To determine whether this was the case, the expression of a spo0H′::′lacZ translational fusion was examined in lonA and clpX mutant cells grown in parallel cultures of DSM-GG. Again, one of the cultures was treated with Tris-HCl at the end of exponential growth to raise the pH. We observed little or no increase in the expression of spo0H in clpX or lonA mutants compared with that observed in wild-type cultures (Fig. 3A and B). The effect of a lon and clpX mutation on σH concentration was probably caused by altered post-translational regulation.
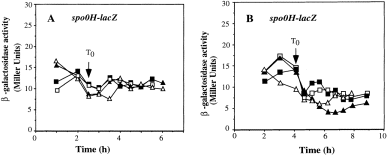
. Expression of spo0H′::′lacZ translational fusion in lonA and clpX mutants. Cultures of wild-type, lonA and clpX mutant strains were grown in parallel DSM-GG cultures with one undergoing Tris-HCl pH adjustment as described in Fig. 2. Samples were collected at 30 min intervals for assay of β-galactosidase activity. A. OR172 (wild type) DSM-GG, ▪; DSM-GGTris-HCl □. ORB3087 (lonA) DSM-GG, ▴; DSM-GGTris, ▵. B. OR172 (wild type) DSMGG, ▪; DSM-GGTris, □. ORB3088 (clpX ) DSM-GG, ▴; DSM-GGTris, ▵.
A mutation in clpX confers low σH activity
We expected to observe an increase in σH-dependent gene expression in lonA and clpX mutant cells grown in DSM-GG with higher levels of σH protein. The mutant strains carrying either the phrC–lacZ or spoVG42–lacZ fusions were grown in parallel DSM-GG cultures, and one was treated with Tris-HCl as described above. Both fusions were constructed from genes that require the σH form of RNAP for their transcription. The phrC gene encodes an extracellular regulatory peptide that stimulates both competence and sporulation (Solomon et al., 1995; 1996; Lazazzera et al., 1997). The spoVG gene functions in the sporulation process (Margolis et al., 1993) and is controlled by σH and the global, transition state repressor, AbrB (Zuber and Losick, 1987; Robertson et al., 1989; Furbaßet al., 1991). The spoVG42 gene bears a single nucleotide substitution upstream of the −35 region that renders spoVG transcription insensitive to AbrB-dependent repression (Zuber and Losick, 1987). Thus, the transcriptional control of spoVG42 is exerted solely by spo0H. The rpoDp4–lacZ fusion contains the P4 promoter of the rpoD operon, transcription from which requires the σH form of RNAP (Carter et al., 1988; Qi and Doi, 1990). The spoIIA–lacZ fusion contains the promoter region of the spoIIA gene encoding B. subtilisσF (Wu et al., 1989; 1991) and requires σH for its transcription.
The spoVG42–lacZ expression in lonA cells was very similar to that of wild-type cells (Fig. 4A). Expression of both phrC–lacZ (data not shown) and spoVG42–lacZ was low in lonA mutant cells of a low-pH culture even though σH levels were high. Both fusions were expressed at a higher level in lonA cells upon the addition of pH stabilizer, as was the case in wild-type cells. The mutation in lonB also did not affect the pattern of σH-dependent gene expression in low-pH culture and after Tris-HCl treatment (Fig. 4B). To test the possibility that elimination of both lonA and lonB might result in higher σH activity in DSM-GG grown cells, a lonA lonB double mutant was constructed, and the regulation of σH activity was examined. DNA from the lonB mutant was used to transform competent lonA mutant cells of strain ORB3102. A SPβspoVG42–lacZ lysogen was constructed, and expression of the phage-borne fusion was examined in DSM-GG and DSM-GGTris. Again, expression of the fusion in the double mutant was very similar to the pattern of expression observed in wild-type cells (data not shown).
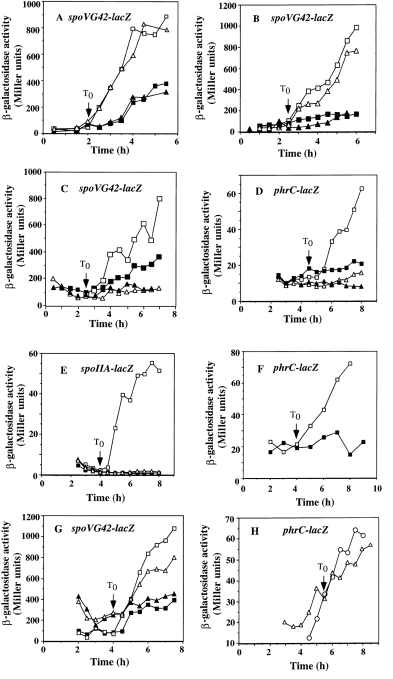
. Expression of σH-dependent genes in lonA, clpX and clpP mutants. Wild-type and mutant strains bearing the indicated lacZ fusions were grown in DSM-GG (with and without Tris-HCl treatment) or DSM (sporulation medium). Samples were collected as described in Fig. 3 and in Experimental procedures for assays of β-galactosidase activity. T0, when exponential growth ends, is indicated by the arrow in each graph. A. ZB256 (wild type) DSM-GG, ▪; DSM-GGTris, □. ORB3095 (lonA) DSM-GG, ▴; DSM-GGTris, ▵. B. ZB256 (wild type) DSM-GG, ▪; DSM-GGTris, □. ORB3001 (lonB ) DSM-GG, ▴; DSM-GGTris, ▵. C. ZB256 (wild type) DSM-GG, ▪; DSM-GGTris, □. ORB3096 (clpX ) DSM-GG, ▴; DSM-GGTris, ▵. D. LAB2525 (wild type) DSM-GG, ▪; DSM-GGTris, □. ORB3130 (clpX ) DSM-GG, ▴; DSM-GGTris, ▵. E. MAB205 (wild type) DSM-GG, ▪; DSM-GGTris, □. ORB3109 (clpX ) DSM-GG, ▴; DSM-GGTris, ▵. F. LAB2525 (wild type) DSM, □; ORB3130 (clpX ) DSM, ▪. G. ZB256 (wild type) DSM-GG, ▪; DSM-GGTris, □. ORB3097 (clpP ) DSM-GG, ▴; DSM-GGTris, ▵. H. LAB2525 (wild type) DSM, ▵; ORB3232 (clpP ) DSM, ○.
In contrast, the expression of phrC–lacZ and spoVG42–lacZ in clpX mutant cells, while low in DSM-GG cultures, showed no increase in activity when Tris-HCl was added to raise the pH (Fig. 4C and D). The same pattern of clpX dependency was observed in the case of rpoDp4–lacZ (data not shown) and spoIIA–lacZ. (Fig. 4E), both of which are transcribed from promoters that are recognized by the σH form of RNAP. Although the elevated concentrations of σH were observed in lonA and clpX mutants, there is another level of control that affected σH activity and was dependent on clpX.
The expression of phrC–lacZ was also examined in DSM culture without added glucose and glutamine to determine whether clpX was required for the induction of σH-dependent gene transcription in cells undergoing sporulation. 4Figure 4F shows that the expression of phrC–lacZ increases as cells enter stationary phase, but no post-exponential induction is observed in the clpX mutant. The level of phrC–lacZ expression in exponential phase was unaffected by the clpX mutation. The same dependency on clpX in cells grown in sporulation medium was observed in the case of spoVG42–lacZ expression (data not shown). Not surprisingly, the clpX mutation also reduced sporulation efficiency by more than 96%, conferring an oligosporogenous phenotype.
The effect of a clpX mutation on the expression of lacZ fusions requiring the σA and σD forms of RNAP was examined using a rpsD–lacZ and a hag–lacZ fusion. The rpsD gene encodes the ribosomal protein S4 and is transcribed from a strong, σA-utilized promoter (Grundy and Henkin, 1992). The hag gene encodes flagellin and is a member of the σD regulon (Mirel et al., 1994). Wild-type and clpX strains bearing either of the two fusions were grown in DSM-GG and subjected to Tris-HCl pH adjustment. The clpX mutation had no effect on the expression of hag–lacZ, whereas it was observed to cause a slight increase in rpsD–lacZ expression compared with wild-type cells (Fig. 5A and B). These results suggest that the ClpX requirement was specific for σH activity and that ClpX does not significantly influence the expression of genes that are transcribed by the σA and σD forms of RNAP.
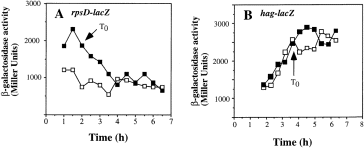
. Expression of rpsD–lacZ and hag–lacZ in a clpX mutant. rpsD- and hag-directed β-galactosidase activity of wild-type and clpX genetic backgrounds was measured in DSM-GG with pH adjustment. A. LAB691 (wild type), □; ORB3099 (clpX ), ▪. B. LAB2607 (wild type), □; ORB3098 (clpX ), ▪.
One interpretation of these results is that there is an inhibitor of σH activity that is degraded by the ATP-dependent ClpXP protease. If this was true, then a clpP mutation would also confer reduced σH activity. We examined the effect of a clpP insertion mutation on the expression of the spoVG42–lacZ fusion under low- and high-pH conditions. As shown in 4Fig. 4G, the pattern of spoVG42–lacZ expression after exponential growth is nearly the same as that of a wild-type strain. The expression of phrC–lacZ in wild-type and clpP mutant cells grown in DSM medium was also examined. Again, a clpP mutation showed no effect on the pattern of σH-dependent phrC expression (Fig. 4H). These data indicate that ClpP is probably not involved in the activation of σH activity.
The clpX–lon region of the B. subtilis genome is a complex locus
How does one reconcile the observation of low σH activity in clpX cells with high levels of σH protein under conditions of low environmental pH? One possibility is that ClpX performs two functions, one is to stimulate, directly or indirectly, σH activity, and the other is to facilitate ClpP-independent degradation of σH in DSM-GG medium. Another explanation can be uncovered from inspection of the clpX–lon locus (Fig. 6). Downstream from clpX is the lonB gene, which appears to encode a shortened version of Lon protease, lacking the N-terminal region conserved among prokaryotic Lon proteases. A mutation in lonB was shown to cause an increase in σH concentration (Fig. 2E). Between clpX and lonB is a stem–loop structure that resembles a rho-independent transcriptional terminator. A small open reading frame (ORF), orf61, overlaps with the N-terminal coding end of lonB (Gerth et al., 1996; Kunst et al., 1997). The ribosome binding site of orf61 resides within the downstream half of the stem–loop structure. It is conceivable that the translation of orf61 prevents the formation of the transcriptional termination sequence, thereby allowing transcription from clpX to proceed into lonB. Thus, it is also possible that the clpX insertion mutation confers a negative, polar effect on lonB expression. The high levels of σH observed in a clpX mutant might be caused, in part, by the reduced lonB transcription resulting from the polarity of the clpX insertion mutation.
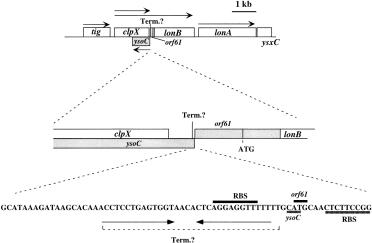
. The organization of the clpX–lon locus. Top: the rectangles represent the coding sequences of the genes of the clpX–lon locus. The shaded box is the ysoC coding sequence on the opposite strand of the clpX–lonB DNA. tig encodes trigger factor or peptide prolyl isomerase. Arrows indicate the direction and length of transcription units. The location of a putative rho-independent transcription termination sequence between clpX and lonB is indicated (Term.). Middle: the locations of the C-terminal coding end of clpX and N-terminal coding ends of ysoC and lonB and the orf61 coding sequence overlapping with the 5′ end of the lonB coding sequence are shown. Bottom: the sequence of the rho-independent transcription termination sequence and flanking DNA are shown. The overlapping ATG start codons of orf61 and ysoC are indicated along with their ribosome binding sites.
Another ORF, ysoC, is located on the opposite strand of DNA containing clpX and orf61 (Kunst et al., 1997). Its ATG translational start codon overlaps with that of orf61, and the coding sequence extends 428 nucleotides into the 3′ half of the clpX coding sequence. The clpX mutation used in our experiments is a deletion of 286 nucleotides that also deletes 135 nucleotides of ysoC. It was therefore a possibility that the gene required for σH activity is ysoC and not clpX.
Complementation of the clpX mutation restores σH-dependent expression
Both LonA and LonB function in turnover of σH at low pH, but the induction of σH activity observed in Tris-HCl-treated DSM-GG and in DSM cultures requires the clpX gene and not the ysoC gene. This was confirmed by a complementation experiment. A polymerase chain reaction (PCR) fragment extending from 79 bp upstream to 70 bp downstream of the clpX coding sequence was inserted into the plasmid pDR67. The fragment contains the clpX promoter region and the stem–loop region between clpX and lonB. It also carries the entire ysoC ORF (Fig. 7A). The clpX–ysoC sequence was inserted into the amyE locus of the chromosome as a result of the amyE DNA of pDR67 that mediates a double recombination event (Ireton et al., 1993). The amyE ::clpX strain was rendered competent and transformed with DNA from the clpX ::spc mutant, yielding the amyE ::clpX/clpX ::spc diploid strain. Cells of the clpX diploid were lysogenized with SPβphrC–lacZ, and the expression of the phage-borne fusion was examined during growth and stationary phase in DSM. The complementation of clpX by the wild-type allele at amyE was incomplete, but phrC–lacZ expression was significantly higher than that observed in the clpX mutant (Fig. 7B). A similar result was observed in clpX cells grown in DSM (see below).
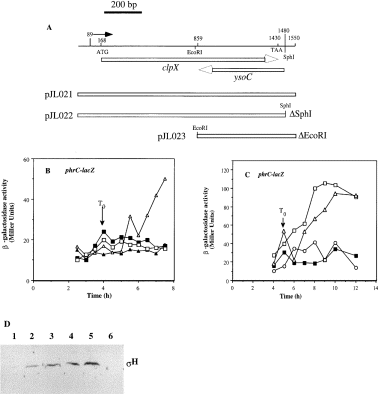
. Complementation analysis of clpX. A. Diagram showing the region of the clpX locus amplified by PCR (line at the top). The numbers above the line indicate nucleotide positions on the PCR fragment. The arrow above the line indicates the location of the transcriptional start site. ATG and TAA are the start and stop codons of clpX respectively. The arrows below the line show the orientation and direction of clpX and ysoC coding sequences. The three rectangles indicate the inserts of plasmids pJL021, 22 and 23. B. Time course of phrC–lacZ expression in cells grown in DSM-GG, with and without Tris-HCl treatment, or in DSM. ORB3130 (clpX ) DSM-GG, ▪; DSM-GGTris, □. ORB3107 (amyE::clpX+/clpX ) DSM-GG, ▴; DSM-GGTris, ▵. C. Time course of phrC–lacZ expression in cells grown in the sporulation medium DSM. LAB2525 (wild type), □. ORB3130 (clpX ), ▪. ORB3122 (amyE ::clpX SphI/clpX ), ▵. OR3127 (amyE ::clpX EcoRI/clpX ), ○. D. Western blot analysis of σH protein in extracts of cells collected from DSM-GG cultures at the times indicated (T1 or T3). Protein (42 μg) was applied to 12% SDS–polyacrylamide gels and resolved by electrophoresis. The protein was blotted onto nitrocellulose, and σH protein was visualized using anti-σH antiserum together with reagents and methods described in Experimental procedures. Lane 1, JH642 (wild type) T3. Lane 2, LAB2876 (clpX ) T1. Lane 3, LAB2876 (clpX ) T3. Lane 4, ORB3106 (amyE ::clpX/clpX ), T1. Lane 5, ORB3106 (amyE::clpX/clpX ) T3. Lane 6, RL102 (spo0H ) T3.
To determine whether ysoC or clpX were required for the σH activity, deletions were made in the clpX–ysoC fragment used for complementation. SphI digestion of the pDR67 derivative, pJL021, released a fragment bearing the ribosome binding site of the ysoC gene and the 3′ end of the clpX–ysoC fragment, but leaving the clpX coding sequence intact. EcoRI digestion released a fragment of the 5′ end of the clpX gene deleting 692 nucleotides of clpX, but leaving the ysoC ORF intact.
The impairment of sporulation and phrC–lacZ expression conferred by a mutation in clpX was alleviated by the introduction of the clpX gene and the SphI deletion derivative at the amyE locus (Fig. 7C). The number of colony-forming units (cfu) after heat treatment at 80°C as a percentage of total cell number was calculated for the wild-type strain, the clpX mutant and the three diploid strains. A sporulation frequency of 70% or greater was observed for JH642, for clpX/clpX diploid cells and for the clpX cells bearing the SphI deletion derivative of the clpX fragment. Less than 4% sporulation frequency was observed for the clpX mutant and the diploid strain harbouring the EcoRI deletion allele of clpX at the amyE locus. Because the ysoC coding sequence is intact in the clpX EcoRI/clpX ::neo diploid, we concluded that ysoC does not complement the clpX ::neo mutation. Only when the intact clpX gene is integrated into the amyE locus is complementation of clpX ::neo observed.
These results clearly show that clpX, not ysoC, is required for sporulation and stimulation of σH activity.
Complementation does not reverse the effect of clpX on σH concentration at low pH
As mentioned above, the high levels of σH protein observed in the clpX mutant under low-pH conditions could be the result of the absence of ClpX-dependent proteolytic activity targeted to σH or it could be caused by the polar effect of the clpX mutation on the expression of lonB, which, along with lonA, is responsible for the low concentrations of σH in DSM-GG. If ClpX functions in the turnover of σH at low pH, then cells of the clpX diploid strain should exhibit low σH concentrations under low-pH conditions as a result of complementation. If, instead, the level of σH at low pH remains high after complementation, then this result would lend support to the hypothesis that the clpX mutation is exerting a polar effect upon the expression of lonB, thereby conferring a lonB phenotype. As shown in 7Fig. 7D, the level of σH protein in cells from the low-pH culture is high in the diploid strain (lanes 4 and 5) as well as in the clpX mutant (lanes 2 and 3), compared with the low concentration of protein in the extract of wild-type cells from the low-pH culture (lane 1). This suggests that the clpX mutation is exerting some other effect on σH concentration at low pH apart from its effect on ClpX activity. Quite possibly, the mutation causes reduced lonB expression.
Discussion
The data presented here show for the first time the essential role that clpX plays in the stimulation of σH-dependent gene expression in sporulating cells of B. subtilis. Evidence is also presented that high levels of σH protein are not sufficient to activate σH-dependent gene transcription. Low concentrations of σH protein and σH activity are observed in low-pH DSM-GG cultures. The introduction of a lon mutation (in either lonA or lonB ) results in a higher σH concentration, but not in higher σH activity, as shown by the low expression of lacZ fused to promoters used by the σH form of RNAP. Dramatic induction of σH-dependent gene expression is observed after the addition of a pH stabilizer to wild-type and lon mutant cell cultures, but this does not occur in a culture of clpX mutant cells. ClpX somehow stimulates σH activity, but only under conditions of nutritional stress, which are encountered after growth resumes following the relief of pH stress, and when cells initiate the sporulation process (in DSM). We postulate that there exists some mechanism regulating the expression of clpX or the activity of its product when cells encounter nutritional stress. Preliminary experiments using a clpX–lacZ fusion suggest that clpX transcription is not induced in DSM or in Tris-HCl-treated DSM-GG (J. Liu and P. Zuber, unpublished results).
Several studies have provided evidence for a post-translational mechanism of σH regulation and its importance in initiating cellular differentiation. The expression of a sporulation gene that requires σH for its transcription initiation was repressed in exponentially growing cells and under conditions of low external pH. These effects were not exerted at the level of spo0H transcription initiation (Healy et al., 1991; Frisby and Zuber, 1994; Cosby and Zuber, 1997). The half-life of σH in exponentially growing cells is shorter than in stationary phase cells (Healy et al., 1991). Other evidence for post-translational control has implicated the regulatory ATPase ClpC (MecB) in the turnover of σH at high temperature and during the late stages of sporulation (Nanamiya et al., 1998). Our studies indicate that σH is subject to additional levels of post-translational control involving the ATP-dependent protease Lon and the regulatory ATPase ClpX.
An insertion mutation in clpX results in elevated σH levels in cells of cultures with low external pH. This is apparently not the result of loss of ClpX activity, as complementation of the clpX mutation did not result in a reduction in σH concentration at low pH characteristic of wild-type cells. More probably, this was due to the reduced expression of the downstream lonB gene caused by polarity of the clpX ::spc insertion.
In a culture of clpX mutant cells, the elevation of pH, which normally stimulates σH-dependent gene expression, does not increase the expression of several lacZ fusions whose transcription requires the σH form of RNAP. This effect of a clpX mutation is also observed in cells grown in DSM in which sporulation is initiated at the end of exponential growth. This requirement for ClpX is specific for genes transcribed by the σH holoenzyme and not by σA or σD. At present, we do not know how ClpX stimulates σH activity and whether the action of ClpX is direct or indirect. One possibility is that ClpX facilitates the association of σH to core polymerase. Previous work has provided evidence that σA competes with σH for core polymerase, with σH overcoming this competition as cultures enter stationary phase, possibly as a result of reduced σA concentration (Hicks and Grossman, 1996). In another study, σA was shown to possess higher affinity for core polymerase than σH (Fujita and Sadaie, 1998b), suggesting that, at higher concentrations, σH could compete with σHin vivo for core polymerase. For σH to compete for core polymerase successfully, ClpX activity might be required. However, preliminary studies designed to examine the amount of σH protein associated with holo-RNAP from wild-type and clpX mutant cells have not revealed a substantial difference in the amount of σH associated with core polymerase in the two strains (J. Liu and P. Zuber, unpublished results). Another possibility is that ClpX is required for σH holoenzyme to bind specifically to promoter DNA. Unlike other sigma subunits, σH has been shown to bind non-specifically to DNA (Fujita and Sadaie, 1998a), which would render σH unavailable to direct transcription from cognate promoters in vivo. ClpX might also be required for catalytic turnover of σH holoenzyme during transcription initiation, perhaps facilitating the release of σH for the transcriptional complex. We cannot rule out the possibility that ClpX activity might operate at the level of RNAP promoter interaction without directly targeting σH. It could act on a repressor of the promoters, which, coincidentally, are also used by the σH form of RNAP. We feel that this is unlikely given the diverse functions that the σH-controlled genes (spoVG, rpoD, spoIIA, phrC ) encode. It is also possible that ClpX acts through one or more intermediate steps in a pathway affecting σH activity.
ClpX is known to function in the proteolytic regulation of RpoS, the stationary phase and stress-induced sigma factor of enteric bacteria (Pratt and Silhavy, 1996; Schweder et al., 1996; Zhou and Gottesman, 1998). In this case, the protease ClpXP, under the control of the response regulator RssB(SprE), rapidly degrades RpoS during exponential growth. The proteolytic activity associated with ClpX function is evidently not involved in the σH activity, as a mutation in clpP did not significantly affect the stimulation of σH-dependent gene expression after pH elevation or in sporulation-promoting medium. That is not to say that we have ruled out ClpXP as being a factor in σH proteolytic regulation. As shown in 4Fig. 4G, the level of σH-dependent gene expression in the exponential phase of growth is higher in a clpP mutant than that observed in a wild-type strain. It is possible that ClpP, in association with ClpX, functions in the exponential phase degradation of σH, thus mimicking its role in RpoS degradation.
There is ample evidence that ClpX performs a dual role in the regulation of DNA processing events (Mhammedi-Alaoui et al., 1994; Levchenko et al., 1995; 1997; Kruklitis et al., 1996; Jones et al., 1998). ClpXP functions in the proteolytic regulation of the Mu repressor, thereby stimulating transposition. ClpX interaction with MuA promotes transposasome disassembly, thereby stimulating phage replication.
The Lon protease is also known to function in the proteolytic regulation of a sigma subunit. In B. subtilis, the inappropriate, premature σG-dependent expression of sigG is observed in lonA mutant cells (Schmidt et al., 1994). The sigG product is the forespore-specific sigma subunit, σG, required for the optimal transcription of its own gene. The results suggest that Lon prevents the accumulation of σG in non-sporulating cells. Our results indicate that both LonA and B negatively regulate σH, proteolytically, under conditions of acid stress, but this function of Lon might be manifested under conditions, besides starvation, that inhibit cell growth. These conditions induce the general stress response that involves, among other regulatory systems, the σB regulon. The lonA gene, although not a member of the σB regulon, is induced under conditions of temperature, osmotic and oxidative stress that inhibit growth but do not induce sporulation (Hecker et al., 1996). The product of lonB is a homologue of prokaryotic Lon proteases and, based on Western analysis, also participates in the degradation of σH in low-pH cultures. The LonA and B proteins may operate in concert, perhaps as a complex to catalyse proteolysis.
From our studies, a model of σH post-translational control can be constructed (Fig. 1). When growth is inhibited by acid stress, Lon is responsible for maintaining a low level of σH protein. Under starvation conditions, Lon-dependent proteolysis of σH is downregulated, but the induction of σH activity in response to conditions that promote sporulation requires ClpX. Questions remain as to whether σH itself is the direct substrate of Lon and ClpX and, if so, how do they distinguish σH from other sigma subunits? How does ClpX affect σH activity? Answers to these questions will further our understanding of the events that trigger cellular differentiation.
Experimental procedures
Bacterial strains
Bacterial strains used in the study are all derived from JH642 and are listed in Table 1. Strain RL102 (provided by R. Losick) bears a deletion of a 96 bp fragment within the spo0H gene that is replaced with a cat gene conferring chloramphenicol resistance. RL106 contains a plasmid-amplified spo0H gene that directs overproduction of σH protein in B. subtilis. The clpX mutation (obtained from A. Grossman) is a replacement of a 286 bp internal segment of the clpX coding sequence with a spectinomycin resistance cassette (spc ). The lonA ::cat mutation (obtained from C. Moran) has been described previously (Schmidt et al., 1994). The ftsH ::erm mutation was isolated from a plasmid disruption of the ftsH gene that conferred a defect in fermentative anaerobic growth (Nakano et al., 1997). The lonB mutation was constructed by transforming JH642 cells with plasmid pJL020, which contains an internal fragment of the lonB gene (see below). A single recombination event resulted in disruption of the lonB coding sequence. The clpP mutation was constructed by M. Ogura by inserting an erm resistance gene cassette into the SacII site of the clpP coding sequence (Ogura et al., 1999). The resulting plasmid, bearing the clpP ::erm allele, was used to transform competent cells of JH642. The clpP ::erm allele was introduced into the clpP gene by double recombination. The strain LAB2525 was constructed by first transforming strain ZB307A (Zuber and Losick, 1987) with plasmid pML109, which carries the phrC–lacZ fusion (see below). Strains harbouring the spoVG42–, spo0H–, rpoDP4–, rpsD– and hag–lacZ fusions have been described previously (Grundy and Henkin, 1992; Hicks, 1993; Chen and Helmann, 1994; Cosby and Zuber, 1997).
Plasmids
Plasmid pML109, which carries a phrC–lacZ transcriptional fusion, was constructed by inserting the 741 bp EcoRI–EaeI fragment of plasmid pLC22-42 (Carter et al., 1990) into EcoR1–SmaI-digested pTKlac (Kenney and Moran, 1991). The EaeI site of cleaved pLC22-42 was filled in by treatment with T7 DNA polymerase and deoxynucleotides. The fragment contains the 3′ end of rapC and the 5′ end to the 12th codon of the phrC gene. Plasmid pJL020 contains an internal segment of the lonB gene. The primers olonB-U and olonB-L (Table 2) were used in a PCR containing B. subtilis chromosomal DNA. A PCR product encompassing 269 bp to 803 bp of the lonB was generated, which was inserted into the integration vector pMMN13. Plasmid pJL021 was created by inserting a PCR fragment containing the clpX gene into plasmid pDR67. The fragment was generated using primers oclpX-U and oclpX-L, which created a SmaI site and a BamHI site, respectively, at the ends of the fragment. The fragment was inserted into SmaI–Bgl II-cleaved pDR67 (Ireton et al., 1993). Two derivatives of pJL020, pJL022 and pJL023, were constructed by cleaving the plasmid with either SphI or EcoRI, followed by intramolecular ligation (Fig. 7A).
Bacterial culture conditions
B. subtilis cultures were grown in liquid or solid Difco sporulation medium (DSM) (Nakano et al., 1988). Liquid cultures were supplemented with 2% glucose and 0.1% glutamine to make DSM-GG (Cosby and Zuber, 1997). E. coli cells were grown in LB for the purpose of plasmid isolation. Antibiotics were used at the following concentrations: ampicillin, 25 μg ml−1; erythromycin/lincomycin, 1 μg ml−1 and 25 μg ml−1 respectively; chloramphenicol, 5 μg ml−1; spectinomycin, 75 μg ml−1; neomycin, 5 μg ml−1.
Transformation and transduction
Preparation of competent cells of B. subtilis and DNA-mediated transformation were carried out as described previously (Hoch et al., 1967; Dubnau and Davidoff-Abelson, 1971; Niaudet and Ehrlich, 1979). Specialized transduction using SPβ phage constructs was carried out as described previously (Zuber and Losick, 1987).
Assay of β-galactosidase activity
All inocula were grown on solid DSM at 37°C overnight with the appropriate antibiotics. The cells were harvested by washing the plate surface with 2 ml of DSM-GG medium and used to inoculate DSM-GG medium to an initial optical density (OD) at 595 nm of about 0.17 or an initial Klett value (red filter) of about 8. The two parallel cultures were grown at 37°C in a shaking water bath. Collection of 1 ml of culture was started and continued at 30 min intervals for β-galactosidase activity assay when the culture OD595 reached 0.4 or a Klett number of around 20. Tris-HCl buffer (1 M, pH 7.5) was added to one of the two flasks to a final concentration of 25 mM when the DSM-GG cultures reached T0 (OD595 = 1.7–2.0). Measurement of β-galactosidase activity was performed on 1 ml samples collected from liquid cultures at 30 min intervals. Methods and assay conditions have been described previously (Nakano et al., 1988).
Sporulation frequency
Wild-type, clpX, amyE::clpX/clpX::spc, amyE::clpX EcoRI/clpX::spc and amyE::clpX: SphI/clpX::spc strains were cultured in DSM medium for 36 h. Serial dilutions were then made and plated on DSM solid medium for total viable cell number. Duplicate samples were heated to 80°C for 20 min and plated on DSM. Sporulation efficiency was recorded as the percentage of the total colony count that arose on DSM plates after treatment at 80°C.
Western blot analysis
Cultures were grown in DSM-GG and DSM-GGTris as described above. Samples were removed and centrifuged at 4°C at T1 (1 h after the end of exponential growth), T2, T3 and T4. The cells were washed once in PBS, centrifuged again and stored at −70°C. The pellets were thawed and resuspended in 20 mM Tris-HCl (pH 7.5), 5 mM EDTA, 1 mM dithiothreitol (DTT), 1.5 mM phenylmethylsulphonyl fluoride (PMSF). Cells were disrupted with a French press as instructed by the manufacturer (Spectronics). Samples of whole-cell extracts were diluted in 2 × SDS loading buffer, boiled for 5 min and applied to a SDS–12% polyacrylamide gel. After electrophoresis, the proteins were electrotransferred to nitrocellulose filters with a Mini Trans-Blot transfer cell (Bio-Rad) and probed with anti-σH antibody (obtained from D. Rudner and R. Losick; Healy et al., 1991). The anti-σH antibody was purified by absorption with a whole-cell lysate of RL102 (spo0H HindIII–EcoRI) (Healy et al., 1991). After incubation with anti-σH antibody, the filters were incubated with secondary goat anti-rabbit alkaline phosphate conjugate and reacted with chromogenic alkaline phosphatase substrate, as recommended by the manufacturer (Gibco BRL).
Acknowledgements
We wish to thank D. Dubnau, A. D. Grossman, J. Helmann, R. Losick, C. P. Moran, Jr, O. Resnekov and D. Rudner for strains and plasmid constructs. We thank R. Losick for anti-σH antiserum. We acknowledge M. LaCelle and Yi Zhu for technical assistance. We are grateful to M. M. Nakano for critical reading of the manuscript. This research was supported by grant GM45898 from the National Institutes of Health.





