Alanine mutants of the Spo0F response regulator modifying specificity for sensor kinases in sporulation initiation
Abstract
Five single alanine substitution mutations in the Spo0F response regulator gave rise to mutant strains of Bacillus subtilis with seemingly normal sporulation that nevertheless rapidly segregated variants blocked in sporulation. The basis for this deregulated phenotype was postulated to be increased phosphorylation of the Spo0A transcription factor, resulting from enhanced phosphate input or decreased dephosphorylation of the phosphorelay. Strains bearing two of these Spo0F mutant proteins, Y13A and I17A, retained a requirement for KinA and KinB kinases in sporulation, whereas the remaining three, L66A, I90A and H101A, gave strains that sporulated well in the absence of both KinA and KinB. Sporulation of strains bearing L66A and H101A mutations was decreased in a mutant lacking KinA, KinB and KinC, but the strain bearing the I90A mutation required the further deletion of KinD to lower its sporulation frequency. The affected residues, L-66, I-90 and H-101, are involved in crucial hydrophobic contacts stabilizing the orientation of helix α4 of Spo0F. The data are consistent with the notion that these three mutations alter the conformation of the β4–α4 loop of Spo0F that is known to contain residues critical for KinA:Spo0F recognition. As this loop has a propensity for multiple conformations, the spatial arrangement of this loop may play a critical role in kinase selection by Spo0F and might be altered by regulatory molecules interacting with Spo0F.
Introduction
Sporulation in Bacillus subtilis is a developmental process initiated in response to environmental, metabolic and cell cycle signals. While the nature of these signals is still unknown, the mechanism by which they are interpreted and transduced to activate developmental transcription is becoming understood. The key transcription factor for sporulation, Spo0A, is activated by phosphorylation through a pathway termed the phosphorelay (Burbulys et al., 1991). The phosphorelay is a signal transduction system closely related to the two-component systems known to be a major means by which bacteria recognize and respond to environmental signals (Stock et al., 1989; Parkinson and Kofoid, 1992). Under laboratory conditions, signal input in sporulation results from the action of two kinases, KinA and KinB (Trach and Hoch, 1993). These kinases phosphorylate a single-domain response regulator Spo0F that serves as a substrate for phosphorylation of Spo0A by means of the Spo0B phosphotransferase. The enhanced complexity of the phosphorelay is designed to allow supplemental signal input (Burbulys et al., 1991). Signals antithetical to sporulation are processed by phosphatases that dephosphorylate either Spo0F∼P (Perego et al., 1994) or Spo0A∼P (Ohlsen et al., 1994). In addition, KinA is regulated by an anti-kinase, which is in turn controlled by an anti-anti-kinase (Wang et al., 1997). The probability that sporulation will occur in any cell is determined by competition between signal input from the kinases and signal cancellation by the phosphatases (Perego and Hoch, 1996; Perego et al., 1996; Perego, 1998). Thus, the phosphorelay serves as the core of a larger signal integration circuit (Ohlsen et al., 1994).
Mutations in the Spo0A transcription factor that allow the cell to bypass the phosphorelay and initiate sporulation in the absence of Spo0F or Spo0B are known (Sharrock et al., 1984; Hoch et al., 1985; Shoji et al., 1988). These mutations occur in the phospho-acceptor domain of Spo0A and allow direct phosphorylation by other cellular kinases (Spiegelman et al., 1990; Kobayashi et al., 1995; LeDeaux and Grossman, 1995). As these mutations bypass all of the checks and balances that the phosphorelay provides, such mutants have a propensity to accumulate additional mutations that block sporulation entirely (Spiegelman et al., 1990). In this study, the biochemical basis for mutations in Spo0F that give rise to phenotypes similar to bypass mutants of Spo0A was determined.
Results
Alanine substitution mutations of Spo0F conferring deregulated sporulation phenotypes
In an alanine mutagenesis study designed to determine the surfaces of interaction of Spo0F with kinases and phosphatases, five Spo0F mutants, Y13A, I17A, L66A, I90A and H101A, were observed to segregate Spo− papillae from an otherwise Spo+ colony (Tzeng and Hoch, 1997). This phenotype is characteristic of phosphorelay mutants that increase the level of sporulation at inappropriate times. In such mutants, the absolute level of sporulation reached by the culture is not affected. The increased propensity to sporulate is manifested during growth and is rapidly selected against by reversion or by the accumulation of mutations in the phosphorelay that prevent sporulation and relieve the pressure to sporulate (Spiegelman et al., 1990). Mutation of Spo0F residue Y-13 to serine is known to render the protein resistant to the RapA and RapB phosphatases, with the consequence of increasing the frequency of sporulation (Perego et al., 1994). A similar mutant, Y13A, was resistant to the RapB phosphatase in vitro, accounting for its deregulated phenotype (Tzeng et al., 1998). The number of Rap phosphatases that affect the phosphorylation level of Spo0F is not known for certain, preventing a definite assignment of phosphatase resistance as the cause of increased sporulation in any mutants in residues other than Y-13. Other causes for the phenotype, such as increased activity with kinases or modified kinase specificity, were tested in this study.
Sporulation of Spo0F alanine mutants with kinase-deficient backgrounds
Sporulation in wild-type strains of B. subtilis under laboratory conditions is dependent on the functioning of two kinases, KinA and KinB (Trach and Hoch, 1993). One of the possible reasons for the deregulated sporulation phenotype of the mutant strains might be increased affinity or reaction rate with these kinases. To test this possibility, sporulation of the mutants was assessed in strains lacking KinA, the major kinase responsible for the initiation of sporulation during stationary phase. The mutant Spo0F alleles gave reproducibly higher sporulation in the wild-type background, and two mutants, Y13A and I17A, were as affected by the loss of KinA as the wild-type strain (Table 1). Thus, these two mutants are still dependent on KinA. As the increased sporulation of Y13A is known to result from resistance to the Rap phosphatases, I17A may be a mutation that affects interaction with KinA or phosphatases or both.
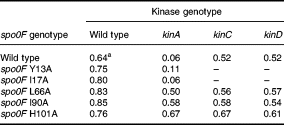
Three mutants, L66A, I90A and H101A, showed high sporulation levels in a kinA mutant, suggesting that they may have acquired the ability to be phosphorylated by other kinases in the cell or were more active with KinB The three mutants gave high levels of sporulation in a kinA kinB double mutant, which rules out increased reactivity with KinB as the reason for the sporulation phenotype (Table 2). The effect of two other kinases, KinC and KinD, was tested. KinC is known to be responsible for the phosphorylation of Spo0A mutants that bypass the phosphorelay (Kobayashi et al., 1995; LeDeaux and Grossman, 1995). KinD (formerly YkvD; Kunst and et al., 1997) was identified in the genome sequencing project as a putative kinase, and further analysis has shown it to be in the same family of kinases as KinA and KinB (Fabret et al., 1999). Strains with these three mutant spo0F alleles constructed in kinC- or kinD-deficient strains sporulated normally (Table 1).
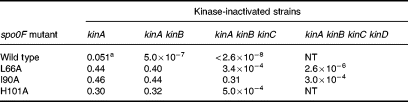
As single-mutant kinases did not decrease sporulation in the L66A, I90A or H101A strains, the alleles were tested in strains lacking more than one kinase (Table 2). A strain expressing the wild-type Spo0F protein was extremely sensitive to the loss of both KinA and KinB in sporulation, confirming that these two kinases were responsible for the initiation of sporulation in the wild-type strain. However, the loss of both of these kinases did not affect the sporulation of strains bearing the three mutant Spo0F alleles. A mutation in KinC, in addition to KinA and KinB, reduced the frequency of sporulation of the L66A and H101A strains 1000-fold, but was without effect on the I90A strain. A quadruple kinase mutant lacking KinD in addition to KinA, KinB and KinC reduced the frequency of sporulation in the I90A strain 1000-fold and also reduced sporulation of the L66A strain an additional 100-fold. As the single mutations in KinC or KinD were not effective in reducing sporulation in strains bearing the spo0F alleles, the actual effect of the mutations on Spo0F was the acquisition of new kinase specificities while retaining reactivity with the old. Furthermore, in vitro studies with purified Spo0F mutant proteins showed that they did not acquire the ability to be phosphorylated with acetyl-phosphate as a possible non-specific source of phosphoryl groups (unpublished data).
Reactivity of kinases on Spo0F mutant proteins in vitro
The altered kinase reactivity of the mutant spo0F alleles determined from genetic studies was tested by in vitro phosphorylation studies. KinC and KinD are both membrane-bound kinases with two transmembrane domains. Therefore, in order to obtain purified enzyme for these studies, maltose-binding protein fusions of the catalytic domains of each were constructed, expressed and the proteins isolated.
KinA, KinC and KinD were tested in vitro for their phosphotransfer activities with Spo0F, Spo0F(L66A), Spo0F(I90A) and Spo0F(H101A). A time course for each reaction was performed, and the quantity of phosphorylated Spo0F was determined by phosphorimaging. KinA was active on all mutants, as predicted from the genetics and, while the initial reaction rate appeared to be similar for all, the final level of phosphorylation was higher for Spo0F(L66A) and Spo0F(I90A) (Fig. 1A). KinD was found to be much more active on all of the mutants than on the wild-type Spo0F for both initial rate and final phosphorylation level (Fig. 1C). KinC was also more active on the mutants than on the wild-type Spo0F (Fig. 1B). Surprisingly, there was less difference between mutants than would have been predicted from the genetics. For example, sporulation frequencies (Table 2) predicted that KinC should be more specific for Spo0F(H101A) than KinD. This paradox could have many explanations, including diminished specificity in the KinC and KinD kinases in which the membrane-spanning domains have been deleted in order to purify the proteins. KinA was 70-fold more active on Spo0F than either KinC or KinD (Fig. 2).
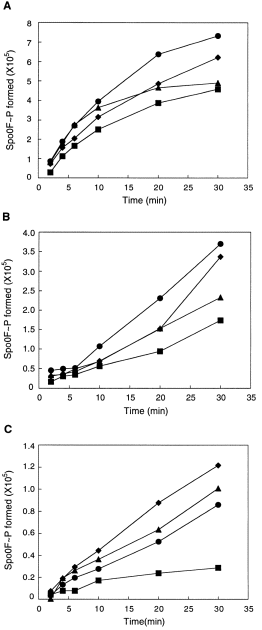
. Time courses of phosphorylation of Spo0F proteins (10 μM) by KinA (A), KinC (B) or KinD (C) (0.5 μM). The reactions were carried out as described in Experimental procedures. The level of Spo0F∼P formed is expressed in pixel values as determined by phosphorimaging analysis of SDS–glycine–polyacrylamide gels using the imagequant software. As the values for Spo0F∼P formed are phosphorimager numbers that differ between experiments, and other reasons, comparisons between (A) and (B) or (C) cannot be made. The Spo0F∼P-values for (B) and (C) were obtained from longer exposures than that for (A) because KinA is much more active than either KinC or KinD (Fig. 2). (▪) Wild-type Spo0F; (•) Spo0F L66A; (♦) Spo0F I90A; (▴) Spo0F H101A.
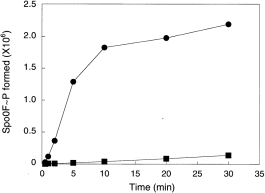
. Time course of phosphorylation of wild-type Spo0F (10 μM) by KinA (•) and KinC (▪) (0.5 μM). The level of Spo0F∼P formed is expressed as in Fig. 1. The reactions were carried out as described in Experimental procedures. The reactivity of Spo0F with KinD was similar to that with KinC and therefore omitted.
Discussion
The Spo0F mutant residues responsible for altered kinase specificity are clustered at a common interface distant from the active site, i.e. the aspartyl pocket (Fig. 3). Residues L-66, I-90 and H-101 are located on α-helix 3, α-helix 4 and β-strand 5 respectively. Each residue makes important van der Waals' contacts between these structure elements. L-66 is one of the two α3 residues making hydrophobic contacts (with S-93) at the interface of α3 and α4, and it is completely buried. The hydrophobic packing between H-101 and I-90 is the sole interaction between the β5 strand and the α4 helix. The imidazole ring of H-101 has been observed to exist in two conformations: one that is buried under the β4–α4 loop; and the other being more solvent exposed (Feher et al., 1997). An aromatic residue always occupies the residue equivalent to H-101 of Spo0F in other response regulators. Y-106, the H-101 equivalent of CheY, has been shown to occupy two conformations and has been implicated in post-phosphorylation activation of CheY (Zhu et al., 1997).
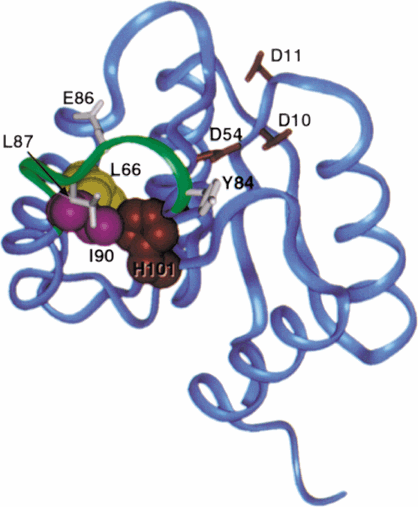
. Ribbon diagram of the Bacillus subtilis Spo0F solution structure (Feher et al., 1997). The side-chains of residues truncated to alanines are shown in CPK representation; L-66 (yellow), I-90 (magenta) and H-101 (red). The β4–α4 loop (green) makes direct contact with I-90 and H-101, and contains several residues found to be important for interactions with KinA (Tzeng and Hoch, 1997), Y-84, E-86 and L-87 (white). The side-chains comprising the aspartyl pocket, D-10, D-11 and D-54 (red), are illustrated for reference. This illustration was generated with the insightII (MSI) and photoshop version 4.0 (Adobe) programs.
All of these residues are involved in the crucial hydrophobic contacts that stabilize the orientation of helix α4. The structure of this helix is consequential to the conformation of the β4–α4 loop, and the changes caused by mutations at residues 66, 90 and 101 would be likely to propagate to the loop. Residues on this loop (Y-84, E-86 and L-87) have been shown to be critical for KinA:Spo0F recognition. Alanine substitution for any one of them diminished this interaction (Tzeng and Hoch, 1997). Mutations in the β4–α4 loop or in helix 4 of PhoB of Escherichia coli were found greatly to enhance the rate of phosphorylation of PhoB by the VanS kinase of Enterococcus faecium (Haldimann et al., 1996). The structure of this loop may be of general importance in kinase–response regulator interaction. As has been pointed out previously (Feher et al., 1998), this is a region with propensity for multiple conformations and with sensitivity to aspartyl pocket perturbation. Spo0F may take advantage of this structural flexibility to accommodate specific recognition with each kinase involved in sporulation initiation. Additionally, I-90 and H-101 are partially surface exposed; truncation of these residues to alanine may reduce steric barriers to allow the binding of additional kinases.
Despite the clustering of the three alanine mutations at a common area, there were subtle variations in structural perturbations mediated through these mutations. Modification of residues H-101 and I-90, which interact with each other according to the structure, showed dissimilar selectivity towards kinases: Spo0F(H101A) was phosphorylated by KinC but not KinD, while KinD was active on Spo0F(I90A).
The effects of the Spo0F mutations were to gain activity for kinases that do not normally play a significant role in sporulation. The single kinase deletion studies suggested that the gaining of new specificities did not greatly diminish activity with KinA. Interestingly, the PhoB mutations that allowed enhanced VanS activity did not diminish PhoR activity and, in some cases, increased it (Haldimann et al., 1996). The KinC and KinD kinases have much in common with KinA and KinB. All these kinases are orphans whose genes are not linked to a response regulator gene and, therefore, their targets for phosphorylation are not obvious. All these kinases are also grouped together in kinase group IIIB, the basis for which is homology around the histidine residue that becomes phosphorylated (Fabret et al., 1999). As the histidine region is certain to make intimate contact with the response regulator active site during the phosphotransfer reaction, the amino acids surrounding the histidine are likely to be a major contributor to the specificity of interaction with the response regulator.
What then do these kinases normally do in the cell, as in the laboratory they do not contribute significantly to sporulation? It is clear that they are expressed and are active enough to suppress the loss of KinA and KinB in certain Spo0F mutants. Therefore, the signal input into these kinases must be sufficient to stimulate autophosphorylation and phosphotransfer despite the poor activity of KinC and KinD on Spo0F and its mutants relative to KinA. Several possibilities exist to explain the apparent paradox of these three Spo0F mutations. The mutations may stabilize the phosphorylated form of Spo0F, allowing low-level phosphorylation by weakly active kinases to suppress the loss of KinA and KinB. This might occur through resistance to dephosphorylation by phosphatase or other enzymes in vivo that is not obvious from the unchanged sensitivity of the mutant phosphorylated Spo0F proteins to RapB phosphatase or to spontaneous dephosphorylation (Tzeng et al., 1998) (Y.-L. Tzeng, unpublished data). Another possibility was suggested from the results. As the loops surrounding the active-site aspartates of Spo0F are highly dynamic and the consequence of the alteration of several buried residues may be a conformational alteration or stabilization of a unique configuration of the β4–α4 loop, perhaps such changes could normally be induced by ligand interactions between Spo0F and specific small molecules or proteins produced under different environmental conditions. Thus, KinC or KinD productive interaction with Spo0F would depend upon Spo0F adopting a configuration allowing interaction, and this configuration could be a result of specific ligands.
Assuming the equilibrium between various conformations of Spo0F is the actual determinant of kinase specificity, cellular environmental conditions may determine the equilibrium and therefore the most productive kinase. Sporulation in laboratory media is certainly not an environment these cells evolved to encounter, and the observed dominance of KinA may be unique to such conditions. Perhaps in the soil or other natural environments, others of this kinase family are more important.
Experimental procedures
Bacterial strains and plasmid construction
The B. subtilis strains used in this study are listed in Table 3. For the construction of strain JH11387, a polymerase chain reaction (PCR)-generated fragment comprising the Pst I–Bgl II region internal to the kinC gene was first cloned in the integrative vector pJM103 (Perego, 1993). The Km resistance cassette from pJM114 (Perego, 1993) was then cloned in the EcoRV site located approximately in the middle of the fragment. The plasmid obtained, pKinCΔkm, was linearized and used to transform the wild-type strain JH642 via a double cross-over integration event. A pJM103 plasmid carrying a PCR-generated fragment of 500 bp internal to the ykvD gene was constructed and used to transform strain JH642. Integration via single cross-over recombination resulted in inactivation of ykvD (kinD ) giving strain JH11467. Site-directed mutagenesis of spo0F was performed as described previously (Tzeng and Hoch, 1997). Plasmids carrying the spo0F mutations Y13A, I17A, L66A, I90A and H101A were linearized and transformed into the recipient strains as described previously (Tzeng and Hoch, 1997).

Protein expression and purification
The wild-type and mutant spo0F genes were cloned in the pET system (Novagen) as described previously (Tzeng and Hoch, 1997) and transformed into the E. coli expression strain BL21(DE3)pLysS. The expression and purification of (His)6-tagged Spo0F proteins was carried out according to Tzeng and Hoch (1997).
For the expression of KinC and YkvD fusions to the maltose-binding fusion protein (MBP), the DNA sequences coding for the C-terminal 363 amino acids of KinC or 231 amino acids of YkvD were obtained by PCR reaction, cloned into the EcoRI–BamHI sites of pMAL-c2 (New England Biolabs) and transformed into E. coli DH5α. The expression of the MBP fusion proteins was obtained by induction with 0.3 mM IPTG at a cell density of OD600 = 0.6. The expressed proteins were purified from amylose columns as described by the manufacturer (New England Biolabs). KinA was purified as described previously (Perego et al., 1989). The protein concentration was determined by BCA protein assay (Pierce) with BSA as standard.
Phosphorylation assays
Time courses of phosphorylation of Spo0F proteins (10 μM) by kinases (0.5 μM) were carried out in a 100 μl reaction volume. Incubation at room temperature was carried out for a period of time indicated in the figure legends, and 15 μl aliquots were withdrawn, quenched by 3 μl of 5 × SDS loading buffer and frozen immediately in dry ice. Samples were loaded on 18% Tris-tricine minigels and run at a constant current of 70 mA. Gels were dried under vacuum and analysed by volume integration using the imagequant software on a Molecular Dynamics phosphorimager.
Spore counts
B. subtilis strains were grown in 5 ml of Schaeffer's medium at 37°C for 24–26 h. Serial dilutions of bacterial culture were made and plated before and after CHCl3 treatment. Sporulation efficiency was determined as the ratio between CHC13 resistant cell count and total viable count.
Acknowledgements
This research was supported, in part, by grants GM19416 and GM55594 from the National Institutes of General Medical Sciences, National Institutes of Health, USPHS. This is publication 12188-MEM from the Department of Molecular and Experimental Medicine at The Scripps Research Institute.




