Identification and characterization of a stress-responsive promoter in the macromolecular synthesis operon of Bacillus subtilis
Abstract
Bacillus subtilis DB1005 is a temperature-sensitive (Ts) sigA mutant. Induction of σA has been observed exclusively in this mutant harbouring extra copies of the plasmid-borne Ts sigA gene transcriptionally controlled by the P1P2 promoters of the B. subtilis macromolecular synthesis (MMS; rpoD or sigA) operon. Investigation of the mechanisms leading to the induction has allowed us to identify a σB-type promoter, P7, in the MMS operon for the first time. Therefore, at least seven promoters in total are responsible for the regulation of the B. subtilis MMS operon, including the four known σA- and σH-type promoters, as well as two incompletely defined promoters. The P7 promoter was activated in B. subtilis after the imposition of heat, ethanol and salt stresses, indicating that the MMS operon of B. subtilis is subjected to the control of general stress. The significant heat induction of P7 in B. subtilis DB1005 harbouring a plasmid-borne Ts sigA gene can be explained by a model of competition between σA and σB for core binding; very probably, the σB factor binds more efficiently to core RNA polymerase under heat shock. This mechanism may provide a means for the expression of the B. subtilis MMS operon when σA becomes defective in core binding.
Introduction
How to overcome environmental stresses is essential for the survival of living cells. In prokaryotes, alternative sigma factors associated with core RNA polymerase are often used to change the pattern of gene expression in response to nutritional and environmental stresses. Examples include the control of the Escherichia coli heat shock regulon by σ32 (Straus et al., 1987), the control of enteric bacteria and Bacillus subtilis chemotaxis and motility regulons by σ28 (Helmann et al., 1988; Arnosti and Chamberlin, 1989; Marquez et al., 1990), the control of the B. subtilis sporulation process by a cascade of at least six different sigma factors (Losick and Pero, 1981; Losick et al., 1986; Losick and Stragier, 1992; Errington, 1993) and the control of B. subtilis general stress (e.g. heat, ethanol, salt or oxidative stress) by σB (Haldenwang and Losick, 1979; Binnie et al., 1986; Igo and Losick, 1986; Duncan et al., 1987; Benson and Haldenwang, 1992; 1993; Boylan et al., 1992; 1993; Voelker et al., 1994; Bernhardt et al., 1997).
The macromolecular synthesis (MMS) operon of B. subtilis consists of three genes in the order of orf23, dnaG and rpoD (sigA) (Wang and Doi, 1986). The first gene orf23 (formerly P23) encodes a non-essential protein (≈ 23 kDa) with an unknown function (Wang and Doi, 1986; Zuberi and Doi, 1990). The second gene dnaG (previously called dnaE ) encodes DNA primase essential for priming the synthesis of Okazaki fragments during DNA replication (Wang et al., 1985). The third gene rpoD (sigA) encodes the σA factor of RNA polymerase (Price et al., 1983). Six promoters have been reported to control the expression of the MMS operon at different developmental stages. The two σA-type promoters, P1 and P2, are expressed during vegetative growth (Wang and Doi, 1987). The P3 and P4 promoters are of σH type and are induced at the start of sporulation (Wang and Doi, 1987; Carter et al., 1988; Qi and Doi, 1990). P5 is induced late during sporulation (T3–T5); however, it is unknown which σ directs transcription of this promoter (Qi et al., 1991). The cascade of σ factors provides a promoter-switching mechanism, which ensures timely expression from this operon during the development of B. subtilis. As dnaG and sigA are so important for cell growth, it is reasonable that cells would also provide multiple means by which the two genes can be expressed appropriately in response to a number of environmental stresses. However, only one promoter, P6, which is located within the coding sequence of dnaG and is not well characterized, appears to be activated under heat shock (Briat et al., 1985; Wang et al., 1985). No other operon promoter was found to control the expression of the MMS operon under any other stresses.
B. subtilis DB1005 is a temperature-sensitive (Ts) sigA mutant containing double amino acid substitutions, I198A and I202A, on the hydrophobic face of the promoter −10 binding helix of σA (Chang and Doi, 1993). The mutant σA is defective in transcription under heat stress and is very easily degraded even at permissive temperature (Chang et al., 1994; 1997). However, the temperature sensitivity of this mutant is not attributed to an insufficient concentration of σA, as it could not be suppressed by overexpression of the Ts σA at elevated temperature. To overexpress σA in B. subtilis, a plasmid (pCX2F or pCX2F5) (Fig. 1A and Table 1[link]) containing either the wild-type (wt) or the Ts sigA gene was introduced into B. subtilis DB2 (wt sigA strain) or DB1005 respectively. Both B. subtilis strains have the increased levels of σA at permissive temperature; however, only in the Ts sigA mutant was σA highly expressed at restrictive temperature. The compensation of the Ts σA factor was also observed in B. subtilis DB1005 with only a chromosomal sigA allele (Chang et al., 1997). Mechanisms for the induction or compensation of σA in the genetic background of B. subtilis DB1005 is intriguing.
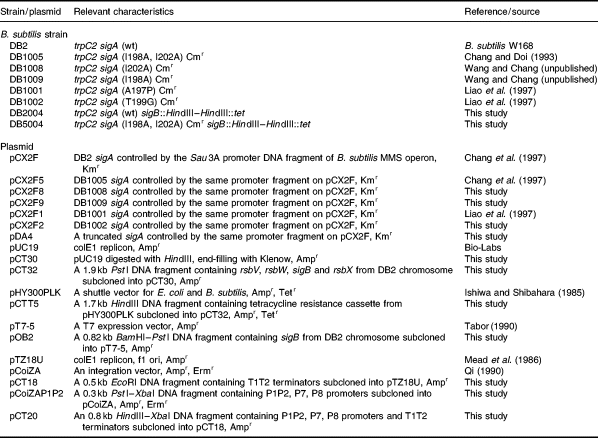
. Maps of plasmids. A. Map of pCX2Fx. The Sau3A DNA fragment upstream of the sigA gene contained the P1P2 promoters of the B. subtilis MMS operon. B. Map of pDA4. The pDA4 plasmid harboured a sigA gene with a base (C) deletion at nucleotide position 748 from the 5′ end of the coding sequence, resulting in a frameshifting event and the synthesis of a C-terminally truncated σA protein of 256 amino acids in length, as revealed from DNA sequencing data. C. Map of pCT20. The 0.5 kb DNA fragment containing the T1T2 terminators of the E. coli rrnB operon (Jurgen et al., 1981) was cut out from the pCoiZA plasmid (Qi, 1990; Qi et al., 1991) with EcoRI and subcloned into pTZ18 U to form pCT18. In parallel, the pCoiZA-P1P2 plasmid (Table 1) was cut with HindIII and XbaI. The resulting 0.8 kb HindIII–XbaI fragment containing the T1T2 terminators as well as the P1P2, P7 and putative P8 promoters of the B. subtilis MMS operon was isolated and subcloned into pCT18 to form pCT20. The pCT20 plasmid also bore an ampicillin resistance gene and an E. coli replication ori. The strong T1T2 terminators locating upstream and downstream of the complex promoters prevented transcription readthrough from promoters at other regions on the vector and allowed transcription from the complex promoters to be terminated at specific sites.
Our efforts to investigate the mechanisms leading to the induction of σA in B. subtilis DB1005 (pCX2F5) at elevated temperature have allowed us to identify a σB-type promoter preceding the three genes of the B. subtilis MMS operon. This promoter is activated in B. subtilis in response to heat, ethanol and salt stresses, or in response to the presence of σA factor defective in core binding, indicating that it plays some physiological roles in B. subtilis. Thus, the MMS operon of B. subtilis is subjected to the control of general stress.
Results
Induction of σA in the B. subtilis Ts sigA mutants harbouring pCX2Fx plasmids is caused by the loss of σA function at high temperature and is mainly from the plasmid-borne sigA
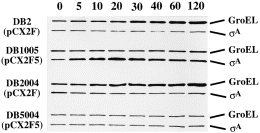
We have reported that there is an induction of σA in B. subtilis DB1005 (pCX2F5) but not in B. subtilis DB2 (pCX2F) under heat stress (Chang et al., 1997). As the chromosomal and the plasmid-borne sigA genes in both B. subtilis strains were controlled by the same transcriptional and translational signals, respectively, we suspected that the difference in σA induction between these two B. subtilis strains was attributed to their difference in σA activity at high temperature. To confirm this idea, the levels of σA and GroEL induction in various B. subtilis strains harbouring the corresponding pCX2Fx plasmids (Fig. 1A and Table 1) were examined by Western blot analyses. The level of GroEL induction was used as an indicator of σA activity, as it is transcriptionally controlled by σA (Chang et al., 1994; Yuan and Wong, 1995). As shown in Fig. 2, the levels of σA induction were high in the Ts sigA mutants such as B. subtilis DB1005 (pCX2F5), DB1001 (pCX2F1) and DB1009 (pCX2F9). However, no clear induction of σA was observed for B. subtilis DB1008 (pCX2F8), DB1002 (pCX2F2) and DB2 (pCX2F), which were relatively resistant to elevated temperature (data not shown). Moreover, no induction of GroEL was observed for the Ts sigA mutants (Fig. 2). These results suggest that the induction of σA is triggered by low σA activity. Presumably, a common regulatory network is adopted by B. subtilis, with which the effect of the defective σA on cells can be overcome at high temperature.
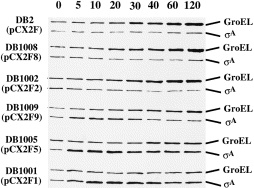
. Levels of σA and GroEL induction in B. subtilis strains harbouring the corresponding pCX2Fx plasmids under heat stress. Methods for sample preparation and Western blot analysis were the same as those described in Experimental procedures. The designated time points (min) for sampling are indicated at the top.
Where was the Ts sigA gene induced? Was it mainly from the chromosomal or the plasmid copy of sigA gene? To answer this question, the pDA4 plasmid (Fig. 1B and Table 1) that bears a truncated sigA gene controlled by the same transcriptional and translational signals as those on the pCX2Fx plasmids was introduced into B. subtilis DB2 and DB1005. The levels of intact and truncated σA thus expressed in the cells of B. subtilis DB2, DB1005, DB2 (pDA4) and DB1005 (pDA4) were then analysed before and after heat shock. Our data showed that the levels of intact σA expressed from the chromosomes of B. subtilis DB2, DB1005 and DB2 (pDA4) were fairly constant during the transition in temperature (Fig. 3). However, the level of truncated σA was induced exclusively in B. subtilis DB1005 (pDA4), indicating that the induction of σA at elevated temperature was mainly from the ‘plasmid-borne’sigA allele.
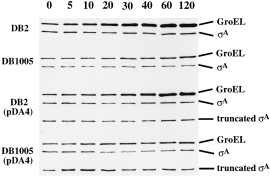
. Induction of intact and truncated σA factors in B. subtilis strains harbouring the pDA4 plasmid under heat stress. Methods for sample preparation and Western blot analysis were the same as those described in Experimental procedures. The designated time points (min) for sampling are indicated at the top.
σB and its cognate promoter are required for the induction of plasmid-borne sigA at elevated temperature
What are the elements responsible for the induction of plasmid-borne sigA? As the sigA genes on the pCX2Fx plasmids were controlled by the same P1P2 promoter-containing Sau3A DNA fragment (Figs 1A and 4A) (Wang and Doi, 1987) and the Ts σAs lost their activities at elevated temperature, we suspected that the induction of σA from pCX2Fx must be dependent on elements other than σA and its cognate P1P2 promoters, similar to that reported for the B. subtilis csh203::Tn917lac mutant in which the minor σ factor, σH, becomes essential for σA expression during vegetative growth (Hick and Grossman, 1995). To prove this idea, we examined the DNA sequence of the P1P2 promoter-containing Sau3A DNA fragment. A nucleotide sequence homologous to the consensus sequence of the B. subtilisσB-type promoters (Moran et al., 1982; Kalman et al., 1990; Boylan et al., 1991; Mueller et al., 1992; Varon et al., 1993; Antelmann et al., 1995; Engelmann et al., 1995; Maul et al., 1995) was found just upstream of the −35 hexamer of the P2 promoter (Fig. 4A); it matched exactly at least at the five positions most important for the activity of the well-characterized σB-dependent ctc promoter (Fig. 4B) (Tatti and Moran, 1984; Ray et al., 1985). This putative promoter was designated P7. We thought that the induction of σA was ascribed to transcription from this promoter by RNA polymerase holoenzyme containing σB. To confirm this idea, we constructed an integration vector, pCTT5, in which the internal HindIII fragment (90 bp long) of sigB was replaced with a tetracycline (tet ) resistance cassette. We then linearized the plasmid and integrated it into the chromosomes of B. subtilis DB2 and DB1005 to obtain the sigB-disrupted mutants (see Experimental procedures). The sigB mutants with the genetic backgrounds of B. subtilis DB2 and DB1005 were designated as B. subtilis DB2004 and DB5004 respectively. The effects of sigB disruption on σA induction were examined in B. subtilis DB2 (pCX2F), DB1005 (pCX2F5), DB2004 (pCX2F) and DB5004 (pCX2F5) under heat stress (Fig. 5). Again, heat induction of σA was observed in B. subtilis DB1005 (pCX2F5). However, the induction was almost completely abolished by sigB disruption, as seen in B. subtilis DB5004 (pCX2F5). The lack of σA induction in B. subtilis DB5004 (pCX2F5) indicates that the induction requires σB, with which P7 can probably be transcribed.
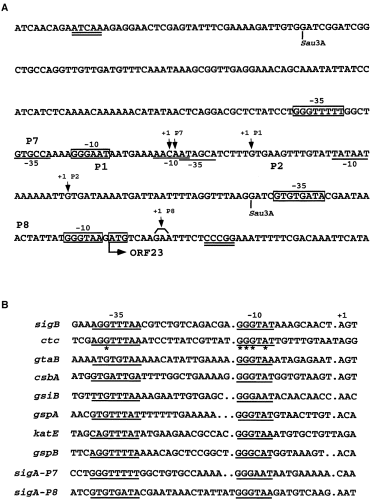
. A. Nucleotide sequence of the regulatory region of the B. subtilis MMS operon. The nucleotide sequence is given in the 5′ to 3′ direction. The −10 and −35 regions of the P1P2 promoters are underlined, while those of P7 and P8 are boxed. The transcription +1 sites of promoters are indicated by downward arrows. The translation initiation codon (ATG) for ORF23 is also boxed. The Sau3A DNA fragment encompassing P1P2 and P7 is the promoter fragment that controls the sigA gene on the pCX2Fx plasmid (see Fig. 1A). The DNA sequence encompassed by the two double-underlined bases is the promoter DNA fragment cloned in the pCT20 plasmid (see Fig. 1C). B. Sequence alignment of known σB-type promoters with P7 and P8. The −10 and −35 regions of promoters are underlined. Bases most important for the function of the ctc promoter are denoted by asterisks.
. Effect of sigB disruption on σA induction in B. subtilis. Methods for sample preparation and Western blot analysis were the same as those described in Experimental procedures. The designated time points (min) for sampling are indicated at the top.
Transcription of P7 by σB-RNA polymerase holoenzyme in vivo
To confirm that P7 was transcribed by σB-RNA polymerase holoenzyme under heat stress, we took advantage of the primer extension assay with which RNA transcripts generated from different initiation sites can be visualized in just a single experiment. In this assay, the total RNA samples isolated from the tested B. subtilis strains before and after heat shock were primed with the synthetic primer pE7B (see Experimental procedures). As shown in Fig. 6, the + 1 sites of RNA transcripts generated from the P2 promoters of all the tested B. subtilis strains lined up at the same position as that reported previously (Wang and Doi, 1987) at both 37°C and 49°C. However, two extra RNA transcripts with the + 1 sites located 39 and 40 nucleotides, respectively, upstream of the P2 initiation site were induced significantly in B. subtilis DB1005 (pCX2F5) at 49°C (lanes M and 5). The two + 1 sites corresponded to an A and a C residue preceded by the P7 promoter DNA sequence, 5′-GGGTTTTT-13 bp-GGGAAT-3′ (Fig. 4). Such induction was also detected in B. subtilis DB1005 (pCX2F5) at 37°C (lane 2) and B. subtilis DB2 (pCX2F) at 49°C (lane 4) after a prolonged exposure of the sequencing gel (data not shown), whereas no such induction was detectable in B. subtilis DB5004 (pCX2F5) (lanes 3 and 6) at both 37°C and 49°C even after a prolonged exposure. These results were consistent with the exclusive σA induction in B. subtilis DB1005 (pCX2F5) at elevated temperature, as shown in Fig. 5. They also demonstrated that there was a σB-type promoter upstream of P2 in the B. subtilis MMS operon. Unexpectedly, P2 induction was also observed in both B. subtilis DB1005 (pCX2F5) and DB5004 (pCX2F5) at 49°C (lanes 5 and 6), and it was more significant with the latter strain. The mechanism responsible for the induction is interesting and will be discussed later.
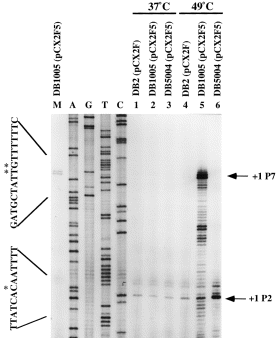
. Primer extension analyses of P7 induction in B. subtilis harbouring the pCX2Fx plasmid. Primer extension analysis was performed as described in Experimental procedures. Lanes 1 and 4, total RNA extracted from B. subtilis DB2 (pCX2F) at 37°C and 49°C respectively. Lanes 2 and 5, total RNA extracted from B. subtilis DB1005 (pCX2F5) at 37°C and 49°C respectively. Lanes 3 and 6, total RNA extracted from B. subtilis DB5004 (pCX2F5) at 37°C and 49°C respectively. Lane M, a diluted sample from lane 5. The sequencing ladders primed with the same primer are shown on the left-hand side. The letters A, G, C or T above each lane indicate the dideoxynucleotide used to terminate the sequencing reaction. The transcription initiation sites are indicated as + 1 and are denoted by asterisks in the DNA sequence. The two DNA sequences shown in the left margin are read directly from the gel and are the sequences of a non-coding strand. The RNA transcripts in lane 5 with sizes smaller than the P7 message were probably the result of either processing of the transcript initiating at P7 or premature termination of the primer extension reaction.
P7 can be transcribed in vitro by reconstituted RNA polymerase holoenzyme containing σB
To confirm the σ specificity of P7, in vitro transcription was performed with reconstituted σB-RNA polymerase holoenzyme. The presence of strong T1T2 terminators (Jurgen et al., 1981) upstream and downstream of P1P2 and P7 on the template plasmid pCT20 (Fig. 1C and Table 1) enabled us to detect the RNA transcripts generated from both P2 (≈ 348 nucleotides) and P7 (≈ 388 nucleotides) precisely. The data are shown in Fig. 7. As expected, the P2 transcript was observed when pCT20 was transcribed with reconstituted RNA polymerase holoenzyme containing wild-type σA at both 37°C and 49°C (lanes 1 and 6). Likewise, the P7 transcript was synthesized in the presence of σB (lanes 3 and 8). Together with the data from σA induction and primer extension assays (Figs 5 and 6), it was clear that P7 was absolutely a σB-type promoter in the B. subtilis MMS operon. Moreover, a higher amount of P2 transcript was obtained when σB was mixed with the wild-type σA (lanes 2 and 7) than with the Ts σA (lanes 4 and 9), suggesting that the Ts σA factor was less efficient than the wild-type counterpart in competition with σB for core binding. To our surprise, a very strong RNA band (278 nucleotides in length) was observed in all assays containing σB (lanes 2, 3, 4, 7, 8 and 9). It seemed that a second σB-type promoter was present in the MMS operon. As the RNA transcript produced from this putative promoter (designated as P8) was shorter than that generated from P2, we assumed that it would be located downstream of P2. As shown in Fig. 4, the putative P8 promoter has a very good −10 consensus sequence despite the possession of a non-conserved −35 sequence and a longer spacer region (15 bp). As no transcript was generated from this promoter in vivo (Fig. 8, lanes 1–8), we thought that it might not be of any physiological significance. However, the synthesis of P8 transcript in vitro without further addition of σB (Fig. 7, lanes 1, 5, 6, 10 and 11) suggests that our core enzyme is contaminated with σB during purification with the cationic exchanger Bio-Rex 70. A similar phenomenon was also reported for the purification of core RNA polymerase using phosphocellulose (Haldenwang and Losick, 1979).
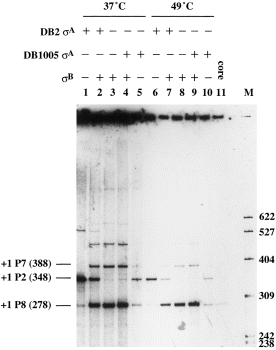
. In vitro transcription analyses of promoters. The pCT20 plasmid containing the complex promoters was used as template for in vitro transcription. Lanes 1 and 6, transcription reactions with reconstituted RNA polymerase holoenzyme containing the wild-type σA of B. subtilis DB2; lanes 2 and 7, transcription reactions with reconstituted RNA polymerase holoenzyme containing wild-type σA and σB; lanes 3 and 8, transcription reactions with reconstituted RNA polymerase holoenzyme containing σB; lanes 4 and 9, transcription reactions with reconstituted RNA polymerase holoenzyme containing the Ts σA of B. subtilis DB1005 and σB; lanes 5 and 10, transcription reactions with reconstituted RNA polymerase holoenzyme containing Ts σA; lane 11, transcription reaction with core enzyme only. Numbers in the left margin indicate the sizes (in bases) of RNA transcripts starting from the +1 sites of the indicated promoters. Numbers in the right margin indicate the sizes (in bases) of DNA markers generated from the MspI-digested pBR322.
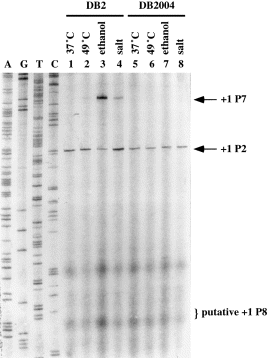
. Induction of P7 under general stresses. Primer extensions were performed as described in Experimental procedures except that 50 μg of total RNA and a primer (PCC25, 5′-CGCACATCCTTTATTATTGTGG-3′) complementary to the template strand of orf23 were used. Total RNA extracted from B. subtilis DB2 and DB2004 grown at 37°C (lanes 1 and 5 respectively), 10 min after the imposition of heat stress (49°C) (lanes 2 and 6 respectively), ethanol stress (lanes 3 and 7 respectively) and salt stress (lanes 4 and 8 respectively). The DNA sequencing ladders obtained with the same primer are shown on the left-hand side. The letters A, G, C or T above each lane indicate the dideoxynucleotide used to terminate the sequencing reaction. The transcription start sites are indicated as +1. The +1 site of the putative P8 promoter is also indicated.
Induction of P7 after the imposition of heat, ethanol and salt stresses
Is the induction of P7 restricted to the Ts sigA mutants under heat stress? Are there other stresses that could also induce the expression of P7 in B. subtilis? To answer these questions, total RNA was extracted from the cultures of B. subtilis DB2 and DB2004 after the imposition of heat, ethanol or salt stress. RNA transcript generated from P7 was then examined using primer extension assays (Fig. 8). Our data showed that P2 was constitutively expressed in B. subtilis DB2 and DB2004 (lanes 1–8); however, no P1 transcript was observable, probably because of its fairly low expression in B. subtilis (Qi et al., 1991). P7 was not expressed until the imposition of heat (lane 2), ethanol (lane 3) and salt (lane 4) stresses, with the least induction observed for heat shock. Minor induction of P7 was also observed in B. subtilis under oxidative stress (data not shown). The induction of P7 was completely abolished when sigB was disrupted (lanes 6–8). Similar results were obtained with B. subtilis DB2 and DB2004 bearing the pCX2F plasmid (data not shown). Thus, it is certain that P7 is transcribed by σB-RNA polymerase holoenzyme and is responsive to stresses.
Discussion
In our method of investigating the mechanisms leading to the induction of σA in B. subtilis DB1005 (pCX2F5) under heat stress (Chang et al., 1997), we discovered for the first time a σB-type promoter (a stress-responsive promoter), in addition to the four known σA- and σH-type promoters and two incompletely defined promoters in the MMS operon of B. subtilis. This promoter is located upstream of the P2 promoter of the operon. Primer extension assays revealed that it is induced in B. subtilis in response to heat, ethanol and salt stresses. Therefore, the MMS operon of B. subtilis is subjected to the control of general stress.
The organization of the MMS operon of B. subtilis is similar to that of E. coli, except that the first gene (orf23) of the B. subtilis MMS operon has an unknown function (Burton et al., 1983; Wang and Doi, 1986). In E. coli, the MMS operon is transcribed from three major operon promoters, P1, P2 and P3, and three minor internal promoters, Pa, Pb and Phs, most of which are assumed to be controlled by RNA polymerase holoenzyme containing σ70 (Lupski et al., 1983; 1984; Taylor et al., 1984). P1, P2 and P3 are expressed during normal growth; Pa and Pb are constitutively but very weakly expressed, while Phs is expressed only under heat stress (Lupski et al., 1983; 1984). The regulation of the B. subtilis MMS operon is much more complicated than that of its E. coli counterpart. At least four operon promoters (P1, P2, P5 and P7) and three internal promoters (P3, P4 and P6) are involved. The P1P2, P3, P4 and P5 promoters are mainly used for cell development. P1P2 (σA type) are expressed during vegetative growth (Wang and Doi, 1987); P3 and P4 (σH type) are expressed in late to early sporulation phase (Carter et al., 1988), while P5 is not expressed until late in sporulation phase (Qi and Doi, 1990; Qi et al., 1991). P6 is not well defined but appears to be transcribed under heat shock (Briat et al., 1985; Wang et al., 1985). The addition of the newly discovered σB-type promoter, P7, to the already known σA- and σH-type promoters of the B. subtilis MMS operon (Fig. 9) indicates that this operon is much more delicately regulated than is the E. coli counterpart. Presumably, B. subtilis, a soil-borne microorganism, needs to face a more complicated environment besides the inherent cellular development event; however, E. coli, an enteric bacterium, lives in a more constant habitat.
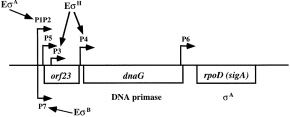
. Overview of the MMS operon of B. subtilis. The MMS operon of B. subtilis contains three genes, orf23, dnaG and rpoD (sigA), encoding a non-essential protein with unknown function, DNA primase and σA factor respectively. Promoters (P) are indicated with arrows above the map along with the forms of RNA polymerase that recognize the promoters. The major vegetative promoters, P1P2, are located upstream of the three genes and are transcribed by RNA polymerase holoenzyme containing σA. P3 and P4 are regulated by σH and are induced during the first 2 h of sporulation. P5 is located just downstream of P1P2 and is induced during sporulation. It is not known which sigma factor controls the expression of P5. P6 is located at the 3′ end of dnaG, and it is not known which sigma factor transcribes this promoter. P7 is induced under general stress and is transcribed by RNA polymerase holoenzyme containing σB.
The discovery of P7 is an accidental event, as heat induction of this promoter is almost undetectable in the wild-type sigA strain, B. subtilis DB2. The induction of P7 in B. subtilis DB1005 (pCX2F5) under heat stress (Fig. 6) can be explained simply by a competition model in which σB and σA compete for binding to core RNA polymerase. Thus, changing the availability of functional σA may affect the efficiency of σB to associate with core and thus the activity of σB-RNA polymerase holoenzyme in the cells. The formation of a multimeric structure of the Ts σA factor at permissive temperature and the aggregation proneness of this factor at elevated temperature in vitro (Y.-D. Wen et al., unpublished) raise the possibility that σB is able to compete with the Ts σA for core binding especially under stresses when σB is activated (Voelker et al., 1994). The low core-binding activity of the Ts σA factor at elevated temperature supports this possibility (Feng, 1997). Thus, the presence of P7 in the B. subtilis MMS operon may provide a means for the expression of this operon when σA becomes defective in core binding. Our observation of a twofold higher expression of lacZ (which was integrated into the chromosome of B. subtilis and was under the control of a DNA fragment containing P7) in B. subtilis DB1005 than in DB2 at 37°C and a higher rate of σA synthesis in DB1005 at 49°C (data not shown) supports this idea. The competition model was also reported for σA and σH, in which a relatively small change in the level of σA would cause a large change in the expression of genes controlled by σH (Hick and Grossman, 1996).
Another important observation in the study is the induction of P2 in B. subtilis DB1005 (pCX2F5) and DB5004 (pCX2F5) under heat stress (Fig. 6). The induction is unexpected as P2 is σA type, and the Ts σA factor is deficient for transcription activity after temperature elevation (Chang et al., 1997). Thus, there should be an unknown regulatory mechanism responsible for this phenomenon. One possible explanation is that there is an unidentified promoter that shares the same transcription initiation site with P2. As the regulatory network is triggered when σA becomes defective and augmented when σB is further knocked out (Fig. 6), we propose that the unidentified promoter must not be of the σA and σB types. Factors responsible for the induction certainly need further investigation. Consequently, the B. subtilis MMS operon is much more delicately regulated than we expected.
Experimental procedures
Bacterial strains, plasmids and culture conditions
The sources and properties of bacterial strains and plasmids used in the study are listed in Table 1. The method for the construction of B. subtilis DB1008 and DB1009 was the same as that reported previously (Chang and Doi, 1993). The overlapping primers for the construction of the two mutant sigA genes were as follows: B8 5′-TCGGCCGCGGCGCGTGTAATCGCC-3′; C8 5′-CACGCGCCGCGGCCGATCAGGCGAGAAC-3′; B9 5′-GGCGCGTGTGGCCGCCTGTCTGATCCAC-3′; C9 5′-GGCGGCCACACGCGCCATTGCCG-3′. Co-transformation and direct DNA sequencing were adopted to confirm the two sigA mutants. B. subtilis was grown in glucose minimal medium (GMM) at 37°C to a cell density of 5 × 107 ml−1 (A550 = 0.4) before the imposition of stress according to the following schemes. Heat shock: the culture was transferred from 37°C to 49°C. Ethanol stress: ethanol was added at a final concentration of 4% (v/v). Salt stress: sodium chloride was added at a final concentration of 4% (w/v). Oxidative stress: hydrogen peroxide was added at a final concentration of 0.005% (v/v). The bacterial culture was sampled before and 10 min after the imposition of stress.
Western blot analyses of σA and GroEL
The method for Western blot analyses of σA and GroEL was similar to that reported previously (Chang and Doi, 1990). In brief, B. subtilis strains were grown in GMM at 37°C to an A550 of 0.4 (referred to as time zero) and then shifted to 49°C. One millilitre of culture was harvested at each designated time point. After resuspending the cell pellet with 50 μl of 0.5 × SET buffer [(20% sucrose, 50 mM Tris-HCl, pH 7.6, 50 mM EDTA, 2 mM phenylmethylsulphonyl fluoride (PMSF)] and digestion of the cell wall with lysozyme, 50 μl of 2 × SAB (125 mM Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 0.002% bromophenol blue) was added to disrupt the cells. Five microlitres of the cell lysate was then used for Western blot analysis. Antibodies against σA and GroEL were prepared as mentioned previously (Chang and Doi, 1990; Chang et al., 1994).
Construction of B. subtilis strains with a disrupted sigB gene
To create the sigB-disrupted mutants, we first constructed the pCT30 plasmid by eliminating the HindIII site on pUC19. Then, a 1.9 kb DNA fragment containing rsbV, rsbW, sigB and rsbX was amplified from the chromosome of B. subtilis DB2 by polymerase chain reaction (PCR) using the two primers, PBO5 (5′-TGACTGCTGCAGAAGCTCATTGAGGAAC-3′) and PBO3 (5′-GTCATACTGCAGAGGATTCGTTAGCAAG-3′) (Kalman et al., 1990). After digestion with PstI, the DNA fragment was ligated with pCT30 to generate pCT32. In parallel, a 1.7 kb tetracycline resistance cassette was PCR amplified from the pHY300PLK plasmid (Ishiwa and Shibahara, 1985; 1986) using the primers PTC5 (5′-ACGTACAAGCTTGGGAACGGAAAAATTATTTTATTAA-3′) and PTC3 (3′-TCGGTGAAGCTTGAACTCTCTCCCAAAGTTGATC-3′). After digestion with HindIII, the DNA fragment was used to replace the 90 bp HindIII fragment located within the sigB gene on pCT32. The resulting plasmid, pCTT5, was linearized and integrated into the chromosomes of B. subtilis DB2 and DB1005 respectively. The sigB-disrupted mutants generated by a double cross-over recombination event were selected on tetracycline plates, verified by PCR, immunoprecipitation and σB activity.
Purification of total RNA
The procedure for the purification of total RNA was the same as that mentioned in the protocol of the High Pure RNA isolation kit from Boehringer Mannheim. The amount of RNA was quantified by determining its OD at 260 nm.
Primer extension assay
The primer used for the study was PE7B (5′-GAGTCGTATTAATTTCGCGGG-3′), which is complementary to the sequence of the T7 phage gene 10 promoter on pCX2F (Chang et al., 1997). Primer extension assay was performed using the following procedure: 5 μg of total RNA was mixed with 9.3 μl of DEPC-treated H2O and 1 μl (1 μg μl−1) of the primer PE7B. After denaturation of the sample at 65°C for 10 min, the mixture was cooled down slowly at 30°C for 1 h to allow the annealing of primer and RNA template. Then, 8.7 μl of reaction cocktail comprising 2 μl of dG labelling mixture (USB sequencing kit), 0.5 μl of RNasin (20 units; Promega), 0.2 μl of avian myeloblastosis virus reverse transcriptase (40 units; Bethesda Research Laboratories), 2 μl of [α-35S]-dATP (>1000 Ci mmol−1; Amersham) and 4 μl of RT buffer (250 mM Tris-HCl, pH 8.3, 375 mM KCl) was added to the annealed sample. The reaction mixture was incubated further at 42°C for 10 min before adding 1 ml of 10 mM dNTP to allow the extension of DNA chain. The reaction was stopped 2 h later by adding 20 μl of stop solution (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol FF). Five microlitres of the final mixture was denatured at 95°C for 3 min and electrophoresed on a 6% polyacrylamide gel containing 8 M urea.
Overproduction of σB
To overproduce σB, we amplified the sigB gene from the B. subtilis DB2 chromosome by PCR using the two linker primers, PSIGB5 (5′-ATCGAGGGATCCAAGGAGATATACATATGACACAACCATCAAAAACTACG-3′) and PSIGB3 (5′-GAGATCCTGCAGTCATTACATTAACTCCATCGAG-3′) (Binnie et al., 1986). The forward primer, PSIGB5, contained the ribosome binding site for the T7 phage gene 10 protein as well as a BamHI site in the 5′ end, while the reverse primer, PSIGB3, contained a PstI site. After digesting the sigB gene with BamHI and PstI, the DNA fragment was cloned into the compatible sites of the overexpression vector pT7-5 (Tabor, 1990) to form the pOB2 plasmid. Overexpression of σB in E. coli BL21(DE3) was performed as reported previously (Chang and Doi, 1990).
Purification of σB and preparation of anti-σB antibody
The cells collected from 1 l of overexpression culture were suspended in 40 ml of buffer L [10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 1 mM dithiothreitol (DTT), 1 mM PMSF, 200 mM NaCl, 10% glycerol, 130 μg ml−1 lysozyme] followed by incubation on ice for 30 min and disruption with a sonicator. The cell debris containing the overexpressed σB protein in the form of an inclusion body was collected by centrifugation at 8000 r.p.m. (4°C for 30 min) and then resuspended with 3 ml of buffer L. To purify σB for antibody preparation, a equal volume of 2 × SAB buffer was added to the protein sample before separation on SDS–polyacrylamide gels. The separated σB protein was cut out from the gel and collected with an eluter (Isco) using TAE buffer (40 mM Tris-acetate, pH 8.0, 1 mM EDTA). The σB protein thus purified had a purity over 95%. After being concentrated, the protein was mixed with adjuvant and injected into rabbit to raise antibody. For in vitro transcription assays, the purified σB protein was precipitated with four volumes of cold acetone (−20°C) by centrifugation at 10 000 r.p.m. for 20 min, and then washed twice with 1 ml of 80% acetone containing 0.15 M NaCl. This would allow the removal of SDS from the protein sample as much as possible. The washed protein pellet was dried, dissolved in 20 μl of 6 M guanidine-HCl and diluted stepwise with an equal volume of dilution buffer (50 mM Tris-HCl, pH 7.9, 0.1 mM EDTA, 1 mM DTT, 0.15 M NaCl, 5% glycerol) until the concentration of guanidine-HCl reached 0.06 M. The diluted protein sample was further incubated overnight at 4°C, concentrated with a Centricon-10 tube (Amicon) and finally dialysed against storage buffer (20 mM Tris-HCl, pH 7.9, 10 mM MgCl2, 1 mM EDTA, 0.1 mM DTT, 1 mM PMSF, 0.2 M KCl, 50% glycerol).
In vitro transcription
Core enzyme and σA were prepared as described previously (Fukuda and Doi, 1977; Halling et al., 1977; Davison et al., 1979; Chang and Doi, 1990). Procedures for the preparation of reconstituted RNA polymerase and in vitro transcription assay were modified from a previously published version (Chang and Doi, 1990). The RNA polymerase holoenzyme was obtained by mixing 15 μl of core enzyme (1 μg) with 10 μl of purified σ (1.5 μg) on ice for 10 min. Afterwards, 25 μl of the supercoiled pCT20 plasmid (1.5 μg) was added to the enzyme solution, and the mixture was further incubated on ice for 10 min. After this, the mixture was transferred to the designated temperature (37°C or 49°C), and 70 μl of reaction cocktail prewarmed at the designated temperature was added to start the transcription reaction. The final concentration of each component in the reaction mixture was 40 mM Tris-HCl, pH 7.9, 10 mM MgCl2, 150 mM KCl, 0.4 mM DTT, 5% glycerol, 0.2 mM each of UTP, CTP, GTP, ATP and 6 μCi [α-32P]-ATP. After incubation of the reaction mixture at the designated temperature for 1 h, the sample was immediately placed on ice. Four micrograms of E. coli tRNA was then added to the reaction mixture before extraction with phenol–chloroform. One hundred microlitres of RNA sample in the upper aqueous phase was pipetted into a tube, and the RNA transcripts were precipitated with 10 μl of 3 M sodium acetate and 250 μl of 100% ethanol at −70°C. The pelleted RNA was then dissolved in stop buffer (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol FF), heated at 95°C for 3 min and run on a denaturing polyacrylamide gel. Autoradiography was performed after the electrophoresis.
Acknowledgements
This research was supported by the National Science Council, Taiwan, Republic of China (NSC 88-2311-B-005-034).




