Heterotrimerization of PII-like signalling proteins: implications for PII-mediated signal transduction systems
Abstract
PII-like signalling molecules are trimeric proteins composed of 12–13 kDa polypeptides encoded by the glnB gene family. Heterologous expression of a cyanobacterial glnB gene in Escherichia coli leads to an inactivation of E. coli's own PII signalling system. In the present work, we show that this effect is caused by the formation of functionally inactive heterotrimers between the cyanobacterial glnB gene product and the E. coli PII paralogues GlnB and GlnK. This led to the discovery that GlnK and GlnB of E. coli also form heterotrimers with each other. The influence of the oligomerization partner on the function of the single subunit was studied using heterotrimerization with the Synechococcus PII protein. Uridylylation of GlnB and GlnK was less efficient but still possible within these heterotrimers. In contrast, the ability of GlnB-UMP to stimulate the adenylyl-removing activity of GlnE (glutamine synthetase adenylyltransferase/removase) was almost completely abolished, confirming that rapid deadenylylation of glutamine synthetase upon nitrogen stepdown requires functional homotrimeric GlnB protein. Remarkably, however, rapid adenylylation of glutamine synthetase upon exposing nitrogen-starved cells to ammonium was shown to occur in the absence of a functional GlnB/GlnK signalling system as efficiently as in its presence.
Introduction
The glnB gene family encodes one of the most highly conserved signalling molecules characterized so far, the PII protein. According to the rapidly expanding sequence information available, members of this family are found in all three domains of life and, as far as is known, they are all involved in nitrogen-related regulation (Merrick and Edwards, 1995; Hsieh et al., 1998). Moreover, genomic sequence information has revealed various bacterial and archaeal species containing multiple glnB-like sequences. Paralogues of PII proteins were first identified in Escherichia coli and Azospirillum brasiliense (van Heeswijk et al., 1996; de Zamaroczy et al., 1996). In addition to the well-characterized PII protein of Escherichia coli (hereafter termed GlnB for clarity) encoded by the glnB gene, the recently discovered paralogue GlnK is structurally very similar to GlnB, as revealed by crystallographic analysis of the two proteins (Jaggi et al., 1996; Xu et al., 1998). Both proteins are trimeric, and each subunit can be uridylylated at a tyrosyl residue (Tyr-51) (Son and Rhee, 1987) that is located at the tip of a solvent-exposed loop (T-loop) (Jaggi et al., 1996).
Signal transduction by GlnB has been studied for the last three decades (Shapiro, 1969; Brown et al., 1971; reviewed by Reitzer, 1996) and represents a paradigm for signal transduction cascades. Recently, a complete reconstitution of the GlnB signal transduction pathways with purified components was achieved (Jiang et al., 1998a, b, c). Depending on its uridylylation status, GlnB interacts with its receptors, NtrB and the adenylyltransferase/removase enzyme (ATase). NtrB is a sensor kinase that also transmits the GlnB signal to the response regulator NtrC; ATase regulates the activity of glutamine synthetase (GS) through adenylylation–deadenylylation. The PII-modifying activity, a bifunctional uridylyltransferase/uridylyl-removing (UTase/UR) enzyme, responds to the cellular glutamine pool, which reflects the cellular N-status. High glutamine levels favour the uridylyl-removing activity, whereas at low glutamine levels, corresponding to nitrogen-poor conditions, the uridylyl-transferase activity dominates. In addition to signalling the glutamine status of the cell via uridylylation, GlnB and probably GlnK synergistically bind the small molecule effectors ATP and 2-oxoglutarate, thereby integrating an additional signal to the system (Kamberov et al., 1995; Xu et al., 1998; Jiang et al., 1998a). High 2-oxoglutarate concentrations, corresponding to relevant physiological conditions, can prevent the productive interaction of GlnB with its signal receptors (Jiang et al., 1998c). The precise function of the recently discovered GlnK protein has still to be established, although it has been shown that GlnK is able to interact with the GlnB receptors (van Heeswijk et al., 1996; Atkinson and Ninfa, 1998). In contrast to GlnB, which is constitutively produced, synthesis of GlnK is highly induced under nitrogen-poor conditions (van Heeswijk et al., 1996; Atkinson and Ninfa, 1998). GlnK of the related genus Klebsiella pneumoniae was recently shown to be involved in the control of nitrogen fixation (Jack et al., 1999). Other PII-like proteins in proteobacteria have been shown to be modified by uridylylation, as in E. coli, and have been shown to be involved in different aspects of nitrogen regulation (de Zamaroczy, 1998).
A PII-like protein with a different type of covalent modification has been discovered in cyanobacteria (Forchhammer and Tandeau de Marsac, 1994). The Synechococcus PII protein (Sc PII) can be phosphorylated at a seryl residue (Ser-49) located just two amino acids N-terminal to the conserved tyrosyl residue 51 (Forchhammer and Tandeau de Marsac, 1995a). Like the E. coli homologues, the cyanobacterial PII protein is a trimer that binds ATP and 2-oxoglutarate in a mutually dependent manner (Forchhammer and Hedler, 1997). In contrast to E. coli, glutamine has no role in Sc PII modification, but the phosphorylation state of Sc PII depends primarily on the effector molecule 2-oxoglutarate (Irmler et al., 1997). The PII protein was shown to be involved in the regulation of nitrate utilization (Forchhammer and Tandeau de Marsac, 1995b), presumably by controlling the nitrate uptake system (Lee et al., 1998).
In a previous investigation, we found that expression of the Synechococcus glnB gene in E. coli cells resulted in a profound perturbation of the PII signalling system in E. coli (Forchhammer and Hedler, 1997). Cells synthesizing Sc PII were unable to repress GS production under conditions of nitrogen excess. Phenotypically, these strains resembled a glnB null mutant. As this effect depended on NtrB, we suggested that Sc PII either interacted directly with NtrB, thereby preventing regulation by the intrinsic E. coli PII paralogues, or interacted with its E. coli homologues, thereby inactivating them. The fact that the cyanobacterial PII protein interfered with the E. coli system indicated some fundamental conservation between these proteins from phylogenetically distinct organisms. Investigation of this process promised to provide new insights into PII-mediated signalling systems.
Results
Investigation of a possible interaction between Sc PII and NtrB
One possible explanation for the derepression of NtrC-dependent transcription caused by Sc PII is the following: Sc PII could bind to NtrB in such a manner that GlnB or GlnK are unable to induce NtrB-phosphatase activity. This possibility was tested by performing an NtrB-dependent phosphorylation/phosphatase assay using purified proteins. NtrB was incubated with the soluble receiver domain of NtrC (His6–NtrCR) together with radiolabelled [γ-32P]-ATP until a constant level of phosphorylated NtrCR was attained. Addition of purified GlnB led to the rapid dephosphorylation of phospho-NtrCR, whereas the addition of Sc PII had no effect on the level of phospho-NtrCR (Fig. 1A). When Sc PII was first added to the reaction mixture and GlnB was added subsequently, a rapid dephosphorylation of NtrCR was observed, as in the absence of Sc PII (Fig. 1B), indicating that Sc PII was not able to inhibit NtrB-phosphatase activity directly. However, when GlnB was preincubated with Sc PII for 20 min before addition to the phosphorylation reaction, dephosphorylation of NtrCR-P induced by GlnB was partially inhibited (Fig. 1C).
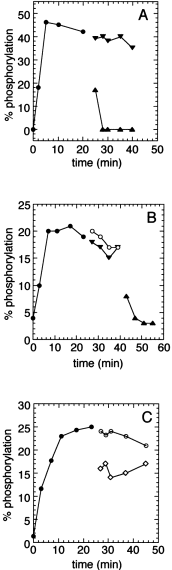
. Dephosphorylation of NtrC-P by Sc PII and GlnB. His6–NtrCR (1 μM) was phosphorylated in the presence of NtrB (100 nM) and [γ–32P]-ATP (0.5 mM) at 37°C. After 23 min (A and B) or 25 min (C), the reaction was split and 7 μl of the following samples was added to each 63 μl sample. A. Sc PII (10 μM) to a final concentration of 1 μM (▾), 3 μM GlnB to a final concentration of 300 nM (▴). B. Buffer containing 0.1 mg ml−1 BSA (○); (▾) to 126 μl of the reaction mixture 14 μl of 10 μM Sc PII was added (final concentration 1 μM) and, after incubation for another 20 min, 10 μl of 3 μM GlnB (to a final concentration of 300 nM) (▴). At the indicated time, 10 μl aliquots were removed, spotted onto glassfibre filters and analysed as described in Experimental procedures. C. Buffer containing 0.1 mg ml−1 BSA (○),10 μM Sc PII and 10 μM GlnB, incubated for 20 min at 37°C in buffer P containing 0.5 mM ATP before addition to the reaction mixture, to a final concentration of 1 μM each (◊).
Heterotrimerization of Sc PII with GlnB and GlnK from E. coli
To analyse whether Sc PII might interfere with the E. coli PII system by the formation of non-functional complexes, an immunological study was performed. Previously, we raised antibodies against the Sc PII protein that did not cross-react significantly with the homologous proteins from E. coli. To be able to detect the E. coli proteins, we now raised antibodies against a highly purified GlnK preparation (see Experimental procedures). The resulting antiserum was highly specific for both E. coli GlnK and GlnB but did not react with Sc PII. With these tools, we performed co-immunoprecipitation experiments with α-GlnK serum using extracts derived from the E. coli glnB glnK double mutant HS9060 carrying plasmids that led to the co-expression of Synechococcus glnB with either glnB or glnK from E. coli or, as controls, strains expressing only one of these genes. The immunoprecipitates were analysed by SDS–PAGE and immunoblotting with α-GlnK or α-Sc PII IgGs. If Sc PII formed heteromers with the E. coli homologues, co-immunoprecipitation in extracts from strains co-expressing the respective genes should be expected. Figure 2 shows that this was indeed the case. Whereas no Sc PII can be detected from the immunoprecipitate of the Synechococcus GlnB-overproducing strain, this protein immunoprecipitated with the GlnK/GlnB-specific serum when overproduced together with GlnB or GlnK. A reciprocal result was obtained when immunoprecipitation was performed with the α-Sc PII IgGs: GlnB and GlnK were immunoprecipitated only when they were co–expressed with Sc PII (data not shown).
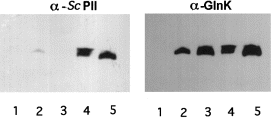
. Co-immunoprecipitation of Sc PII with GlnB and GlnK. Extracts from cells of strain HS9060 carrying either pMP1B (lane 1), pMP2 (lane 2), pMP3 (lane 3), pMP4 (lane 4) or pMP5 (lane 5) were subjected to immunoprecipitation using the α-GlnK/GlnB antiserum. The immunoprecipitates were then analysed by immunoblotting using the α-Sc PII and subsequently the α-GlnK/GlnB serum.
To prove that the different PII homologues form heterotrimeric structures, the migration of these proteins was analysed by immunoblot analysis after non-denaturing PAGE. This approach separates proteins of equal size according to charge differences and was used successfully to resolve different phosphorylated species of Sc PII (Forchhammer and Tandeau de Marsac, 1994), uridylylated forms of GlnB (Atkinson et al., 1994) and heterotrimers composed of wild-type and mutant GlnB polypeptides (Jiang et al., 1997). First, extracts were analysed from cells that were grown under nitrogen-excess conditions (no PII uridylylation) and that synthesized Sc PII together with GlnB. In addition to the unmodified homotrimeric forms, there appeared two novel species, which migrated between the slower migrating homotrimeric Sc PII and the faster migrating GlnB homotrimer (Fig. 3A). These intermediate forms reacted both with the α-Sc PII IgGs and with the α-GlnK serum, which indicated that these species were indeed heterotrimers. From their electrophoretic property, the slower migrating novel species corresponds to a heterotrimer containing two subunits of Sc PII and one subunit of GlnB, and the faster migrating species corresponds to a heterotrimer composed of one Sc PII subunit and two GlnB subunits. A similar observation was made by analysing the Sc PII and GlnK-overproducing strain (Fig. 3B). The heterotrimers appear as bands with an intermediate mobility between the two homotrimeric forms. A plasmid was constructed that leads to the production of all three homologues. In this case, all possible intermediate forms could be detected, including a putative Sc PII/GlnB/GlnK heterotrimer (Fig. 3C).
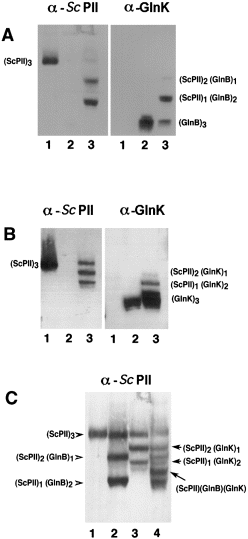
. Analysis of different PII heterotrimers by non-denaturing PAGE and immunoblotting. A. Visualization of heterotrimers formed between Sc PII and GlnB. Cell-free extracts (2.5 μg of protein each) from strain HS9060 carrying either pMP1B (1), pMP2 (2) or pMP4 (3) were separated by non-denaturing PAGE and analysed by immunoblotting using α-Sc PII IgGs and, after stripping off the antibodies, the blots were probed with the GlnK/GlnB-specific serum. B. As (A), except that cell-free extracts from strain HS9060 carrying either pMP1B (1), pMP3 (2) or pMP5 (3) were used for the analysis. C. Comparison of heterotrimers formed between Sc PII, GlnB and GlnK. Cell-free extracts from strain HS9060 carrying either pMP1B (1), pMP4 (2), pMP5 (3) or pMP6 (4) were analysed as described in (A), and the immunoblot was probed with the Sc PII-specific IgGs.
Uridylylation of heterotrimers
In vivo modification of the different heterotrimeric molecules was analysed by non-denaturing gel electrophoresis of extracts from cells producing the molecular species of interest, followed by immunodetection of the different forms using a mixture of α-Sc PII and α-GlnK IgGs to visualize all possible molecular species concomitantly. 4Figure 4A shows an experiment in which modifications of GlnB/Sc PII heterotrimers were analysed. Extracts containing unmodified homotrimeric GlnB (lane 1), partially uridylylated GlnB (showing all possible modified species, lane 2) and the same extract after phosphodiesterase treatment (lane 3) were used as controls. Phosphodiesterase partially removed the faster migrating forms, which corresponded to trimeric GlnB containing one, two or three uridylylated subunits (Atkinson et al., 1994). Modification of GlnB/Sc PII heterotrimers was analysed in HS9060 cells carrying plasmid pMP4 (Synechococcus glnB and E. coli glnB). In nitrogen-rich medium, the GlnB/Sc PII heterotrimers were unmodified (lane 4), but 2 h after transfer to nitrogen-poor conditions (lane 5) and even more pronounced 16 h after the transfer (lane 6), faster migrating forms appeared that were as sensitive to phosphodiesterase as uridylylated GlnB (lane 7). Note that the heterotrimeric species were apparently modified more slowly than homotrimeric GlnB. A similar experiment was conducted to reveal modification of GlnK/Sc PII heterotrimers (Fig. 4B). The control (lanes 1–3) shows modification and phosphodiesterase sensitivity of homotrimeric GlnK. Modification of GlnK/Sc PII heterotrimers was analysed using cells of HS9060 carrying pMP5 (Synechococcus glnB and E. coli glnK ). Modification of these heterotrimers was already visible after 1 h in nitrogen-poor medium (lane 5) and was more pronounced after 2 h (lane 6). Note that the putative twofold uridylylated species of the (Sc PII)2(GlnK)1 heterotrimer migrates at nearly the same position as unmodified GlnK (lane 6) but can be removed by phosphodiesterase treatment (lane 7). The same type of experiment was carried out in the UTase-deficient strain YMC26. There, the faster migrating bands did not appear during nitrogen starvation, further confirming that these bands correspond to uridylylated forms (data not shown).
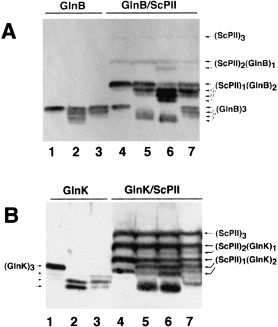
. Modification of heterotrimers of Sc PII with GlnB (A) or GlnK (B). A. Extracts from the following cells were analysed by non-denaturing PAGE and immunoblotting. Lanes 1–3, controls showing uridylylation of GlnB and phosphodiesterase sensitivity of the modified species: (1) YMC26 pMP2 grown in nitrogen-excess LBGln medium producing unmodified GlnB; (2) HS9060 pMP2 grown under semi-nitrogen-poor conditions (GGln medium); (3) extract from lane 2 after 20 min incubation in the presence of 0.5 μg of snake venom phosphodiesterase (SVD) under conditions described previously (Forchhammer and Tandeau de Marsac, 1994). Lanes 4–7: HS9060 pMP4 cells producing GlnB/Sc PII heterotrimers grown in (4) nitrogen-excess LBGln medium; (5) 2 h after transfer to nitrogen-deficient G-N medium; (6) grown in GArg medium for 16 h; (7) extract from lane 6 treated with SVD as described for lane 3. B. Extracts from the following cells were analysed as described above. Lanes 1–3, controls showing uridylylation of GlnK and phosphodiesterase sensitivity of the modified species: (1) YMC26 pMP3 grown in LBGln medium producing unmodified GlnK; (2) HS9060 pMP3 grown in GGln medium; (3) extract from lane 2 after 20 min treatment with SVD as described in (A). Lanes 4–7: HS9060 pMP5 cells producing GlnK/Sc PII heterotrimers grown in (4) nitrogen-excess LBGln medium; (5) 1 h after transfer to nG-N medium or (6) 2 h after transfer to G-N medium; (7) extract from lane 6 treated with SVD as described above.
Uridylylation of Sc PII in E. coli
In a previous study, we have shown that Sc PII, which was expressed in E. coli strain RB9060 (ΔglnB), could be uridylylated in a glnD-dependent manner under nitrogen-deficient conditions (Forchhammer and Hedler, 1997). The results shown above suggested that Sc PII might have formed heterotrimers with GlnK, which were uridylylated on the GlnK subunit. Using the analytical method employed above, we could show that, indeed, heterotrimers formed between the chromosomally encoded GlnK and the plasmid-borne Sc PII when cells were grown under nitrogen-deficient or nitrogen-poor conditions (Fig. 5A). This result emphasizes the dependence of glnK expression on the nitrogen status reported previously (van Heeswijk et al., 1996; Atkinson and Ninfa, 1998). To clarify whether homotrimeric Sc PII can be uridylylated in E. coli, the GlnB/GlnK-deficient strain HS9060 carrying pMP1 was incubated in nitrogen-depleted medium or in nitrogen-poor GArg medium. Only after 3 h incubation in the nitrogen-free medium did a faster migrating species of Sc PII appear (Fig. 5B, lane 2). This species disappeared after phosphodiesterase treatment (lane 5), which was in accordance with being a uridylylated form of Sc PII. As a final proof that the Sc PII protein was modified by uridylylation, an in situ [α-32P]-UTP labelling experiment was performed. HS9060 cells carrying plasmids pMP2, pMP3 or pBluescript were labelled as controls. Cell lysates were separated by SDS–PAGE and blotted onto nitrocellulose. The blot was first autoradiographed (Fig. 5C, top), and then the position of GlnB, GlnK and Sc PII was determined immunologically using a mixture of α-Sc PII and α-GlnK IgGs (Fig. 5C, bottom). As shown in 5Fig. 5C, a weak label appeared at the position of Sc PII, whereas the control reactions with GlnB or GlnK were much more strongly labelled, supporting the conclusion that Sc PII is uridylylated, but with a very low efficiency.
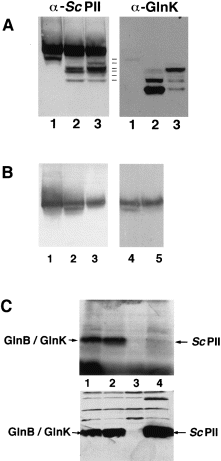
. Uridylylation of Sc PII in Escherichia coli. A. Cells of the glnB mutant RB9060 carrying pMP1B were grown in LB medium (1) and then transferred to nitrogen-depleted G-N medium (2) or nitrogen-poor GArg medium (3) and incubated for 3 h each. Subsequently, extracts were prepared and analysed by non-denaturing PAGE and immunoblotting. First, the blot was probed with the α-Sc PII IgGs. Then, the antibodies were stripped off, and the blot was reprobed with α-GlnK antibodies. Heterotrimers are revealed as species reacting with both antisera. The multiplicity of bands results from uridylylation. B. Cells of strain HS9060 carrying pMP1B were grown under the following conditions: (1) nitrogen-excess LBGln medium; (2) transferred to G-N medium for 3 h or (3) to nitrogen-poor GArg medium and then analysed for Sc PII modification by non-denaturing PAGE and immunoblotting. The extract from cells incubated in nitrogen-depleted medium (lane 2) was either treated with 2 μg of SVD for 1 h (5) or incubated in SVD buffer (4) and analysed as above. C. Radiolabelling of GlnB, GlnK and Sc PII with [α–32P]-UTP. The labelling experiment was performed as described in Experimental procedures. The labelled proteins were separated by SDS–PAGE, blotted onto nitrocellulose and visualized by autoradiography (top). Lanes: HS9060 strain carrying pMP2 (1); pMP3 (2); pBluescript (3) or pMP1B (4). The position of Sc PII, GlnK and GlnB was checked immunologically using a mixture of α-Sc PII and α-GlnK antibodies (bottom). The immunoblot was heavily overexposed to detect cross-reactivities with other cellular proteins, which shows that the lanes were loaded with equal amounts of protein.
Heterotrimerization of E. coli GlnB with GlnK
The results obtained in this study suggested that GlnB and GlnK could form heterotrimeric species in E. coli. To examine this prediction, a plasmid (pBK1) carrying both the glnK and the glnB gene was introduced in the UTase-deficient strain YMC26. This strain was chosen to avoid uridylylation, which could interfere with the interpretation of multiple bands in non-denaturing gels. As controls, YMC26 cells carrying pMP2 or pMP3 were used. Extracts of these cells were analysed by immunoblot analysis of non-denaturing gels (Fig. 6). Co-expression of glnK and glnB indeed resulted in the appearance of bands of intermediate mobility between homotrimeric GlnK and GlnB (lane 2). The mobility of these species was clearly different from the uridylylated GlnK species that were loaded in lane 4. Interestingly, YMC26 cells expressing only glnK from the plasmid (lane 3) exhibit a GlnK/GlnB cross-reactive material migrating like a putative (GlnB)1(GlnK)2 heterotrimer in addition to the dominant homotrimeric GlnK species. This species could be produced by heterotrimerization of GlnK with the chromosomally encoded, constitutively synthesized GlnB (Liu and Magasanik, 1993).
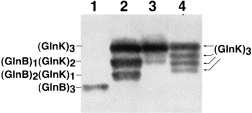
. Formation of heterotrimers between GlnB and GlnK. Extracts were analysed by non-denaturing PAGE and immunoblotting using the α-GlnK antibodies from cells of the UTase- deficient YMC26 strain grown in LB and carrying either (1) pMP2; (2) pBK1 or (3) pMP3. To compare the resulting GlnB/GlnK cross-reactive species with the different uridylylated forms of GlnK, an extract was loaded from HS9060 pMP3 cells that were grown in GGln medium and exposed to 2 mM ammonium chloride for 5 min to deuridylylate the modified GlnK species partially and to reveal unmodified and all modified GlnK species (lane 4).
Effect of the PII heterotrimers on glutamine synthetase adenylylation/deadenylylation
Heterotrimerization of chromosomally encoded GlnB or GlnK with overproduced Sc PIIin vivo offers a new approach to investigate the regulation of ATase by PII, in particular the role of trimerization. Under these conditions, the E. coli paralogues will be distributed preferentially into heterotrimers with Sc PII. In a first series of experiments, we studied the deadenylylation of GS upon shifting cells from a nitrogen-excess medium (LBGln) to nitrogen-depleted (G-N) conditions. Cells that were grown in nitrogen-excess medium until mid-exponential phase (OD600 = 0.4) were rapidly transferred to the N-depleted medium by filtration, and aliquots were removed at different times and assayed for GS adenylylation. The strains carrying the vector control showed, as expected, that GS deadenylylation depended on GlnB. As shown in Fig. 7, wild-type cells deadenylylated GS almost to completion within the first 5 min. In contrast, the glnB mutant RB9060 exhibited only a very slow deadenylylation of GS, and the double mutant HS9060 was apparently unable to activate GS by deadenylylation. Sc PII could not complement the deadenylylation deficiency of this strain. On the contrary, expression of Synechococcus glnB slowed deadenylylation further in RB9060. The most pronounced effect of Synechococcus glnB expression was observed in the wild-type strain: GS deadenylylation was as slow as in the glnB-deficient strain. This suggested that GlnB-UMP, which was trapped in heterotrimers with Sc PII, was unable to stimulate the adenylyl-removing activity of ATase.
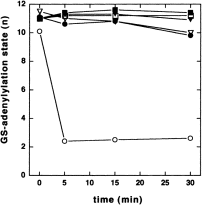
. Deadenylylation of GS-AMP upon transferring cells from nitrogen-excess conditions (LBGln) to nitrogen-depleted G-N medium. Nitrogen stepdown was performed as described in Experimental procedures. Open circles, YMC10 pBluescript (wild type); filled circles, YMC10 pMP1B; open triangles, RB9060 pBluescript; filled triangles, RB9060 pMP1B; open squares, HS9060 pBluescript; filled squares, HS9060 pMP1B.
By analogy with the above study, the effect of PII heterotrimerization on GS adenylylation was investigated. As some of the strains used do not grow in the nitrogen-poor GArg medium, cells were first grown in LB medium and then transferred to nitrogen-free medium and incubated under these non-growing conditions. During prolonged nitrogen starvation, the adenylylation state of GS gradually decreased, even in those strains that were shown above to be unable to deadenylylate GS rapidly. After 3 h under nitrogen-depleted conditions, the extent of GS deadenylylation allowed a subsequent analysis of GS adenylylation. Adenylylation of GS was triggered by the addition of 5 mM ammonium chloride to the cultures and, at different times, the adenylylation state of GS was examined. In the wild type, GS was almost completely adenylylated within a few minutes after ammonium shock (Fig. 8A). Surprisingly, expression of Synechococcus glnB did not alter this response. Similarly, the glnB deletion strain responded like the wild type, regardless of whether Synechococcus glnB was expressed or not. This was a first indication that this process proceeded independently of a PII system. To test this possibility further, the experiment was performed with the glnB glnK double mutant (Fig. 8B). In this strain, the initial GS adenylylation was significantly higher than in the other strains, probably because of the requirement of PII for GS deadenylylation. Consequently, the amplitude of the increase in GS adenylylation was lower than in the other strains. Nevertheless, the response to ammonium was unambiguous: adenylylation of GS increased to maximal levels within the same time period as in the other strains. As a further confirmation that the observed rapid adenylylation of GS is independent of the PII system, the ammonium shock experiment was carried out with a glnD mutant strain that lacks the PII-modifying UTase/UR enzyme. Upon addition of ammonium, GS adenylylation increased as rapidly as in the wild type (Fig. 8C). Again, expression of Synechococcus glnB had no effect on this response.
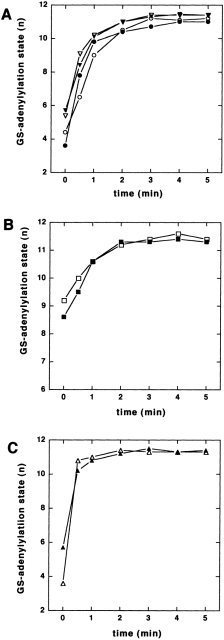
. Adenylylation of GS upon exposing nitrogen-starved cells to 5 mM NH4Cl. Cells grown in LB medium to mid-exponential phase (OD600 of 0.4) were transferred to nitrogen-depleted G-N medium and incubated as prior to the shift for 3 h. Then, the cultures were supplemented with 5 mM NH4Cl and, after different times, aliquots were removed, and the adenylylation state of GS was determined. A. Open circles, YMC10 pBluescript; filled circles, YMC10 pMP1B; open triangles, RB9060 pBluescript; filled triangles, RB9060 pMP1B. B. Open squares, HS9060 pBluescript; filled squares, HS9060 pMP1B. C. Open triangles, YMC26 pBluescript; filled triangles, YMC26 pMP1B.
Discussion
A surprising result of this investigation was the finding that the rapid adenylylation of GS upon exposing nitrogen-starved cells to ammonium did not require the GlnB/GlnK signalling system, which seems to disagree with the established model of GS regulation (Reitzer, 1996). Previously, it has been recognized that regulated adenylylation of GS was not completely impaired in a glnB-deficient mutant (Bueno et al., 1985; van Heeswijk et al., 1996); however, the remaining regulation was attributed to the recently discovered GlnB paralogue GlnK (Atkinson and Ninfa, 1998). Furthermore, it was known that the bifunctional ATase has a bias to adenylylate GS in the absence of PII (Rhee et al., 1985; Jiang et al., 1998a); this intrinsic activity is responsible for the constitutively high degree of GS adenylylation in the glnB glnK double mutant, but it was not considered to be of physiological relevance with respect to rapid adaptation to ammonium shock. ATase was recently characterized in vitro (Jaggi et al., 1997) and was shown to be composed of two homologous domains, one of which catalyses the deadenylylation reaction and the other the adenylylation of GS. The isolated deadenylylation domain was shown to be regulated by the uridylylation status of PII, whereas the isolated adenylylation domain was only regulated by glutamine but not by PII. However, adenylylation of GS by the entire ATase required PII, which was taken as evidence that the activity of the adenylylation domain is controlled by the binding of PII to the deadenylylation domain. The in vitro study by Jiang et al. (1998c) concluded that GS adenylylation can be triggered by glutamine alone but is 15-fold accelerated in the presence of PII and low concentrations of 2-oxoglutarate. We identify here for the first time a physiological condition under which the GlnB/GlnK signalling system is not required for the regulated adenylylation of GS, as shown by three lines of evidence. (i) The regulated GS adenylylation upon ammonium shock was not affected in a strain in which GlnB function was impaired through heterotrimerization with Sc PII. (ii) The time course of GS adenylylation was not altered in the glnB /glnK double mutant strain. (iii) GS adenylylation was unimpaired in a UTase/UR-deficient strain, in which PII cannot be uridylylated and, thus, cannot transmit the nitrogen signal to ATase. The fact that, in a glnD mutant strain, GS will be partially deadenylylated during prolonged nitrogen starvation has not been reported before but can be explained by the glutamine dependence of ATase activity (Rhee et al., 1978). As the internal glutamine pools drop during nitrogen starvation (Ikeda et al., 1996), ATase activity will decrease, and de novo synthesized GS escapes from adenylylation. After ammonium exposure of these cells, the deadenylylated GS could be adenylylated as fast as the wild type, although the nitrogen-sensing enzyme UTase/UR was absent. It is tempting to speculate that GS adenylylation was triggered directly by increased glutamine levels, as suggested by in vitro data (Jiang et al., 1998c). Depending on the particular growth conditions, there might be PII-dependent and -independent pathways leading to GS adenylylation. Further studies are required to elucidate the relevant physiological conditions under which this occurs.
The present study also reveals another novel aspect of PII-mediated signalling systems: the formation of heterotrimers between different PII-like proteins. Heterotrimerization between PII-like proteins from phylogenetically distinct organisms reveals that the structure of the oligomerization domain of these proteins is highly conserved. The high conservation of subunit interaction is probably an indication that it is of functional importance and may be involved in allosteric regulation, as discussed below. In a previous study, we have shown that the stoichiometry of metabolite binding increases with the metabolite concentration (Forchhammer and Hedler, 1997), suggesting antico-operative interactions between the subunits. A recent re-examination of the metabolite binding characteristics of GlnB reported strong anticooperativity between unmodified subunits of homotrimeric GlnB upon 2-oxoglutarate binding (Jiang et al., 1998a). The recently resolved crystal structure of E. coli GlnK with bound ATP revealed that the ATP binding sites were located in the clefts between adjacent subunits. The high conservation of quaternary structure between Sc PII and GlnB/GlnK could thus be related to the highly conserved metabolite binding characteristics of these proteins.
A previous approach using mutant GlnB heterotrimers aimed to characterize single subunit interactions and the role of the T-loop in GlnB function (Jiang et al., 1997). In that study, the wild-type GlnB polypeptides that were distributed in heterotrimers with mutant polypeptides (defective T-loop structures) were not impaired in their activity towards UTase/UR and the PII receptor proteins NtrB and ATase, suggesting that the PII receptors interact with single T-loop structures. In contrast, the activity of the GlnB or GlnK polypeptides towards the PII receptors NtrB and ATase (deadenylylating) was abolished in heterotrimers with Sc PII. Heterotrimer formation with the evolutionary distinct Synechococcus PII homologue could affect the T-loop structure of GlnB via allosteric interactions, thus preventing productive interaction with its receptors. If this were the case, the uridylylation of GlnB or GlnK subunits in heterotrimers with Sc PII would indicate that UTase is less specific towards subtle changes in the T-loop structure. Indeed, Jiang et al. (1998a) showed that UTase, in contrast to NtrB or ATase, does not discriminate between the GlnB trimer liganded to one, two or three 2-oxoglutarate molecules. A lower specificity of UTase towards its substrate is also plausible, as this enzyme can to some extent uridylylate the only distantly related cyanobacterial PII protein.
Evidence presented in this study show that GlnB and GlnK have the potential to form heterotrimers. If the formation of such heterotrimers occurs in the same way with the chromosomally encoded gene products, one has to conclude that the synthesis of homotrimeric GlnB is inversely correlated to the synthesis of GlnK, which is strongly induced under nitrogen-poor conditions. This conclusion is consistent with the recently reported dependence of GS deadenylylation on growth conditions. GlnK is much less efficient in stimulating GS deadenylylation than GlnB (van Heeswijk et al., 1996; Atkinson and Ninfa, 1998). Under growth conditions resulting in high-level expression of glnK (low N), the deadenylylation of GS in vivo is much slower than under conditions of glnK repression (N excess) (van Heeswijk et al., 1996). According to our interpretation, under low-nitrogen conditions, the highly active GlnB protein could be diluted into heterotrimers with the less efficient GlnK protein, resulting in GlnB/GlnK heterotrimers in which the GlnB subunit is less active, similar to the GlnB/Sc PII heterotrimers. Consequently, organisms with multiple PII paralogues could exhibit an additional level of regulation. Through differential expression of the genes encoding PII-like proteins resulting in the formation of different heterotrimers, the efficiency of the PII signalling system could be adjusted to various environmental conditions.
Transformation of E. coli cells with the Synechococcus glnB gene is an example of horizontal gene transfer, which results in novel regulatory capabilities of the recipient organism. By expressing the Synechococcus glnB gene, the cells are able to inactivate or modulate their PII signalling system selectively. Such a process might have been a driving force in the evolution of organisms with multiple PII proteins.
Experimental procedures
Bacterial strains and growth conditions
The bacterial strains and plasmids used in this work are listed in Table 1. The construction of HS9060 will be described in detail elsewhere. Briefly, in HS9060, glnK was deleted between the EcoRI site (located 22 bp upstream of the start codon for glnK ) and the ClaI site of glnK (bp 220), such that the σ54-dependent promoter for the glnKamtB operon was retained. The chloramphenicol resistance gene was inserted between basepairs 3354 and 3365 of mdl (Allikmetz et al., 1993). Nitrogen-excess medium (LBGln) was Luria–Bertani medium (Sambrook et al., 1989) supplemented with 0.2% (w/v) glutamine; nitrogen-poor GArg medium was a 0.4% (w/v) glucose-containing minimal medium supplemented with 0.2% arginine, and GGln medium contained 0.2% glutamine, instead, as described by Bueno et al. (1985). The nitrogen-depleted medium (G-N) was the same glucose-containing minimal medium but lacking a nitrogen source. For maintenance of plasmids, all media were supplemented with 100 μg ml−1 ampicillin. Cells were grown routinely at 37°C under aerobic conditions. Nitrogen stepdown experiments were performed by growing cells to an optical density (OD) at 600 nm of the culture of approximately 0.4. Cells from 20 ml of culture were harvested by filtering through a 0.45 μm polyvinylidene difluoride (PVDF) membrane filter (Millipore; type HVLP), washed with 20 ml of nitrogen-free G-N medium on the filter and finally resuspended in 20 ml of G-N medium, transferred back to sterile conical flasks and incubated under the previous conditions. The whole procedure was completed within 1 min and is thus more rapid than centrifugation and washing of cell sediments.
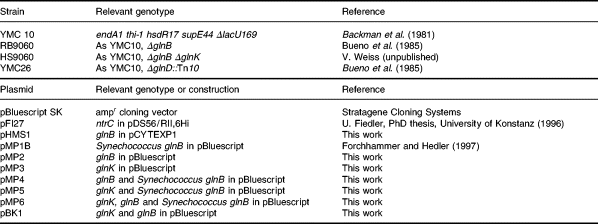
Construction of plasmids
For the construction of plasmids pMP2, pMP3, pMP4, pMP5 and pMP6, the glnB and glnK coding regions together with the respective ribosomal binding sites were amplified by polymerase chain reaction (PCR) from E. coli chromosomal DNA using the following oligonucleotides: glnB5′ (5′-GAGGATCC AGATCAAAAGACAGGCGAC-3′); glnB3′ (5′-GCTCTAGAT CCGGCAACCCTTGACGC-3′); glnK5′ (5′-CTGGATCCATT ACCGAATTCTGACCG-3′); glnK3′ (5′-GTGGATCCTGTTG CTGTGTGCCAG-3′).
The E. coli glnB–Synechococcus glnB-carrying plasmid pMP4 was constructed by restricting the 0.4 kb glnB amplification product with BamHI and XbaI and cloning it upstream of the Synechococcus glnB gene of pMP1B into the BamHI/XbaI restriction sites, such that both genes are arranged in the same orientation. Transcription is driven from an inducible lac promoter, which is located in front of the multiple cloning site of the vector pBluescript SK (Stratagene) on which pMP1B is based. Similarly, the glnK–Synechococcus glnB-carrying plasmid pMP5 was constructed by restricting the 0.4 kb glnK amplification product with BamHI and cloning into the BamHI site of pMP1B, upstream of the Synechococcus glnB gene. Constructs with the glnK gene in the same orientation as the Synechococcus glnB gene were identified by restriction analysis. Plasmid pMP2, carrying only the E. coli glnB sequence, was derived from pMP4 by deleting the Synechococcus glnB gene. pMP4 was restricted with StyI (cuts in the 5′ terminal end of the Synechococcus DNA insert) and EcoRV. The 1.3 kb fragment of cloned Synechococcus DNA was eliminated, and the remaining 3.3 kb fragment containing E. coli glnB and the pBluescript sequences were religated. pMP3 was constructed by cloning the BamHI-restricted 0.4 kb glnK amplification product directly into the BamHI restriction site of pBluescript and screening for constructs with the glnK gene in the same orientation of transcription as the lac promoter. Plasmid pMP6 was derived from pMP4 by ligating the 0.4 kb BamHI-restricted glnK fragment from pMP3 to BamHI-restricted pMP4 and screening for constructs in which glnK has the same orientation as glnB. Plasmid pBK1 was derived from pMP6 by deleting the original Synechococcus DNA insert containing the Synechococcus glnB gene by StyI/XhoI digestion and religation of the remaining plasmid DNA. All constructed plasmids were checked by DNA sequencing. Standard cloning techniques were performed according to Sambrook et al. (1989).
For the construction of pHMS1, glnB was amplified by PCR from plasmid pGS27 using the primers 5′-CGGAAGCTTCATATGAAAAAGATTGA TGCGATT-3′ and 5′-CGGAATTCG GGCAACCCTTGACGC-3′. The fragment was cut with NdeI and EcoRI and ligated to the large EcoRI/NdeI fragment of pCYTEXP1 (Belev et al., 1991).
pFI27 (U. Fiedler) carries ntrC (bp 1–369) cloned into the BamHI site of pDS56/RII,6Hi (B. Bukau). The encoded His6–NtrCR comprises amino acids 1–123 from NtrC fused to an N-terminal His-tag with the sequence MRGSHHHHH HGSTQ.
Purification of GlnK and generation of a GlnK-specific antiserum
For the purification of GlnK, E. coli cells RB9060 carrying pMP3 were grown in 2 l of Luria broth supplemented with 100 μg ml−1 ampicillin overnight at 37°C under aerobic conditions. All subsequent steps were performed at 0–5°C. Cells were harvested by centrifugation and washed once with 50 mM Tris-Cl, pH 7.4, containing 4 mM EDTA. The cell paste was resuspended with 20 ml of buffer A [50 mM Tris-Cl, pH 7.4, 50 mM KCl, 5 mM MgCl2, 2 mM EDTA, 2 mM benzamidine and 1 mM Pefablock (Boehringer Mannheim)], and the cells were broken by three consecutive passages through a French press cell (110 kPa in−1). Unbroken cells and debris were removed by centrifugation at 5000 × g and 20 000 × g respectively. The lysate was clarified by protamine sulphate precipitation (0.2% w/v) and was then fractionated by ammonium sulphate precipitation. The fraction between 30% and 50% ammonium sulphate saturation was collected and dissolved in 9 ml of buffer B [10 mM HEPES, pH 7,0, 50 mM NaCl, 0.4 mM EDTA, 1 mM MgCl2, 1 mM dithiothreitol (DTT)] and dialysed against the same buffer. After centrifugation at 10 000 × g for 10 min, the soluble material was passed through a 10 ml heparin–agarose column (Bio-Rad). The column was developed with a 200 ml 50–400 mM NaCl gradient in buffer B, and the fractions containing GlnK were detected by immunoblot analysis using antibodies raised against Azospirillum brasiliense GlnB-derived oligopeptides (de Zamaroczy et al., 1996). Fractions containing GlnK were pooled, and the proteins were precipitated by the addition of ammonium sulphate to 50% saturation. The precipitate was collected, dissolved in buffer C (10 mM Tris-Cl, pH 7.4, 50 mM NaCl, 0.4 mM EDTA. 1 mM MgCl2, 1 mM DTT) and passed through a Superdex 75 HR 16/60 FPLC gel filtration column (Pharmacia) equilibrated with buffer C. Fractions containing GlnK were detected as described above, pooled and further chromatographed on a 5 ml High Q anion exchange column (Bio-Rad) that was equilibrated with buffer C. The column was developed with a 100 ml gradient (50–400 mM NaCl). Fractions containing GlnK were pooled, and the material was finally purified by chromatography through a 5 ml High S cation-exchange column (Bio-Rad), which was equilibrated in buffer D (20 mM potassium phosphate, pH 7.4, 0.4 mM EDTA, 1 mM DTT). Elution was performed in the same buffer with a NaCl gradient of 0–500 mM. The final GlnK preparation was electrophoretically homogeneous according to a silver-stained SDS–PAGE analysis. The protein was dialysed against buffer D and concentrated by ultrafiltration. Approximately 0.4 mg of pure GlnK protein was sent to Eurogentec (Seraing) to raise a polyclonal serum in rats against GlnK.
Purification of GlnB, His6–NtrCR and NtrB
For the purification of GlnB, E. coli cells RB9060 carrying pHMS1 were grown at 28°C in LB medium supplemented with 0.2% (w/v) glutamine and 100 mg ml−1 ampicillin to an OD578 of 0.9. Overproduction of GlnB was induced by increasing the temperature rapidly to 42°C. After 3 h of incubation at 42°C, cells were harvested, resuspended in 50 mM Tris-Cl, pH 7.5, 1 mM phenylmethylsulphonyl fluoride (PMSF), disrupted in a French pressure cell and centrifuged at 16 000 × g. Streptomycin sulphate was added to the supernatant to a final concentration of 2% (w/v), followed by centrifugation at 12 000 × g for 30 min. Protein was precipitated from the resulting supernatant with ammonium sulphate at 50% saturation. After centrifugation, the precipitated protein was dissolved in 20 mM Tris-Cl, pH 7.5, containing 1 mM PMSF and applied to a heparin column (Heparin Sepharose CL6-B; Pharmacia) equilibrated in 50 mM Tris-Cl, pH 7.5, 50 mM NaCl and 1 mM EDTA. GlnB was eluted with a linear gradient from 50 mM NaCl to 600 mM NaCl. Pooled fractions were concentrated by precipitation with ammonium sulphate at 75% saturation and dialysed overnight against 30 mM Tris-Cl, pH 7.5, 70 mM KCl, 0.1 mM EDTA, 2 mM MgCl2, 1 mM DTT and 1 mM PMSF.
For purification of His6–NtrCR, FI8202 (Fiedler and Weiss, 1995) carrying pFI27 was grown at 37°C in LB medium supplementedwith 0.2% (w/v) glutamine and 100 mg ml−1 ampicillin. At an OD578 of 0.6, overexpression of His6–NtrCR was induced with 1 mM IPTG. After 2 h at 37°C, cells were harvested, washed with 50 mM NaH2PO4, pH 8.0, 20 mM imidazole, 300 mM NaCl, 5 mM MgCl2, 1 mM PMSF, disrupted in a French pressure cell and centrifuged at 4000 × g. The supernatant was applied to a Ni2+-NTA-agarose column (Qiagen). His6–NtrCR was eluted with a linear gradient from 20 to 250 mM imidazole. Pooled fractions were concentrated by precipitation with ammonium sulphate at 40% saturation. Purified His6–NtrCR was dialysed overnight against 50 mM Tris-Cl, pH 8.0, 300 mM NaCl and 5 mM MgCl2.
NtrB was purified as described previously (Fiedler and Weiss, 1995).
Protein concentrations were determined from their absorbances at 280 nm using A1% = 2.04 for GlnB, A1% = 2.1 for Sc PII, A1% = 3.79 for NtrB and A1% = 10.06 for His6–NtrCR, as calculated by the amino acid composition (Gill and von Hippel, 1989), and are expressed in the molarity of the trimer for GlnB and Sc PII, in the molarity of the dimer for NtrB and in the molarity of the monomer for His6–NtrCR.
Immunological methods
Immunoprecipitation experiments were carried out as described previously (Forchhammer and Tandeau de Marsac, 1994). Analysis of PII migration in non-denaturing gels was performed as described previously (Forchhammer and Tandeau de Marsac, 1994), except that the acrylamide concentration was 7.5% (w/v) instead of 10% (w/v). The proteins were blotted onto nitrocellulose (Optitran BA-S 83; Schleicher & Schuell), and the blots were probed with α-GlnK antibodies, which were subsequently visualized using an α-rat IgG peroxidase conjugate and a chemoluminescent substrate (Boehringer Mannheim). Subsequently, the bound antibodies were stripped from the membrane by incubating it in Tris-Cl, pH 6.8, 100 mM β-mercaptoethanol, 1% SDS (50 mM) for 20 min at 37°C. Then, the blot was washed twice in each case for 10 min in TBS and reprobed with the Sc PII-specific antibodies.
In situ labelling of cells with [α32P]-UTP
In situ labelling of cells with [α-32P]-UTP was performed according to the procedure of Colonna-Romano et al. (1993) with the exception that the labelling buffer contained 1 mM 2-oxoglutarate and 0.2 mM ATP. After SDS–PAGE separation of the labelled extracts, proteins were blotted on nitrocellulose, and the blot was exposed to an X-ray film, followed by immunodetection of GlnB/GlnK and Sc PII.
Glutamine synthetase (GS) assays
To follow precisely the rapid adenylylation/deadenylylation kinetics of GS, 2 ml samples were withdrawn from the shifted cultures into microcentrifugation tubes and chilled for 7 s in liquid nitrogen. Then, the cells were spun down, and the sediment was frozen in liquid nitrogen until the enzyme assay was conducted. GS activity and adenylylation state was determined principally as described by Pahel et al. (1982). The adenylylation state of GS was calculated from the quotient (Q) of GS (transferase) activity in the presence or absence of 67 mM MgCl2 in the assay according to the equation given by Rhee (1984): n (number of adenylylated subunits of GS) = 12–13.95 × Q.
Coupled NtrB/NtrC phosphorylation-phosphatase assay
The coupled NtrB/NtrC phosphorylation-phosphatase assay was performed according to Ninfa and Magasanik (1986). His6–NtrCR (1 μM) was phosphorylated at 37°C in the presence of NtrB (100 nM) and [γ-32P]-ATP (0.5 mM; specific activity 1 Ci mmol−1) in buffer P (40 mM Tris-Cl, pH 7.5, 50 mM NaCl, 1 mM EDTA, 10 mM MgCl2, 50 mM 2-oxoglutarate and 16% glycerol). After 25 min, the reaction was split, and samples containing Sc PII, GlnB or BSA were added to the reaction mixtures, as described in the figure legends. At the indicated time, aliquots were removed and spotted on glassfibre paper (Whatman), immediately immersed in ice-cold 10% TCA containing 1% sodium pyrophosphate, and washed for 4 h in 5% TCA containing 1% sodium pyrophosphate. The amount of phosphorylated His6–NtrCR was determined by liquid scintillation counting.
Footnotes
Acknowledgements
We would like to thank U. Fiedler for plasmid pFI27, and G. Sawers for critical reading of the manuscript. This work was carried out in the laboratories of A. Böck and W. Boos and was supported by grants from the Deutsche Forschungsgemeinschaft to K.F. and to V. Weiss and H. Bujard.




