Characterization of the SarA virulence gene regulator of Staphylococcus aureus
Abstract
Staphylococcus aureus is a potent human pathogen that expresses a large number of virulence factors in a temporally regulated fashion. Two pleiotropically acting regulatory loci were identified in previous mutational studies. The agr locus comprises two operons that express a quorum-sensing system from the P2 promoter and a regulatory RNA molecule from the P3 promoter. The sar locus encodes a DNA-binding protein that activates the expression of both agr operons. We have cloned the sarA gene, expressed SarA in Escherichia coli and purified the recombinant protein to apparent homogeneity. The purified protein was found to be dimeric in the presence and absence of DNA and to consist mostly of α-helices. DNase I footprinting of SarA on the putative regulatory region cis to the agr promoters revealed three high-affinity binding sites composed of two half-sites each. Quantitative electrophoretic mobility shift assays (EMSAs) were used to derive equilibrium binding constants (KD) for the interaction of SarA with these binding sites. An unusual ladder banding pattern was observed in EMSA with a large DNA fragment including all three binding sites. Our data indicate that SarA regulation of the agr operons involves binding to multiple half-sites and may involve other sites located downstream of the promoters.
Introduction
Staphylococcus aureus can cause a diverse array of diseases, ranging from relatively superficial infections of the skin to life-threatening osteomyelitis, endocarditis and toxic shock syndrome (reviewed by Projan and Novick, 1997). The potency of this pathogen can be attributed to the co-ordinated, temporally regulated expression of a wide array of virulence factors. Early in infection, the expression of surface proteins predominates, e.g. the collagen and fibronectin adhesins and protein A. The surface proteins allow the organism to attach to host tissues and evade the immune system. However, when the concentration of S. aureus cells at the site of infection becomes high, surface protein expression is reduced, and exoprotein expression increases. The temporal regulation of surface proteins and exoproteins can be recapitulated in laboratory culture growth models, in which early log phase growth represents an early infection and stationary phase represents late infection. Using this model system, two pleiotropically acting regulatory loci that govern the temporal expression of surface proteins and exoproteins have been identified: agr, for accessory gene regulator (Recsei et al., 1986; Morfeldt et al., 1988; Peng et al., 1988); and sar, for staphylococcal accessory gene regulator (Cheung et al., 1992; Cheung and Projan, 1994).
The agr operon encodes four protein products comprising a quorum-sensing apparatus that is homologous to many two-component signal transduction systems found in prokaryotic organisms (summarized recently by Ji et al., 1997). AgrD is exported from the cell by the membrane-bound AgrB protein. AgrD serves as a peptide pheromone and is specifically recognized by the AgrC membrane-bound receptor. Once the extracellular concentration of AgrD reaches a particular level, AgrC initiates a signal transduction pathway that is believed to include AgrA. By a mechanism that has yet to be revealed, cytoplasmic AgrA is thought to activate the expression of the agr operon (RNA II) and the divergently expressed RNA III. Mutations in any of the agr open reading frames (ORFs A, B, C and D) eliminate the upregulation of RNA II and RNA III expression (Novick et al., 1995). Additionally, agrA mutants have dramatically reduced virulence in animal models of staphylococcal arthritis, osteomyelitis, endocarditis and endophthalmitis (Abdelnour et al., 1993; Cheung et al., 1994a; Booth et al., 1995; Gillaspy et al., 1995).
Production of the three distinct transcripts arising from the sar operon are regulated temporally (Bayer et al., 1996; Blevins et al., 1999). However, all three transcripts include the SarA ORF. Like agrA mutations, transposon insertions in the SarA ORF also eliminate induction of RNA II and RNA III in late-phase growth and result in reduced staphylococcal virulence in animal models of disease (Cheung et al., 1994a,b; Booth et al., 1997). In seminal biochemical work in this area, SarA has been shown to be a DNA-binding protein that is capable of binding DNA fragments containing cis-regulatory elements for the promoters of both the agr operon (RNA II, P2 promoter) and the RNA III operon (P3 promoter) (Morfeldt et al., 1996). Heptad repeats have been identified upstream of both P2 and P3 promoters and have been proposed to be SarA binding sites (bold type in Fig. 1). A DNA fragment containing the RNA III gene and 93 bp upstream of the transcription start site, including the heptad repeats, was sufficient for regulated expression of RNA III (pEX085 in Fig. 1). Furthermore, removal of the distal half of the sequences upstream of the P3 promoter, including one heptad, eliminated appropriate expression of RNA III (pEX082 in Fig. 1). In addition, a synthetic DNA fragment including the repeats was bound by SarA in electrophoretic mobility shift assays (EMSAs) in vitro using S. aureus extracts and was used to purify SarA successfully from extracts by DNA-affinity chromatography (Morfeldt et al., 1996).
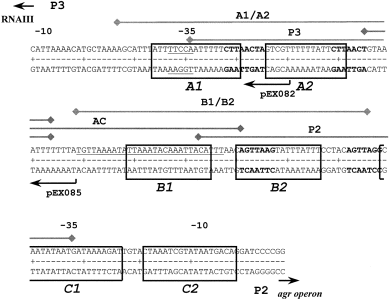
. Sequence of the P2–P3 promoter region, footprints from DNase I studies and DNA fragments used in this study. The diagram shows the DNA sequence of the P2 and P3 promoters and the intervening region. The protected areas from the DNase I footprinting experiments are indicated by the boxes and are labelled A1, A2, B1, B2, C1 and C2. The regions corresponding to nucleotide sequences used for quantitative EMSA are indicated by the lines above the sequence and are labelled A1/A2, B1/B2, P2, P3 and AC. The heptad repeats reported by Morfeldt et al. (1996) are shown in bold type. The DNase I footprint reported by Chien and Cheung (1998) is underlined. The upstream ends of the RNA III/P3 promoter constructs, pEX082 and pEX085, used by Morfeldt et al. (1996) for complementation studies are indicated below the sequence.
In a more recent report, SarA, expressed as a GST-fusion protein in E. coli and purified, was observed to have relatively low affinity for DNA fragments containing the heptad repeats (Chien and Cheung, 1998). Furthermore, DNase I footprinting revealed a primary binding site for SarA in the interpromoter region (underlined type in Fig. 1) that did not include the heptad repeats in the fragment cis to the P3 promoter shown to be sufficient for appropriate expression of RNA III by Morfeldt et al. (1996).
To clarify the interactions of SarA with the P2–P3 regulatory region and to begin to reveal the mechanism by which SarA regulates virulence gene expression in S. aureus, we have undertaken a biochemical characterization of SarA and its interaction with DNA. We expressed full-length SarA in E. coli without heterologous fusions and purified the protein to apparent homogeneity. We found SarA to be a dimer in the presence or absence of DNA and to be composed primarily of α-helices. The combined results of our DNase I footprinting and quantitative EMSA experiments indicate that three SarA binding sites exist. Two of the footprints overlap elements of the P2 and P3 promoters. All of the protected sequences included portions of the heptad repeats described by Morfeldt et al. (1996). One SarA dimer was found to bind each binding site with very high affinity. Three dimers bind the entire region and produce an unusual laddering pattern in EMSAs.
Results
Oligomeric state and secondary structure of SarA
SarA was expressed in E. coli and purified as described in Experimental procedures. Stoichiometric binding analysis with the P3 DNA fragment (described in Fig. 1) revealed that our SarA preparations are 90–95% active (data not shown). The concentration of dimeric (described below) active SarA was used in all subsequent experiments.
The stoichiometry of the predominant SarA complex was established by using two independent techniques: chemical cross-linking and dynamic light scattering (DLS). For the chemical cross-linking experiments, SarA was exposed to increasing concentrations of cross-linking agents (see Experimental procedures for details). The reactions were quenched, and the products were resolved by tricine-SDS–PAGE. In 2Fig. 2A a representative gel after detection of SarA by staining with Coomassie blue is shown. Increasing the concentration of the cross-linking agent increased the relative amount of a species that was the appropriate molecular weight to be a SarA dimer (calculated 29.4 kDa). The data in 2Fig. 2A were generated with DSS (disuccinimidyl suberate); however, other cross-linking agents (DSP and BS3) gave equivalent results. No other species were detected, even at the highest concentration of DSS when over 50% of SarA had been cross-linked. The same results were observed in the presence of saturating concentrations of P2–P3 promoter DNA and Mg2+, indicating that the species probably binding DNA is a dimer (data not shown). In addition, cross-linking for longer periods of time and under different conditions did not result in the detection of higher molecular weight species.
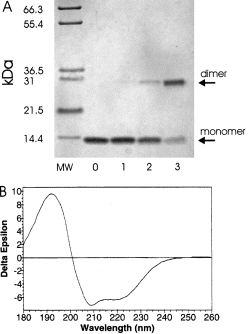
. A. Determination of the oligomeric state of SarA by chemical cross-linking. Purified SarA (10 μM) was treated with various concentrations of cross-linking agent DSS for 1 min and quenched. The products of the reactions were resolved by tricine-SDS–PAGE and detected by staining with Coomassie blue. MW, molecular weight standards; 0, no DSS; 1, 5 μM DSS; 2, 50 μM DSS; 3, 500 μM DSS. B. Analysis of SarA secondary structure with circular dichroism. The CD spectrum of SarA (1.8 mg ml−1) was determined in phosphate buffer from 180 nm to 260 nm. The secondary structure elements were calculated from this spectrum.
Dynamic light scattering (DLS) was also used to examine the oligomeric state of SarA. DLS reveals the homogeneity and oligomeric state of proteins based on the diffraction of visible light. The oligomerization state of SarA at two concentrations (3.3 and 11.7 mg ml−1) was analysed using a 2001 DynaPro DLS instrument and the attendant software dynamics. The bimodal analysis of the scattering revealed a monodisperse solution with a macromolecular species of molecular weight 34 kDa. This is consistent with scattering from a slightly elongated SarA dimer (calculated 29.4 kDa). Taking the cross-linking and DLS experiments together, the predominant form of SarA is a dimer. Therefore, SarA concentration is expressed in terms of dimer concentration for all quantitative analyses in this study.
To begin to examine the structural aspects of SarA, circular dichroism was used to determine the secondary structure elements. The circular dichroism spectrum was taken from 260 nm to 180 nm. The spectrum showed large negative ellipticity at 208 nm and 220 nm, indicative of a high helical content (Fig. 2B). Analysis of the spectrum from 260 nm to 190 nm revealed SarA to be largely alpha-helical (55%), with very little β-strand (6%) and modest amounts of turn (15%) and random coil (27%). Thus, it was concluded that the structure of SarA consists predominantly of alpha-helices.
DNase I footprint analysis of SarA binding sites in the P2–P3 region
The results of genetic experiments from Arvidson's group indicated that ≈60 bp upstream of the P3 promoter was sufficient for SarA-dependent expression of RNA III (Morfeldt et al., 1996). However, footprinting results from Cheung's group indicated a primary SarA binding site that did not overlap the putative regulatory region (Chien and Cheung, 1998). To begin to clarify the exact SarA binding site(s) and to resolve the discrepancy described above, we performed DNase I footprinting with purified SarA. The DNA target for footprinting studies, the entire interpromoter region, was polymerase chain reaction (PCR) amplified from a plasmid clone (pBKH50) using 32P-labelled −40 and reverse M13 primers. Representative results of the DNase I analysis in the presence of increasing concentrations of SarA are shown in 3Fig. 3A. The data were analysed digitally after phosphorimaging. Analysis of both strands revealed the same protected regions, and a summary of these results is presented in Fig. 1. Three sets of footprints were revealed by this analysis. Each set consisted of two footprints of ≈18 bp separated by 4–5 bp, which have an identical pattern of DNase I protection. The concomitant protection of the pairs of sites suggests that each set (A, B and C) consists of two half-sites. Sites B1 and B2 were fully protected at the lowest concentration of SarA, 6.4 nM, which is indicative of the highest affinity binding site. The C1/C2 and A1/A2 sites were fully protected at a slightly higher SarA concentration, 12.8 nM. Hypersensitive sites were observed routinely on the outermost portion of the DNA fragments tested. The sequences of the six regions protected were aligned using pileup, and a consensus sequence was derived using PRETTY, programs resident in the GCG software package (Devereux et al., 1984). The results of this analysis are shown in 3Fig. 3B. All six DNA sequences are over 79% AT.
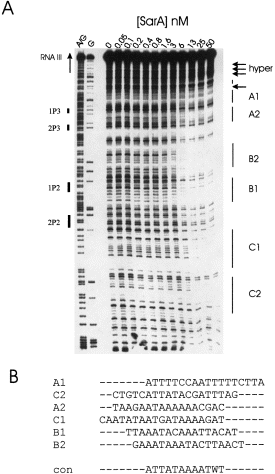
. DNase I protection analysis of SarA interactions with the P2–P3 promoter region. A. Representative DNase I result. A 32P-labelled DNA fragment was equilibrated with various concentrations of SarA before treatment with DNase I. Samples were resolved by denaturing gel electrophoresis and detected by phosphorimaging. A/G, G, chemical sequencing standards. Solid bars on left side (e.g. 1P3, 2P3, etc.) indicate the position of the heptad repeats; solid bars on the right side (e.g. A1, A2, etc.) indicate protected regions, and the arrows indicate hypersensitive sites. B. Alignment and consensus sequence of protected regions.
Interactions of SarA with the entire region between the P2 and P3 promoters
In the EMSA data reported by Morfeldt et al. (1996) using large DNA fragments between the P2 and P3 promoters, an unusual ladder pattern of SarA–DNA complexes was observed. As the SarA-containing protein sample used in that study was a crude lysate of S. aureus, we asked whether our recombinant SarA had similar activity. The 180 bp region used in the DNase I experiments described above was amplified by PCR with 32P-labelled primers and used in EMSA experiments. The unbound and SarA-bound DNA fragments were quantified by phosphorimaging. A representative gel is shown in Fig. 4. Indeed, a ladder pattern of protein–DNA complexes was generated, very similar to that shown by Morfeldt et al. (1996). One conclusion from this experiment is that the native SarA and the recombinant SarA have very similar activities in EMSA. The high concentration of DNA used in these experiments (200 nM) allows us to determine the stoichiometry of binding. The concentration of SarA required for 50% and 100% of the DNA to be bound was observed to be a protein–DNA ratio of 3:1 (300 nM and 600 nM respectively). This result supports the concept of one SarA dimer binding to each set of footprints identified in the DNase I analysis. The total number of resolvable bands was 12, with the first seven bands having a uniform laddering pattern and shifting preferentially. Given a 3:1 SarA–DNA stoichiometry at saturation, the simplest interpretation of this result is that the bands observed in this analysis represent different conformations of SarA–DNA complexes. However, a more complicated model for interactions of SarA with this DNA fragment might explain the data better. One such model, originally described by Morfeldt et al. (1996), would postulate that SarA alters the superhelicity of the bound DNA (described fully in Discussion ).
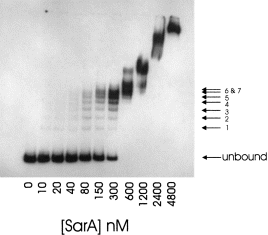
. Stoichiometric binding of SarA to the 180 bp region of the P2–P3 promoter. A 32P-labelled DNA fragment corresponding to the entire interpromoter region was equilibrated with purified SarA, resolved by native PAGE and detected by phosphorimaging. Numbering on the right side indicates well-resolved complexes.
Determination of equilibrium binding constants for SarA binding site fragments using EMSA
Whereas the DNase I footprint analysis described above indicates the relative affinity of three proposed SarA binding sites, we performed quantitative EMSAs to derive the equilibrium binding constants (KD) for synthetic DNA fragments containing those sites, as well as sites described by Chien and Cheung (1998) and Morfeldt et al. (1996). The promoter region was divided into five fragments of DNA (P3, P2, AC, A1/A2 and B1/B2), as shown in Fig. 1. A limiting concentration of 32P-labelled DNA (< 10 pM) was mixed with various concentrations of purified SarA and subjected to native gel electrophoresis. The amount of DNA in the unbound and bound state was determined by phosphorimaging. Representative gels of SarA with the fragments are shown in 5Fig. 5A. The SarA–DNA complexes with fragments P2, P3 and AC were observed to be a unique species in the gels. However, the complexes formed with either the A1/A2 or B1/B2 fragments yielded two distinct species. The significance of this result is addressed in the Discussion. In 5Fig. 5B, the percentage of the total DNA bound by SarA is plotted against the concentration of SarA present in the mixture. The data were fitted using the non-linear least squares program bioeqs as described in Experimental procedures. The data fitted best to a model of one stable protein species (dimer) binding to each of the 45 bp DNA fragments. However, a monomer–dimer equilibrium is most probably involved in SarA binding to the A1/A2 and B1/B2 DNAs (addressed in the Discussion ). A summary of the calculated values for ΔG and KD are presented in Table 1. SarA binding affinity for the DNA fragments correlates with the number of half-sites present in the DNA fragment tested. The DNA fragments containing a pair of intact half-sites, A1/A2 and B1/B2, were bound with highest affinity (KD = 10 and 7 pM respectively). The P3 and P2 fragments have 1.5 half-sites (KD = 220 pM), and the AC fragment has one half-site (KD = 1 nM). As a negative control, SarA binding affinity for a heterologous DNA fragment (the multiple cloning site of pUC118) was tested using EMSA. No specific SarA–DNA complexes were resolved, and the apparent KD for the interaction was at least 3 nM, which is 300- to 400-fold higher than the A1/A2 or B1/B2 fragments (data not shown).
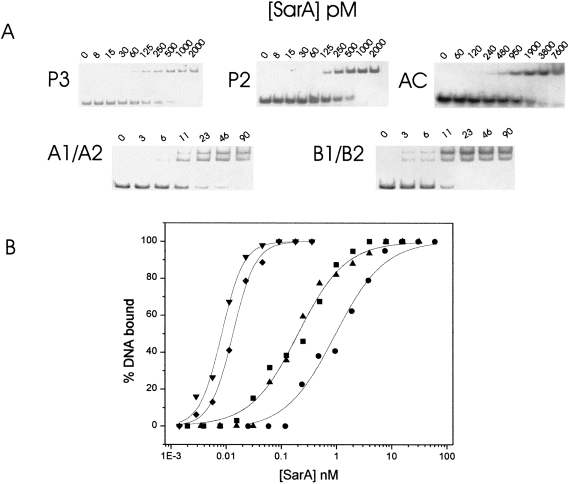
. Quantitative analysis of SarA binding to regions within the P2–P3 promoter by EMSA. A. Representative EMSA of SarA and various DNA fragments used in this study. 32P-labelled DNA fragments were equilibrated with purified SarA, resolved by native PAGE and detected by phosphorimaging. The DNA fragments used are indicated to the left side of the data. B. Binding isotherms of EMSA data from (A). The concentration of bound DNA was calculated and plotted against the concentration of SarA present in the sample. DNA fragments used: P3(▴), P2(▪), AC(•), A1/A2(♦), B1/B2(▾).

In addition, we performed EMSA of SarA binding to these fragments under stoichiometric binding conditions (> 10 nM DNA; data not shown). In each case, the analysis revealed a stoichiometry of 1:1. These data lend further support to a model of one SarA dimer binding to two half-sites. The data were also analysed using a sigmoidal power logistic function resident in origin software, which yields a value, ρ, reflecting the slope of the curve or co-operativity of binding (Czernik et al., 1996). Fragments P2, P3 and AC have ρ values of 1, indicating no co-operativity in SarA binding. Whereas the fragments bound with the highest affinity, A1/A2 and B1/B2, had ρ values of 2, reflecting considerable apparent positive co-operativity. As the stoichiometry of SarA–DNA for all fragments tested was 1:1, we conclude that, at the very low concentrations of SarA needed to bind the A1/A2 and B1/B2 fragments, there must be considerable SarA monomer present. The presence of DNA serves to drive the equilibrium towards dimer formation.
Discussion
Two pleiotropically acting regulatory loci have been identified that affect the temporal regulation of virulence gene expression in Staphylococcus aureus : agr and sar. The product of the sar gene, SarA, is a DNA-binding regulatory protein that activates expression of the two operons in the agr locus. One operon expressed from the P2 promoter encodes a quorum-sensing system that affects the switch from surface protein expression when the population density is low, as in an early infection, to exoprotein expression when population density is high, which occurs in later stages of infection or in stationary phase laboratory cultures. The other operon in the agr locus is expressed from the P3 promoter. The most important product of its expression is a regulatory RNA molecule (RNA III) that directly upregulates the expression of many exoproteins. One of the long-term goals of our research in this area is to characterize the interactions between SarA and agr to reveal the mechanism of regulation imposed on virulence gene expression. Two published reports of SarA interactions with the agr region have inconsistent conclusions regarding the binding sites for SarA in the agr locus (Morfeldt et al., 1996; Chien and Cheung, 1998). In the earlier report, ≈60 bp upstream of the P3 promoter was shown to be sufficient for appropriate, regulated expression of RNA III expression in S. aureus (Morfeldt et al., 1996). In addition, Morfeldt et al. (1996) proposed that SarA probably interacts with the regulatory regions containing the 7 bp repeats immediately upstream of the P3 and P2 promoters (see Fig. 1). However, in the later report a DNase I footprint in the interpromoter region using a recombinant fusion of GST-SarA was observed that had no overlap with the regulatory region described in the earlier report (Chien and Cheung, 1998).
To clarify the nature of the SarA binding site in the agr region and to establish a solid base for our future experiments, we performed a biochemical characterization of the interaction of purified SarA with the DNA region between the divergent promoters for the agr operons P2 and P3. In DNase I analysis, three sets of SarA-dependent footprints were detected with the highest affinity sites, B1 and B2, in the interpromoter region more proximal to the P2 promoter. Two pairs of footprints with slightly lower affinity for SarA, A and C, which overlapped to the −35 positions of the P2 and P3 promoters, were also observed. Determination of binding stoichiometry by EMSA of the entire interpromoter region revealed a SarA–DNA ratio of 3:1, indicating that each pair of binding sites was composed of two half-sites. It is interesting to note that the half-sites are two turns of the DNA helix apart, in contrast to the binding sites for many other well-studied prokaryotic regulatory proteins, e.g. trp repressor, λ repressor, cro, lac repressor, which are one turn of the helix apart. The binding sites determined by our DNase I footprinting do not overlap all the heptad repeated sequences observed by Morfeldt et al. (1996). However, the A1 and A2 sequences are within the construct pEX085, which was reported by this group to contain the minimal sequence required for SarA-dependent transcription of RNA III (see Fig. 1). In addition, our results do not concur completely with Chien and Cheung (1998). The discrepancies between results presented here and those of Cheung's group can be explained most easily by the nature of the recombinant SarA protein. Whereas our protein is expressed as a full-length, unmodified form, their SarA was expressed as an N-terminal GST fusion. We have found the N-terminus to be very sensitive to modifications (B. K. Hurlburt, unpublished results).
Quantitative EMSA was used to test the affinities and stoichiometry of SarA for the sites described above, as well as those of sites proposed by others using synthetic DNA fragments. The KD values derived from analysis of the results show SarA binding affinity was highest for DNAs containing intact binding sites and decreased when only partial sites were present. The stoichiometry of SarA binding to all the DNA fragments we tested was 1:1. The KD values determined in this study are dramatically lower than those reported by Chien and Cheung (1998). Our current model of SarA interactions with the agr region has a stable dimer of SarA binding preferentially to three specific sites, each containing two half-sites, upstream of the regulated P2 and P3 promoters. Only one half-site (B1) is contained within the DNase I footprint reported by Chien and Cheung (1998).
Whereas a consensus site was derived from the six half-sites, the optimal sequence for a SarA binding site is still not obvious. Identification and characterization of other sites in genes that are directly regulated by SarA, e.g. cna (Gillaspy et al., 1997; Blevins et al., 1999), will contribute to this knowledge. Significant homology has been observed among sequences cis to genes with altered expression in SarA mutants (e.g. tst, spa, hlb, seb, hla ) with the binding site described by Chien and Cheung (1998), which contains the B1 half-site (Chan and Foster, 1998). However, none of these genes has been shown to be regulated by SarA directly. A common theme among our SarA-protected sequences was AT abundance (79–89%). The significance of a highly AT-rich binding site in an AT-rich genome is unclear, but may indicate that the binding site for SarA may be dependent upon DNA structure.
The multiband pattern of SarA–DNA complexes observed in EMSA experiments with the larger fragments of DNA are similar using SarA in extracts of S. aureus (Morfeldt et al., 1996) and recombinant, purified SarA. These multiple bands are only present when the DNA fragment used in EMSA contains at least two intact half-sites. As mentioned above in Results, a simple interpretation of this phenomenon would have different SarA–DNA conformations using different sets of half-sites. However, several lines of evidence lead to a more complicated yet interesting model, namely that SarA induces changes in the superhelicity of the DNA fragment (originally proposed by Morfeldt et al., 1996). First, the spacing between the −10 and −35 regions of the P2 and P3 promoters is ≈3 bp too far. Deletion of 3 bp from the P3 promoter resulted in constitutive, SarA-independent expression of RNA III (Morfeldt et al., 1996). Thus, one effect of SarA is to overcome this spacing. Next, the EMSA with the A1/A2 and B1/B2 fragment shows two bandshifts occurring simultaneously, although the protein–DNA ratio is 1:1, and only two half-sites are present. These two bands might correspond to differences in structure resulting from SarA binding an AT-rich sequence and changing the DNA conformation. Lastly, the gene encoding the collagen adhesin (cna ) is repressed by SarA in late-stage cultures, the time when SarA activates the P2 and P3 promoters (Gillaspy et al., 1997; Blevins et al., 1999). The spacing of −10 and −35 regions for the cna promoter is nearly optimal. Thus, SarA binding may serve to overwind regulated promoters acting as both a repressor and an activator. Precedence for the modification of DNA structure has been clearly shown for the MerR protein in E. coli (Ansari et al., 1992; 1995). One requirement of such a model may be that SarA binds downstream of the regulated promoter as well as upstream. We have preliminary data indicating the existence of multiple sites located downstream of the P3 promoter. Their importance is currently being tested.
Experimental procedures
Expression and purification of SarA
The SarA coding region was amplified using PCR from S. aureus strain DB with primers incorporating NdeI and BamHI restriction enzyme sites:
NdeI 5′-GGGAGGTTTTACATATGGCAATTACAAAAATC-3′;
BamHI 5′-GTTTAATAGAATGGATCCTCTATCAAACTTCACC-3′.
S. aureus chromosomal DNA was isolated as described by Smeltzer et al. (1996). The PCR products were restricted with NdeI and BamHI and ligated into similarly restricted pET9A (Novagen) to yield pET-DB. The fidelity of the construct was determined by sequence analysis.
Recombinant SarA expression was induced in E. coli strain BL21(DE3)pLysS when the OD of the culture was 0.4 at 600 nm for various times using a final concentration of 1 mM IPTG in LB broth at 37°C. The levels of expression were checked by SDS–PAGE of crude lysates. Maximal expression occurred within 3 h of induction. Cells were collected by centrifugation (5000 × g, 10 min, 4°C), and cell pellets were frozen at −20°C. Cells were lysed in 50 mM Tris-HCl, pH 7.5, 1 mM dithiothreitol (DTT), 1 mM EDTA, 1 mM phenylmethylsulphonyl fluoride (PMSF) on ice. Chromosomal DNA was sheared with ultrasound treatment, and the insoluble debris was removed by centrifugation (15 000 × g, 30 min, 4°C). The cleared lysate was brought to 70% saturation with solid ammonium sulphate, and the insoluble material was removed by centrifugation (15 000 × g, 30 min, 4°C). The soluble supernatant was twice subjected to dialysis against at least 100 volumes of HSB-150 (HSB = 20 mM Tris-HCl, pH = 7.6, 1 mM EDTA, 1 mM DTT and 150 mM NaCl). The resultant solution was loaded on a heparin-Sepharose column (50 ml bed volume) and washed with at least 300 ml of HSB-150. Proteins were eluted with a linear gradient of HSB-150 to HSB-1500 (HSB with 1.5 M NaCl). The total volume of the gradient was 400 ml. Column fractions were analysed for the presence of SarA using tricine-SDS–PAGE, and peak fractions were pooled. Purified SarA was stored at −20°C and is stable for at least 6 months without noticeable loss of activity. The typical yield of SarA was 1–3 mg l−1 of culture. The concentration of SarA was determined spectrophotometrically at 280 nm using an extinction coefficient of 7740 M−1 (Gill and von Hippel, 1989).
To determine the activity of the purified SarA, we used stoichiometric binding conditions in EMSA with DNA fragment P3 (Fig. 1). At DNA concentrations that are very high relative to the equilibrium dissociation constant (KD), the amount of protein required to bind 50% of the available DNA is used to determine the concentration of active protein using the equation KD = [P]1/2 − 1/2[DNA]o, where [P]1/2 is the protein concentration at 50% saturation, and [DNA]o is the total DNA concentration (Riggs et al., 1970; Hurlburt and Yanofsky, 1990). Under these conditions ([DNA] > 10 nM), KD is insignificant, and the equation simplifies to [P]1/2 = 1/2[DNA]o. Assuming that SarA binds to the P3 DNA fragment as a dimer (described in Results ), the activity of SarA in our preparations is routinely 90–95% active of the value determined spectrophotometrically. The concentration of SarA reported in our data reflects the concentration of active protein.
Chemical cross-linking of SarA
SarA in 50 mM sodium phosphate (pH 8.0), 150 mM NaCl, 1 mM EDTA and 1 mM DTT was treated with protein cross-linking agents bis-(sulphosuccinimidyl)suberate (BS3), disuccinimidyl suberate (DSS) and dithiobis(disuccinimidylpropionate) (DSP). In the case of DSP, DTT was excluded from the buffer. The reaction mixture contained 10 μM SarA and cross-linker in a total reaction volume of 30 μl. Reactions were allowed to proceed for 1 min at 4°C and quenched by the addition of 10 μl of 1 M Tris-Cl (pH 8.0). Denaturing loading dye was added to each reaction and incubated at 95°C for 20 min. Reaction products were analysed on a 12% tricine-SDS–polyacrylamide gel and visualized by Coomassie blue staining. A product consistent with a SarA dimer was observed. In the optimization of the reaction conditions, concentrations of cross-linker (5–500 μM), time (1 min to 1 h) and temperature were varied. No oligomeric species were detected exceeding a dimer. In addition, 5 mM MgCl2 and 15 μM DNA containing the P2 and P3 promoter regions were included in the reaction mixture. Again, the only product detected was a SarA dimer.
Dynamic light scattering
The oligomerization state of purified SarA was examined in the presence and absence of 10 mM MgCl2 by dynamic light scattering (DLS) using a 2001 DynaPro Dynamic Light Scattering Instrument and analysis software, dynamics, version 3.30. In each experiment, SarA (3.3 mg ml−1) was buffered by solutions containing 50 mM Tris-HCl, pH 7.5, 2 mM DTT, 500 mM NaCl and 1 mM EDTA. Twenty measurements were taken at 22°C for each analysis. The bimodal analysis of the scattering revealed a monodisperse solution with a macromolecular weight of 34 kDa. This is consistent with the scattering from a slightly elongated SarA dimer (calculated molecular weight of 29.4 kDa).
Circular dichroism
The protein concentration used for this study was 1.8 mg ml−1 in a solution of 50 mM potassium phosphate, pH 7.5. The circular dichroism spectrum was taken from 260 nm to 180 nm. The spectrum showed large negative ellipticity at 208 nm and 220 nm, indicative of a high helical content. Using the spectrum from 260 nm to 190 nm, the percentage of each secondary structure element was calculated.
DNase I protection assay
A 180 bp DNA fragment, which spans the −10 regions of the P2 and P3 promoters, was used as the target for DNase I protection studies. This region of DNA (shown in Fig. 1) was cloned from the genomic DNA of the S. aureus strain DB by PCR amplification. The primers contained BamHI sites, and the PCR product was cloned into the BamHI site of the plasmid pUC118 to form pBKH50. A 240 bp DNA fragment containing the P2–P3 promoter region was PCR amplified from the plasmid pBKH50 with the 32P end-labelled primers, −40 and M13 reverse. SarA was allowed to bind to the DNA in a reaction mixture containing 40 pM 32P-labelled DNA, 10 mM HEPES (pH 7.6), 5 mM MgCl2, 1 mM CaCl2, 1 mM DTT and 100 mM KCl at room temperature for 30 min. An amount of DNase I that produced ≈50% non-nicked DNA was added to the reaction mixture and incubated for 2 min. The reaction was quenched by the addition of stop solution (80% EtOH, 1 μg ml−1 tRNA, 0.3 M NH4OAc) and immediately put into a dry ice/EtOH bath for 30 min. A visible pellet was obtained after centrifugation and washed with 70% ethanol. After denaturing gel electrophoresis, the DNA fragments were detected and quantified by phosphorimaging. The radioactivity in the bands for samples containing SarA was subtracted from the no-protein control using Microsoft Excel. The concentration of SarA required to protect a cleavage site fully was determined. To identify the regions of protection within the interpromoter region, purine sequencing was included in the analysis. Chemical sequencing was performed according to standard procedures.
Electrophoretic mobility shift assay (EMSA)
The sequences of DNAs used in EMSA are illustrated in Fig. 1. DNAs P2, P3, A1/A2 and B1/B2 were designed to test the importance of the heptad repeats and the footprints revealed in this study respectively. The AC fragment is exactly the same as a fragment used by Chien and Cheung (1998) and was used to test the importance of the footprint reported in that study. The oligonucleotides were synthesized, end-labelled with 32P and purified after denaturing polyacrylamide gel electrophoresis. Complimentary DNAs were annealed, purified by native gel electrophoresis and used in the binding experiments. Quantitative EMSA was performed with these synthetic DNA fragments to determine both the stoichiometry of SarA binding and equilibrium dissociation constants (KD). DNA concentrations greater than 10 nM were used for stoichiometric binding analysis. Limiting concentrations of DNA (< 10 pM) were used when determining KD values. Various concentrations of SarA were incubated in a 20 μl reaction buffer with 32P-labelled DNA and in buffer containing 10 mM HEPES, pH 7.6, 1 mM EDTA, 2 mM DTT, 50 mM KCl, 0.05% Triton X-100 and 5% glycerol. Binding reactions were allowed to equilibrate for 30 min before electrophoresis. Bound products were separated from free DNA on 6% native polyacrylamide (50:1 acrylamide–bisacrylamide) in 0.5 × Tris borate–EDTA. Gels were run at 200 V, and temperature was maintained at 16°C with a circulating water bath. Resolved gels were dried and the products quantified by phosphorimaging. The amount of bound DNA was calculated from the reduction in the unbound DNA and plotted against the respective SarA concentrations. Stoichiometric determinations were performed as described above. For KD determinations, the resulting data were fitted to various potential models of SarA–DNA interactions including: SarA SarA2 SarA2-DNA; SarA2 SarA2-DNA; 2SarA2 2SarA2-DNA, using the numerically based algorithm bioeqs (Royer et al., 1990; 1993; Royer and Beechem, 1992). Based on the Gibbs free energies of dissociation (ΔG), bioeqs solves directly for the species population. The equilibrium dissociation constants (KD) were calculated from the ΔG values using the formula, ΔG = −RTlnKD. Co-operativity values were determined as described by Czernik et al. (1996) using the sigmoidal power logistic function resident in origin (Microcal). The ρ value is indicative of the slope of the curve. A ρ value greater than 1 indicates positive co-operativity, and less than 1, negative co-operativity. If ρ = 1, then no co-operativity is apparent.
Footnotes
Acknowledgements
We are indebted to Staffan Arvidson, Sam Mackintosh, Soheila Maleki and Kristen Sterba for critical reading of the manuscript. We also thank Jessica Ray and Alan Gies for technical assistance. The work was supported by grants from the Arkansas Science and Technology Authority and the US Public Health Service (AI43356).




