Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli
Abstract
Cyclopropane fatty acid (CFA) formation is a post-synthetic modification of the lipid bilayer that occurs as cultures of Escherichia coli and many other bacteria enter stationary phase. We report the first distinct phenotype for this membrane modification; early stationary phase cultures of strains lacking CFA (as a result of a null mutation in the cfa gene) are abnormally sensitive to killing by a rapid shift from neutral pH to pH 3. This sensitivity to acid shock is dependent on CFA itself because resistance to acid shock is restored to cfa mutant strains by incorporation of CFAs from the growth medium or by introduction of a functional cfa gene on a plasmid. The synthesis of CFA depends in part on the RpoS sigma factor, but the role of RpoS in resistance to acid shock involves additional factors because strains with null mutations in both cfa and rpoS are more sensitive to acid shock than either single mutant strain. Exponential phase cultures of E. coli are much more sensitive to acid shock than stationary phase cultures, but survival is greatly increased if the exponential phase cultures are exposed to moderately acid conditions (pH 5) before shift to pH 3. We show that exposure to moderately acid conditions gives a marked increase in cfa transcription. The efficiency of the survival of acid shock is extremely strain dependent, even among putative wild-type strains. Much, but not all, of this variability can be explained by the partially or totally defective RpoS alleles carried by many strains.
Introduction
Cyclopropane fatty acids (CFAs) are a major component of the phospholipids of many species of bacteria (Grogan and Cronan, 1997). These acids are formed by addition of a methylene group, derived from the methyl group of S-adenosyl methionine, across the carbon–carbon double bond of unsaturated fatty acids (UFAs) (Grogan and Cronan, 1997). The methylene group is transferred to mature phospholipid molecules already present in membrane bilayers and does not involve free fatty acids or intermediates in phospholipid biosynthesis. Furthermore, CFAs are typically produced at the onset of the stationary phase of bacterial culture growth (Wang and Cronan, 1994; Grogan and Cronan, 1997). CFA formation can, therefore, be considered a conditional and post-synthetic modification of bacterial membrane lipid bilayers.
This modification is noteworthy in several respects. It is catalysed by a soluble enzyme, CFA synthase (Grogan and Cronan, 1984; Wang et al., 1992), although one of the substrates, the UFA double bond, is normally sequestered deep within the hydrophobic interior of the phospholipid bilayer. The CFA synthase reaction is energetically expensive (synthesis of one S-adenosyl methionine requires three ATPs in E. coli ) and virtually all of the UFAs are converted to CFAs, and in Gram-negative bacteria this includes the phospholipids of both the inner and outer membranes. The expense, timing and extent of the UFA-to-CFA conversion plus the widespread distribution of CFA synthesis among bacteria suggests an important physiological role for this phenomenon, yet its rationale has remained unclear despite experimental tests of a variety of hypotheses (Grogan and Cronan, 1986; Grogan and Cronan, 1997).
One unusual physiological aspect is the timing of CFA synthesis. Maximal CFA synthesis occurs during the transition from the late log phase to the stationary phase of the cell growth (Wang and Cronan, 1994). This has been explained by the presence of two cfa gene promoters P1 and P2. The proximal promoter P2 is active only during the log to stationary phase transition and activity is dependent on the sigma factor encoded by the rpoS gene, whereas the distal promoter P1 is a standard σ70 promoter and is active throughout the growth curve (Wang and Cronan, 1994).
Recently, a strong correlation between the resistance of various wild-type E. coli strains to a rapid and drastic decrease in pH and the level of CFA present in the cell membrane phospholipids of these strains was reported (Brown et al., 1997). Those strains having high levels of CFA survived acid shock much more efficiently than those strains with low CFA levels. In this paper, we directly demonstrate that E. coli cells containing CFAs survive better in a strongly acid environment than isogenic strains that lack these modified acids in their membrane phospholipids. Therefore, CFA formation is one of the factors that protects E. coli from acid shock.
Results
E. coli strains lacking CFA are more sensitive to acid shock
Acid tolerance is very strongly dependent on growth phase, with stationary phase cultures being much more resistant than log phase cultures (Small et al., 1994; Lee et al., 1995; Slonczewski and Foster, 1996; Bearson et al., 1997). We tested the acid tolerance of early stationary phase cultures of two wild-type E. coli strains MG1655 and ZK126. We chose these two strains because strain ZK126 is known to have the full RpoS response (Zambrano et al., 1993) and is derived from W3110 (Bohannon et al., 1991), the strain that provided the original genomic restriction map and much sequence information, whereas the MG1655 genome is that completely sequenced by Blattner et al., (1997). We found that, although both are considered wild-type strains, the sensitivity of the two strains towards extreme acid (pH 3) was very different, and the two strains had differing dependencies on the test protocol used. In the course of this work, we transduced null mutations in either cfa or rpoS (or both) into the two strains. The rpoS null mutation used is the rpoS ::Tn10 insertion of strain UM122 (Mulvey et al., 1988) whereas the cfa ::kan mutation was constructed as described in the Experimental procedures. It should be noted that the cfa insertion mutation cannot have polar effects on downstream genes because cfa is expressed as a monocistronic mRNA (Wang and Cronan, 1994) and the gene immediately downstream (which encodes a riboflavin synthase subunit) is divergently transcribed (Blattner et al., 1997). Strains carrying the cfa ::kan mutation lacked detectable CFA synthase activity and made no CFA detectable by gas chromatography (Cronan et al., 1974) or collision-induced dissociation electrospray mass spectroscopy (Sweetman et al., 1996) (data not shown) as shown for other null mutants (Grogan and Cronan, 1986).
Two general types of protocols have been used for acid shock of bacterial cultures (Small et al., 1994; Lee et al., 1995; Bearson et al., 1997; Brown et al., 1997) and we have tested both types. In protocol A, cultures grown in Luria–Bertani (LB) medium are simply adjusted to pH 3 with HCl, whereas in protocol B the cultures were grown in glucose minimal medium E. These cells were pelleted, washed and then resuspended in medium E salts at either pH 7 or pH 3. In both protocols, the cell suspensions were held at pH 3 for various time periods and samples taken, diluted in neutral medium and spread on RB plates.
We first tested early stationary phase cultures of strain ZK126 and its derivatives because this strain has the full RpoS response, which is known to play a major role in acid shock tolerance (Slonczewski and Foster, 1996). When tested by protocol A, strain ZK126 was very resistant to acid and had a survival of 0.6–0.7 upon exposure to pH 3 for 40 min at 37°C. In contrast, when protocol B was used, the survival was only about 0.01 (Fig. 1B). Because the major difference in the protocols was the medium used, we performed a hybrid experiment in which the cultures were grown as in protocol A, then acid shocked as in protocol B (Fig. 1A). Strain ZK126 again survived this treatment well, suggesting that the growth medium plays a major role in survival of this strain (Fig. 1A). However, when strain YYC1272, the cfa null mutant of strain ZK126, was examined, a different pattern was seen. When tested by protocol A, there was little or no difference in survival of YYC1272 and ZK126, whereas when tested by protocol B the cfa strain survived at a 100-fold lower rate (Fig. 1B). When tested by the hybrid protocol, there was a 20-fold difference in survival (Fig. 1A). Therefore, the presence of LB medium before and during acid shock increased the survival of the cfa strain. Strain ZK126 seems to have at least two mechanisms to survive acid conditions, one requiring the presence of LB medium both before and during acid shock and a second dependent on CFA. We attribute the LB medium effect to the known acid defence mechanisms, one of which, increased expression of basic amino acid decarboxylases (Olson, 1993; Lin et al., 1995; Park et al., 1996; Slonczewski and Foster, 1996), requires the presence of amino acids. When cultures were grown in minimal medium, these defences were inoperative and the effects of CFA content were unmasked. We then turned to early stationary phase cultures of strain MG1655 to test the generality of these observations and found the behaviour of this strain to be quite different, although the cfa derivative was always more sensitive to acid shock than the wild-type strain (Fig. 1C).
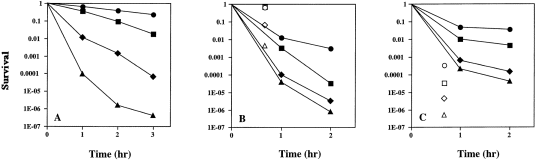
. Acid survival of strains ZK126 and MG1655 plus their cfa and rpoS derivatives after acid shock in various media. A. All strains were grown to early stationary phase in LB medium (see Experimental procedures ) and subjected to acid shock (pH 3.2) by protocol B. Symbols: • strain ZK126 (wild type); ▪, strain YYC1272 (cfa::kan ); ♦, strain YYC1121 (rpoS::Tn10 ); and ▴, the double mutant strain YYC1274 (cfa::kan rpoS::Tn10 ). The survival of each strain was determined (see Experimental procedures ). B. Acid survival of strain ZK126 and its cfa and rpoS derivatives grown in minimal medium and acid shocked by protocol B (filled symbols) or grown in LB medium and acid shocked by protocol A (open symbols). The strains and symbols are as in A. The cultures were grown in minimal medium E plus glucose or in LB medium (see Experimental procedures ) to early stationary phase and subjected to acid shock. C. The experiment was carried out as in B except that the strains were MG1655 and its derivatives. Symbols: • strain MG1655 (wild type); ▪, strain YYC1273 (cfa::kan ); ♦, strain YYC1276 (rpoS::Tn10 ); and ▴, the double mutant strain YYC1275 (cfa::kan rpoS::Tn10 ). The acid survival for each strain was determined. The open symbols are for the strains described as above except that strains were grown in LB and acid shocked by protocol A. Other conditions are described in the Experimental procedures.
These results indicated that other physiological processes can mask the effects of CFA content on acid shock survival. Because rpoS mutants are known to be defective in acid shock survival (Small et al., 1994; Slonczewski and Foster, 1996), it seemed likely that these other processes might be regulated, at least in part (as is cfa expression), by RpoS. If so, we would expect that strains lacking RpoS as well as CFAs should be more sensitive than either single mutant strain. The results of analysis of strain ZK126 and its derivatives confirmed this prediction, the double mutant was more sensitive to acid shock than either single mutant strain when tested by any of our protocols. We attribute the lesser sensitivity of the rpoS strain relative to the double mutant to the P1 cfa promoter, which functions in the absence of RpoS (Wang and Cronan, 1994). The double mutant strain therefore completely lacked cfa expression, whereas residual cfa expression remained in the rpoS strain. Cultures grown on either LB or minimal medium both gave striking differences in survival when the mutant cultures were compared with the wild-type strain, although overall survival values were generally increased if the cultures were grown in LB (Fig. 1A and B). Strain MG1655 and its derivatives gave very similar results except that this strain and its derivatives survived acid shock much better when protocol B was used rather than protocol A (the opposite of the results obtained with strain ZK126 and its derivatives). The rpoS derivative of MG1655 (YYC1276) was almost 100-fold more acid sensitive than its wild-type parent (Fig. 1C), whereas the rpoS cfa derivative had an acid survival rate 700-fold lower than the wild parental strain when tested by protocol A (Fig. 1C). Protocol B gave the same order of acid sensitivity: wild type > cfa > rpoS > cfa rpoS (Fig. 1C).
We then tested whether the defect in survival of the cfa strains was directly due to the loss of this gene by introducing the cfa plasmid pAYW19 into these strains. Both of the transformed derivatives of strains ZK126 and MG1655 became as acid resistant as their wild-type parental strains (data not shown), indicating that the effect of the cfa null mutation was specific to the loss of the cfa gene product. An acid shock experiment was also performed on a mixed culture of strain YYC1273 (cfa::kan ) and its parental strain MG1655 as a further test. The number of surviving cells of each genotype was determined by using media with or without kanamycin. Only the cfa mutant strain could form colonies on kanamycin-supplemented medium, and thus plating on these media differentiated the wild type and cfa::kan null mutant survivors. The survival rate of both strains were the same as when they were grown and acid shocked separately (data not shown), excluding the possibility that molecules produced by one strain diffuse through the medium and influence acid shock survival of the other strain.
Incorporation of exogenous CFAs renders cells more acid resistant
The above experiments indicated that cells lacking CFA synthase were more sensitive to acid shock. It remained possible that these results were due to lack of the enzyme protein per se or of an enzyme product other than CFA (e.g. S-adenosylhomocysteine) rather than the lack of CFA. To test these possibilities, we bypassed the cfa null mutation by providing chemically synthesized CFA in the growth medium. To demand efficient incorporation of the exogenous acids, we used a strain unable to make unsaturated fatty acids (UFAs). Such strains are UFA auxotrophs and require an appropriate UFA or CFA for growth (in the absence of supplementation cultures of these, strains lyse) (Magnuson et al., 1993). The strains used carried the cfa::kan null mutation (to prevent conversion of the UFA supplement to CFA) and the temperature-sensitive fabA2 mutation (fabA encodes the enzyme that introduces the double bond of the UFAs). We grew fabA cfa derivatives of strains ZK126 and MG1655 (strains YYC1283 and YYC1284 respectively) in LB medium supplemented with either a CFA or the UFA analogue for many generations of growth at 42°C (a restrictive temperature for the fabA2 allele) to ensure that the added fatty acid was the sole CFA (or UFA) component of the membrane lipids. Early stationary phase cultures of these cells then were subjected to acid shock at pH 3 for 20 min at 42°C (Fig. 2). Strain YYC1283, the fabA cfa ZK126 derivative, had showed a 330-fold greater survival when grown with C17 CFA supplement than when the strain was grown with the corresponding UFA, which was palmitoleic acid (C16:1) (Fig. 2A). The longer chain length homologues gave a lesser effect; cells grown in LB media supplemented with the C19 CFA survived only fivefold better than those grown with the corresponding UFA (C18:1). Cultures of strain YYC1284, a fabA cfa derivative of strain MG1655, survived fivefold better when supplemented with either C17 CFA or C19 CFA than when supplemented with the corresponding UFA (Fig. 2B). These data therefore directly demonstrate that cells with membranes containing CFA resist acid shock better than the corresponding UFA-containing cells. We analysed the fatty acid compositions of the supplemented cultures by gas chromatography and found that the anticipated UFAs were present when the cultures were supplemented with either palmitoleic acid or oleic acid, whereas only the anticipated CFA was found in the CFA-supplemented cultures (data not shown).
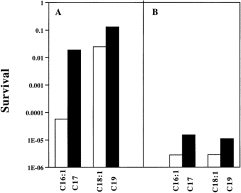
. Effects of exogeneously supplied fatty acids on acid survival. A. Cultures of strain YYC1283 (A) (cfa::kan fabA2 derivative of ZK126) grew in LB medium supplemented with UFA, palmitoleic acid (C16:1) or oleic acid (C18:1), or the CFAs, cis-9,10-methylenehexadecanoic acid (C17) or cis-9,10-methyleneoctadecanoic acid (C19), and the acid survivals were determined. The growth conditions and the acid shock protocol (essentially protocol A) are described in the Experimental procedures. B. The same experiment as in A carried out with strain YYC1284, a cfa::kan fabA2 derivative of MG1655.
Acid adaptation and CFA synthesis
The tolerance of early log phase cultures of enterobacteria to pH 3 treatment is greatly increased if cultures are adjusted to a moderately acid pH and allowed to grow for a period before shift to pH 3 (Foster and Hall, 1990; Slonczewski and Foster, 1996). This phenomenon, called acid adaptation or acid habituation, requires active metabolism during the incubation (Foster, 1991; 1993; Lee et al., 1994). Previous workers (Brown et al., 1997) showed that cellular CFA levels increase during acid adaptation, and they attributed this increase to the known accumulation of RpoS protein that would increase cfa expression by activating the RpoS-dependent P2 promoter (Wang and Cronan, 1994). We tested this premise by use of the cfa–lacZ transcriptional fusions constructed previously (Eichel et al., 1999). Early log phase cultures of strain ZK126 showed only low-level cfa gene expression in either a wild-type or rpoS::kan background. However, when the wild-type cultures were subjected to mild acid (pH 5) exposure for one generation, expression from the RpoS-dependent P2 promoter was increased 10-to 25-fold depending on whether the lacZ fusion construct included the interpromoter region (IPR) or not, whereas expression from the RpoS-independent P1 promoter was only slightly increased (Fig. 3A). In the absence of RpoS, no cfa expression from the P2 promoter was seen, whereas LacZ expression from the P1–lacZ and P1–P2–lacZ fusions was increased two- to threefold under adapted conditions (Fig. 3A). Surprisingly, the level of cfa expression of the P1–P2–lacZ fusion in the rpoS::kan background reached almost the same level as that of the wild-type strain (Fig. 3A). The reason for this is unclear, but suggests that a factor that stimulates expression from the P1 promoter accumulates during acid adaptation. Nevertheless, it seems that during acid adaptation of rpoS::kan strains, expression of cfa from the P1 promoter can produce enough CFA to account for protection from the extreme acid shock.
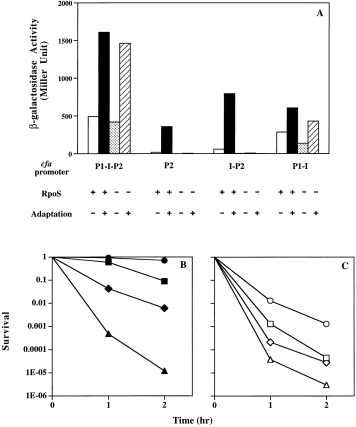
. Effects of acid adaptation on cfa expression and survival of acid shock. A. β-Galactosidase activities of various strains carrying different cfa promoter–lacZ fusions. The cultures of strains (strains YYC1113, YYC1114, YYC1115 and YYC1116) carrying a wild-type rpoS gene (Table 1) or the cultures of the rpoS:kan strains YYC1117, YYC1118, YYC1119 and YYC1120 (Table 1) were grown in LB medium and acid adapted at pH 5.0 or left without adaptation as described in the Experimental procedures. The β-galactosidase activities of adapted cultures or of cultures without adaptation were then determined. The structures of the cfa promoter–lacZ fusions were described previously (Eichel et al., 1999). All strains are lambda lysogens carrying a single copy of cfa–lacZ fusion within a lambda prophage. The first two columns (open and solid columns) are lambda lysogens of the wild-type strain ZK126 carrying different cfa–lacZ fusions, whereas the third and fourth columns (stippled and striped columns) are lambda lysogens of the rpoS::kan strain ZK1000. B. Acid survival of strain ZK126 and its cfa and rpoS derivatives after acid adaptation. Strain ZK126 (wild type, •), strain YYC1272 (cfa::kan, ▪), strain YYC1121 (rpoS::Tn10, ♦) and strain YYC1274 (cfa::kan rpoS::Tn10, ▴) were grown in minimal medium E plus glucose, acid adapted at pH 5 as described in the Experimental procedures, then subjected to pH 3 acid shock protocol B and survival was determined. C. Survival to acid shock of the cultures that were not given the adaptation treatment. The strains are denoted as open symbols of the same shapes as the solid symbols used to depict the adapted cultures. Conditions are described in the Experimental procedures.
We also studied the acid survival of strain ZK126 and its three mutant derivatives using both of the acid adaptation protocols described in the Experimental procedures. When these strains were adapted at pH 5 in LB medium and subjected to pH 3 acid shock, all four strains had similar survival values (about 0.5; data not shown). It should be noted that, without acid adaptation, early log phase cultures of these strains survive acid shock very poorly (survival of about 10−6). The high-acid survival values obtained after adaptation were in part due to the use of acid shock protocol A. When these four strains were grown on glucose in minimal medium E, acid adapted in the same medium at pH 5, and then subjected to acid shock protocol B, the survival values differed greatly (Fig. 3B). The survival rates of cfa::kan or rpoS::Tn10 derivatives of strain ZK126 were one or two orders of magnitude lower, respectively, than the survival of the wild-type strain, whereas the rpoS::Tn10 cfa::kan derivative of ZK126 survived even more poorly (Fig. 3B). The survival rates had the same dependence on rpoS and cfa alleles seen in the acid shock experiments; wild type > cfa > rpoS > cfa rpoS. For example, in two separate experiments with adapted strains, the wild type, cfa, rpoS and cfa rpoS strains had survival values (± standard deviation) of 0.70 (± 0.06), 0.09 (± 0.016), 0.0061 (± 0.0014) and 0.000012 (± 0.000005), respectively, after 2 h of exposure to pH 3.0. We also found that when cultures at low cell densities (A600 = 0.2) were acid shocked without adaptation the cfa or rpoS derivatives of strain ZK126 showed lower acid survival rates than the wild-type strain, whereas the double mutant had the lowest acid survival rate (Fig. 3C). After adaptation, survival of all strains except the double mutant strain increased greatly. We conclude that during acid adaptation the induced expression of cfa and the subsequent synthesis of CFA in cell membrane phospholipids is an important factor in protection of cells from death by extreme acid shock.
Other strains lacking CFA show different degrees of acid sensitivity: the role of RpoS
The acid resistance of strain ZK126 was three or four orders of magnitude greater than strain MG1655 when tested by acid shock protocol A. An obvious explanation for this difference would be the presence of different rpoS alleles in the two strains. Indeed, there are differences in the rpoS DNA sequences for the two strains reported in GenBank that result in changes in two amino acid residues in the N-terminal half of the protein plus a frameshift such that the MG1655 protein is 66 amino acids shorter than that of strain ZK126. In order to test whether the differences in acid sensitivity between these two strains was owing to the activities of their RpoS proteins, we transformed strain MG1655 with the rpoS plasmid pMMKatF2 (Mulvey et al., 1988) (Fig. 4). The rpoS gene of this plasmid is reported to have the same sequence as that of strain ZK126 (Zambrano et al., 1993). The strain carrying the rpoS plasmid did indeed become 100-fold more acid resistant, however the introduced rpoS gene did not increase the acid resistance of strain MG1655 to the level seen for strain ZK126 under the same conditions (Fig. 1), indicating that strains MG1655 and ZK126 differed in a factor(s) other than RpoS that plays an important role in acid resistance.
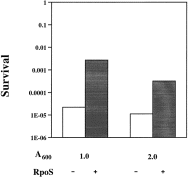
. Acid survival of strain MG1655 carrying vector or rpoS plasmid. Strain MG1655 carrying vector pBR322 (open columns) or carrying rpoS plasmid pMMKatF2 (filled columns) were grown in LB medium to cell densities at A600 of 1.0 or 2.0 and the acid shock was carried out according to protocol A. Other conditions are as described in the Experimental procedures.
We also tested the acid shock response of a number of other E. coli strains carrying a wild-type cfa gene or the cfa::kan allele. Previous workers in this laboratory (Taylor and Cronan, 1976; Grogan and Cronan, 1986) used strain FT1 and a derived cfa mutant strain FT17 for study of CFA function. We found that FT1, like MG1655, was much more acid sensitive (100- to 1000-fold) than strain ZK126 (Fig. 5). Strain YYC1106, a cfa::kan derivative of strain FT1, had a survival only two- to threefold lower than that of strain FT1 (Fig. 5) when tested by acid shock protocol A. We also constructed rpoS::Tn10 and cfa::kan rpoS::Tn10 derivatives of strain FT1 (strains YYC1256 and YYC1257 respectively), which were found to be 100-fold and 1000-fold more sensitive to acid than the parental strain FT1 (Fig. 5). We attribute the behaviour of FT1 and its derivatives to a deficiency of RpoS function because strain FT1 acquired an acid resistance similar to that of strain ZK126 upon introduction of the rpoS plasmid pMMKatF2 (data not shown). Therefore, the different acid sensitivities of strains FT1 and ZK126 can be attributed solely to RpoS function.
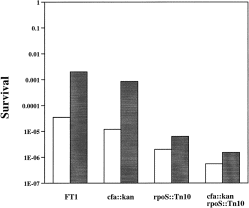
. Acid survival of strain FT1 and its derivatives. Strain FT1 (cfa wild type), YYC1106 (cfa::kan ), YYC1256 (rpoS::Tn10 ) and strain YYC1257 (cfa::kan rpoS::Tn10 ) were grown in LB medium to cell densities at A600 of 1.5 (open columns) or A600 of 2.0 (filled columns) and subjected to acid shock according to protocol A. The survival was determined for each strain at the times given. Other conditions are described in the Experimental procedures.
Finally, to examine the generality of the role of CFA in protection from acid shock, we examined one additional strain, K19, which is a direct descendent of Y-Mel, one of the earliest strains in the E. coli K-12 lineage. Strain K19 was found to be 100-fold more sensitive than strain ZK126 to acid shock protocol A, and the cfa::kan derivative of K19, strain JWC255, was five- to 10-fold lower in acid survival than strain K19 when tested by acid shock protocol A and 100-fold lower when tested by acid shock protocol B (data not shown). Therefore, CFA also plays an important role in this strain.
Discussion
Our data indicate that CFA formation plays a major role in protection of E. coli from acid shock. Although this lipid modification is clearly not the only mechanism that gives efficient survival, CFA formation probably provides the greatest protection against acid shock of any single structural molecule. This protection may also apply to other bacteria. For example, among Helicobacter isolates, those identified as gastric colonizers tend to make large amounts of CFA, whereas those isolates identified as intestinal colonizers generally do not (Haque et al., 1996). Also, many years ago, mutants of Enterococcus faecalis resistant to a folic acid antagonist were found to be CFA deficient and more acid sensitive than the parental strain (Jungkind and Wood, 1974). Although the strains tested were not isogenic and the connection between folate metabolism and CFA content is likely to reflect a general and pleiotropic disruption of single carbon metabolism, these results are consistent with ours. Several models can be envisioned for the role of CFA. The most straightforward role would be a decrease in proton permeability of the membranes due to conversion of UFAs to CFAs in the phospholipid component. Other possibilities would involve the interaction of CFA-containing phospholipids (but not UFA-containing phospholipids) with membrane proteins. These interactions could passively decrease proton permeability or perhaps actively increase proton efflux. At present, we favour the bilayer permeability model as Deamer and co-workers (Paula et al., 1996) have shown that proton permeability in lipid bilayers is inversely proportional to bilayer thickness. If the conversion of UFA to CFA had the same effect as increased bilayer thickness, this could produce acid resistance. The bilayer permeability model also is consistent with our observation that exogeneously supplied CFAs impart protection against acid shock. However, a comparison of the degree of protection between the exogeneously supplied CFAs and the natural mixture of acyl chains in membrane is complicated by the fact that chemically synthesized CFAs are a racemic mixture whereas the acids from biological sources are a single stereoisomer (although the handedness is unknown because there is no detectable optical rotation) (Craven and Jeffery, 1960; Silvius and McElhaney, 1979). This caveat aside, supplementation with the C17 CFA seems to provide more efficient protection from acid than supplementation with the C19 homologue, and this is consistent with the results of Brown et al. (1997) in which the CFAs were synthesized in vivo.
The magnitude of the protection towards acid shock given by CFA formation varies greatly with the bacterial strain examined as well as with the protocol and medium used in the test. Some of this variability is due to the numerous mechanisms that can be utilized by a given strain. E. coli, Salmonella typhimurium and other enterobacteria have developed a complex array of constitutive and inducible strategies to cope with environmental pH fluctuations. Known acid stress response mechanisms include regulatory factors such as RpoS (Small et al., 1994), Fur (Bearson et al., 1997), PhoP/PhoQ (Bearson et al., 1998) and virulence factors (Bearson et al., 1996). Specific acid survival mechanisms include pH homeostasis by inducible lysine and arginine decarboxylases (Lin et al., 1995; Park et al., 1996; Slonczewski and Foster, 1996), DNA repair, and other less defined systems (Slonczewski and Foster, 1996). Indeed, Foster and co-workers (Lin et al., 1995) have defined two kinds of acid responses, called acid tolerance response and acid resistance. The acid tolerance response in S. typhimurium involves 50 or so acid-induced proteins synthesized by growing cultures during a mild acid (pH 5–6) treatment that protects cells from a subsequent low pH shock (pH 3), whereas acid resistance refers to the survival mechanisms of stationary phase cultures that can give resistance to pH values as low at 2.5. Given the number of defence systems, it does not seem surprising to see different levels of acid survival responses for different bacterial species as well as within a single species such as E. coli.
We have observed acid shock survival rates varying over four orders of magnitude for several common laboratory strains previously thought to possess the wild-type response. The medium in which the cells were grown and/or acid challenged can also have a large strain-dependent effect (Fig. 1). Strain ZK126 possesses an efficient survival mechanism that depends on LB medium because the survival rate of strain ZK126 and its cfa mutant were similar in this medium, but very different in minimal medium (Fig. 1B). We believe the effect of CFA deficiency in this strain background was overshadowed by other acid protection mechanisms that depend on an exogenous amino acid supply and the basic amino acid decarboxylases seem likely candidates, although induction of other processes can also depend on the medium used (Slonczewski and Foster, 1996). Another difficulty with the use of LB medium is that the pH of the culture increases with growth and can become as high as pH 8. Foster and others have shown that cultures grown at basic pH values have increased sensitivity to acid shock (Small et al., 1994; Slonczewski and Foster, 1996). Therefore, growth in LB can have negative as well as positive effects on acid shock survival. However, using other experimental conditions, a strong effect of CFA formation on survival after acid shock can be seen in all of the E. coli strain backgrounds we have examined.
Much of the strain to strain variation we observed seems to be the result of naturally occurring rpoS mutants. There is known to be impressive variation in RpoS activity among E. coli K-12 strains (Jishage et al., 1996). This extends even to cultivars of a given strain. Jishage and Ishihama (1997) have reported that stocks of strain W3110 carried in different Japanese laboratories contain at least three different rpoS alleles that encode no RpoS (because of a transcriptional defect), a full-length RpoS, or a truncated protein species. The RpoS entries in the various data bases show a variety of deduced sequences for the protein, and the initiation codon has not been directly identified. If the sequence of the allele of strain ZK126 is taken as wild type, then a variety of missense mutations as well as truncated and extended products resulting from frameshifts have been reported. Indeed, it seems impossible to define the wild-type E. coli K-12 RpoS sequence at this time. Even the least mutated of the wild-type E. coli strains, such as the progenitor strains EMG2, MG1655 and W1485, have defective RpoS alleles (Jishage et al., 1996; Slonczewski and Foster, 1996) as do such ‘modern’ strains as MC4100, a strain often used to study stress responses. At present, it seems that the most active allele of RpoS will have to be considered as the wild-type allele. If so, the rpoS allele of strain ZK126 is wild type. One explanation for RpoS heterogeneity comes from the observation of Zambrano et al. (1993) that strains having a wild-type RpoS allele survive poorly under starvation conditions, whereas strains encoding compromised RpoS proteins survive well. Hence, the now largely obsolete practice of storing strains as agar stab cultures in sealed tubes at room temperature would have provided a strong selection for rpoS mutants. However, it should be noted that even recent isolates of such strains as the pathogen E. coli 0157:H7 show heterogeneity in their RpoS alleles (A. Ferreira, M. Wiedmann and K. J. Boor, unpublished data cited in GenBank accession nos AF002204–AF002209). It should also be noted that the presence of a wild-type rpoS gene does not ensure normal RpoS function. The intracellular levels of RpoS are regulated by a very large and complex series of factors, including translation of the rpoS mRNA and the half-life of both the mRNA and RpoS itself (Hengge-Aronis, 1996). Indeed, a protein has been reported that functions to release RpoD from RNA polymerase to allow RpoS to bind core RNA polymerase and to function. Thus, regulation could be exerted even at this final stage (Jishage and Ishihama, 1998). Finally, it should be noted that a strain having a compromised RpoS previously selected by carbon starvation might survive a subsequent acid shock by upregulation of a constitutive defence system.
It should also be noted that CFA may provide protection against other forms of environmental stress. The Enterococcus faecalis CFA-deficient strains mentioned above also showed increased sensitivity to high salt concentrations and deoxycholate (Jungkind and Wood, 1974). In the case of E. coli, our previous work showed that cfa strains are more sensitive to dilute ethanol solutions than the parental strains (Grogan and Cronan, 1986). Moreover, Harley et al. (1978) showed that cells of an E. coli unsaturated fatty acid auxotroph grown on CFA are more resistant to killing by hyperbaric oxygen than cells grown on the analogous UFA (although a major fraction of the UFA in these cells was converted to CFA, thus making a causal interpretation problematic). However, in both cases, it is difficult to conceive of natural environments where E. coli would encounter these stresses.
Experimental procedures
Strains, media and chemicals
All bacterial strains were derivatives of E. coli K-12 and are listed in Table 1. Genetic markers were transferred among strains by phage P1vir transduction. Strains carrying the cfa::kan mutation were made by P1 transduction from strain JC7623 containing the kanamycin insertion within the cfa gene. Strains carrying the rpoS::Tn10 mutation were made by P1 transduction from strain UM122, which was kindly provided by P. Loewen (Mulvey et al., 1988). Strains YYC1283 and YYC1284 carry the fabA2 temperature-sensitive mutation, which was transduced from strain DC308 (Clark et al., 1983) into strains YYC1272 and YYC1273, respectively, with selection for tetracycline resistance followed by screening for the temperature-sensitive fabA2 phenotype.
The liquid media used were LB or medium E (Vogel and Bonner, 1956). When minimal medium E was used, glucose (0.4%) was added as carbon source. The solid medium was RB agar, a version of LB containing less yeast extract (Chang and Cronan, 1982). The concentration of antibiotics used in media were 50 mg l−1 kanamycin sulphate, 12 mg l−1 tetracycline hydrochloride, and 100 mg l−1 sodium ampicillin.
Tergitol NP-40 detergent, palmitoleic acid (C16:1) and oleic acid (C18:1) were from the Sigma Chemical. The 17-carbon CFA, cis-9,10-methylenehexadecanoic acid (C17-CFA), was chemically synthesized from palmitoleic acid in this laboratory by the method of Goff (1964) followed by purification by argentation chromatography (DeVries, 1963). The sample of cis-9,10-methyleneoctadecanoic acid (dihydrosterculic acid) (C19-CFA) was obtained from Supelco.
Fatty acids were first neutralized with 2 N KOH in 80% ethanol to form the more soluble potassium salt that was mixed with a volume of 20% Tergitol NP-40 (sufficient to give a final NP-40 concentration of 0.1%) and this solution was then added to LB medium.
Acid shock, acid adaptation and enzyme assay
Acid shock of cell cultures was carried out by two different protocols. In protocol A, HCl (6 N) was gradually added to cultures grown in LB medium until a pH of 3.1–3.2 was reached. The pH values were monitored by pH measurements on an identical, but separate, culture. After acid addition, the cultures were shaken at 37°C for 40 min. The untreated control (neutral pH) cultures were immediately diluted and plated for colony formation. The cultures were 25-fold dilutions of a overnight culture that were grown into early stationary phase (approximate A600 of 2.0 or 1–2 × 109 ml−1). Early stationary phase was defined as 1 h after the turbidity of the culture ceased to increase. Cultures grown longer into stationary phase gave erratic results, presumably because of heterogeneity of the cells of the culture.
In protocol B, 1 ml samples of cultures grown in minimal medium E supplemented with glucose were harvested by centrifugation at room temperature in an Eppendorf minicentrifuge. The cells were then washed with medium E at pH 3 (adjusted with HCl) and resuspended in 1 ml of pH 3 medium E. The cell suspensions were shaken at 37°C for 1, 2 or 3 h, and the cells were diluted into medium E at pH 7 and plated on RB agar medium. The early stationary phase cultures were 100-fold dilutions of an overnight culture grown to an approximate A600 of 1.5, when the growth of the cultures were beginning to slow from exponential growth. Control samples received the same treatment except that medium E at pH 7 was used throughout the procedure. Survival is defined as the ratio of colonies formed on RB agar medium after acid treatment to the number of colonies formed on the same medium by cultures that were not treated with acid. The survival values given were the average of at least two experiments and the range of the values were ± 50% of the values. We also performed protocol B with the resuspended cells at 1 × 108 cells ml−1 as carried out by others (Lee et al., 1994; Small et al., 1994; Lin et al., 1995) and obtained results consistent with those obtained using the more concentrated cell suspensions.
When the effects of fatty acid-supplemented medium were tested, strains YYC1283 and YYC1284 were first grown in LB medium at 30°C overnight and the cells were diluted 100-fold into LB medium containing the proper fatty acid supplements and grown for 16 h at 42°C. The cells were subsequently subcultured by a 10-fold dilution into the fatty acid-supplemented LB medium and grown to early stationary phase (approximate cell density at A600 of 1.7–2.0) at 42°C. Acid shock to pH 3 was carried out on fatty acid-supplemented cultures by protocol A at 42°C for 20 min.
Acid adaptation requires early log phase cultures, and when performed in LB medium the cells were subcultured three times at 37°C while in early log phase (a total dilution of 105) and the final culture was grown to early log phase (A600 of about 0.15). The cultures were adjusted to pH 5 by gradual addition of 6 N HCl and allowed to continue growth to an approximate A600 of 0.3 and then subjected to pH 3 acid shock using protocol A. The control and unadapted cultures were treated similarly to the adapted cultures, except that the cultures were maintained at pH 7 and, at an approximate A600 of 0.3 (about 3 × 108 cells ml−1), the unadapted cultures were acid shocked to pH 3. When acid adaptation was carried out in minimal glucose medium, the cells were subcultured twice (a total dilution of 103) and the cultures grown to an A600 of 0.10. The adapted cultures were adjusted to pH 5 and, after one doubling, these cultures were subjected to pH 3 acid shock using protocol B. The control and unadapted cultures were treated similarly to the adapted cultures, except that the cultures were maintained at pH 7 throughout and, at an approximate A600 of 0.2, the unadapted cultures were acid shocked to pH 3.
β-Galactosidase activity was used to measure the hydrolysis of o-nitrophenyl-β-d-galactopyranoside as described by Miller (1972) and is expressed as Miller units.
CFA synthase activity was assayed as described previously by Taylor and Cronan (1976).
Construction of the cfa insertion null allele
Plasmid pAYW60 carrying a kanamycin-resistant gene in the middle of the cfa gene was constructed by Dr A.-Y. Wang of this laboratory. A 2 kbp BamHI DNA fragment encoding the kanamycin resistance determinant of plasmid pHP45Ω-Km (Fellay et al., 1987) was converted to blunt ends by T4 DNA polymerase and ligated to linearized plasmid pAYW19 (Wang et al., 1992) that had been cut with KpnI (a site within the cfa gene) and converted to blunt ends by T4 DNA polymerase. This disruption would block transcription (Fellay et al., 1987) of the cfa gene after codon 17 of the 382-codon cfa gene and thus none of the regions conserved in this protein family (Grogan and Cronan, 1997) would be expressed. This plasmid, pAYW60, was linearized by digestion with SacI (a site located outside the cfa gene), transformed into strain JC7623 and kanamycin-resistant ampicillin-sensitive colonies were isolated (Winans et al., 1985).
The construction of the cfa promoter–lacZ fusion lambda phages in which lacZ expression is driven by P1–I–P2 (the intact cfa promoter), P2 alone, or I–P2 and P1–I, respectively, was described previously (Eichel et al., 1999). These phages were then used to lysogenize the wild-type strain ZK126 and its isogenic rpoS strain ZK1000 (see Table 1).
Acknowledgements
This work was partly supported by NIH grant GM26156.




