Reconstruction of the proteolytic pathway for use of β-casein by Lactococcus lactis
Abstract
Amino acid auxotrophous bacteria such as Lactococcus lactis use proteins as a source of amino acids. For this process, they possess a complex proteolytic system to degrade the protein(s) and to transport the degradation products into the cell. We have been able to dissect the various steps of the pathway by deleting one or more genes encoding key enzymes/components of the system and using mass spectrometry to analyse the complex peptide mixtures. This approach revealed in detail how L. lactis liberates the required amino acids from β-casein, the major component of the lactococcal diet. Mutants containing the extracellular proteinase PrtP, but lacking the oligopeptide transport system Opp and the autolysin AcmA, were used to determine the proteinase specificity in vivo. To identify the substrates of Opp present in the casein hydrolysate, the PrtP-generated peptide pool was offered to mutants lacking the proteinase, but containing Opp, and the disappearance of peptides from the medium as well as the intracellular accumulation of amino acids and peptides was monitored in peptidase-proficient and fivefold peptidase-deficient genetic backgrounds. The results are unambiguous and firmly establish that (i) the carboxyl-terminal end of β-casein is degraded preferentially despite the broad specificity of the proteinase; (ii) peptides smaller than five residues are not formed in vivo ; (iii) use of oligopeptides of 5–10 residues becomes only possible after uptake via Opp; (iv) only a few (10–14) of the peptides generated by PrtP are actually used, even though the system facilitates the transport of oligopeptides up to at least 10 residues. The technology described here allows us to monitor the fate of individual peptides in complex mixtures and is applicable to other proteolytic systems.
Introduction
Lactococci have a limited capacity to synthesize amino acids and are therefore dependent on the use of exogenous nitrogen sources for optimal growth. When growing in milk, these organisms degrade milk proteins (αS1-, αS2-, κ- and β-casein) to fulfil their needs for amino acids. The proteolytic system of L. lactis exemplifies a pathway present in several bacteria that use exogenous proteins as the nitrogen source. It is used not only by lactic acid bacteria used in the dairy industry but also by various spoilage organisms and food pathogens.
The first step in the use of milk proteins is the degradation of these proteins to oligopeptides by an extracellular proteinase (PrtP). In vitro studies with purified proteinase have indicated that under optimal conditions and extensive incubation this enzyme is capable of hydrolysing β-casein into more than 100 different oligopeptides, ranging from 4 up to at least 30 amino acid residues (Juillard et al., 1995). However, the purification procedure involved the release of the proteinase from the cell wall through an autoproteolytic event by incubation of cells in Ca2+-free buffers, and it is possible that this treatment has changed the kinetic properties and substrate specificity of the enzyme.
In the second step, peptides are taken up by the oligopeptide transport system (Opp), but it is unknown which peptides from the casein hydrolysate are transported. Mutants lacking Opp are unable to grow in milk (Tynkkynen et al., 1993) and do not accumulate β-casein-derived amino acids intracellularly (Kunji et al., 1995). Previous experiments have shown that Opp is capable of transporting peptides of four up to eight amino acid residues in length (Tynkkynen et al., 1993; Kunji et al., 1993), but it cannot be excluded that longer ones are transported as well.
In the third step, the intracellularly accumulated peptides are degraded by a multitude of peptidases (see for references Kunji et al., 1996a). The general aminopeptidases PepN and PepC are able to release N-terminal amino acid residues from a wide range of di-, tri- and oligopeptides. The aminopeptidases PepV and PepT also have broad specificities but are specific for di- and tripeptides respectively. The endopeptidases PepO, PepO2, PepF and PepF2 hydrolyse internal peptide bonds of oligopeptides. More specific sequences are recognized and cleaved by PepA [Glu/Asp↓(X)n], PepR [Pro↓X], PepI [Pro↓(X)n], PepQ [X↓Pro], PepP [X↓Pro-(X)n], and PepX [X-Pro↓(X)n] (cleavage sites are indicated by arrows; Kunji et al., 1996a). Mutants lacking PepX, PepT, PepO, PepC and PepN are severely impaired in their ability to use milk proteins as a source of nitrogen and grow 10 times more slowly in milk than the wild type (Mierau et al., 1996). In addition, these mutants accumulate peptides intracellularly during growth in milk. The complexity of the protein and peptide contents of milk has thus far made it impossible to identify these peptides and to determine their source.
In this study, the use of purified β-casein by L. lactis was studied by separating the three steps through the use of well-defined mutants and by monitoring the peptide pools by liquid chromatography coupled to ion-spray mass spectrometry (LC/MS). These experiments have revealed the substrate specificity of the cell-wall-associated proteinase and oligopeptide transport system, and indicate that only a limited number of peptides in the β-casein hydrolysate is used by the cell after uptake via Opp.
Results
Degradation of β-casein by the cell-wall-associated proteinase PrtP
In the first step of β-casein use, the substrate is degraded by the cell-wall-associated proteinase (PrtP). L. lactis MG1363 was genetically manipulated to study proteinase activity in vivo without interference of peptidase activity and transport by deleting the genes that specify the oligopeptide transport system (Kunji et al., 1996b) and the autolysin AcmA (Buist et al., 1995). Deletion of the autolysin enabled us to prolong the incubation times of cells in buffer up to 6 h without detectable release of intracellular peptidases (Buist et al., 1997). The genotype and phenotype of the autolysin and oligopeptide transport deficient mutant (L. lactis GF100), carrying pLP712 for expression of the proteinase GF200, were confirmed by Southern hybridization, proteinase and transport assays, and growth experiments with purified peptides (data not shown). The properties of the mutant were in agreement with the anticipated features of the mutations (Tynkkynen et al., 1993; Buist et al., 1995; Kunji et al., 1995).
To study β-casein degradation by the cell-wall-attached proteinase, L. lactis GF200 was incubated for prolonged periods of time in buffer containing purified β-casein. After removal of cells, whole β-casein and high-molecular-weight products, the samples were analysed by high-performance liquid chromatography (HPLC). Figure 1 shows the time-dependent increase in peptides upon addition of β-casein. Approximately 70 different peaks could be discriminated in the hydrolysate. LC/MS was used to identify these peptides (1, 2). As extracellular fractions of cells are likely to contain other compounds, we have used the fragmentation pattern of peptides at high nozzle voltages for identification (see Experimental procedures). As the fragmentation pattern depends on the composition of the peptide, the identity can be established even when peptides have a similar mass or elute at similar retention times. To indicate the probability of identification of a particular peptide, the number of positively identified fragment ions relative to the total (calculated) number is indicated in Fig. 2. Additional information for identification was obtained by studying the HPLC index and the mass/charge relationship of the peptide at a nozzle voltage of 70.
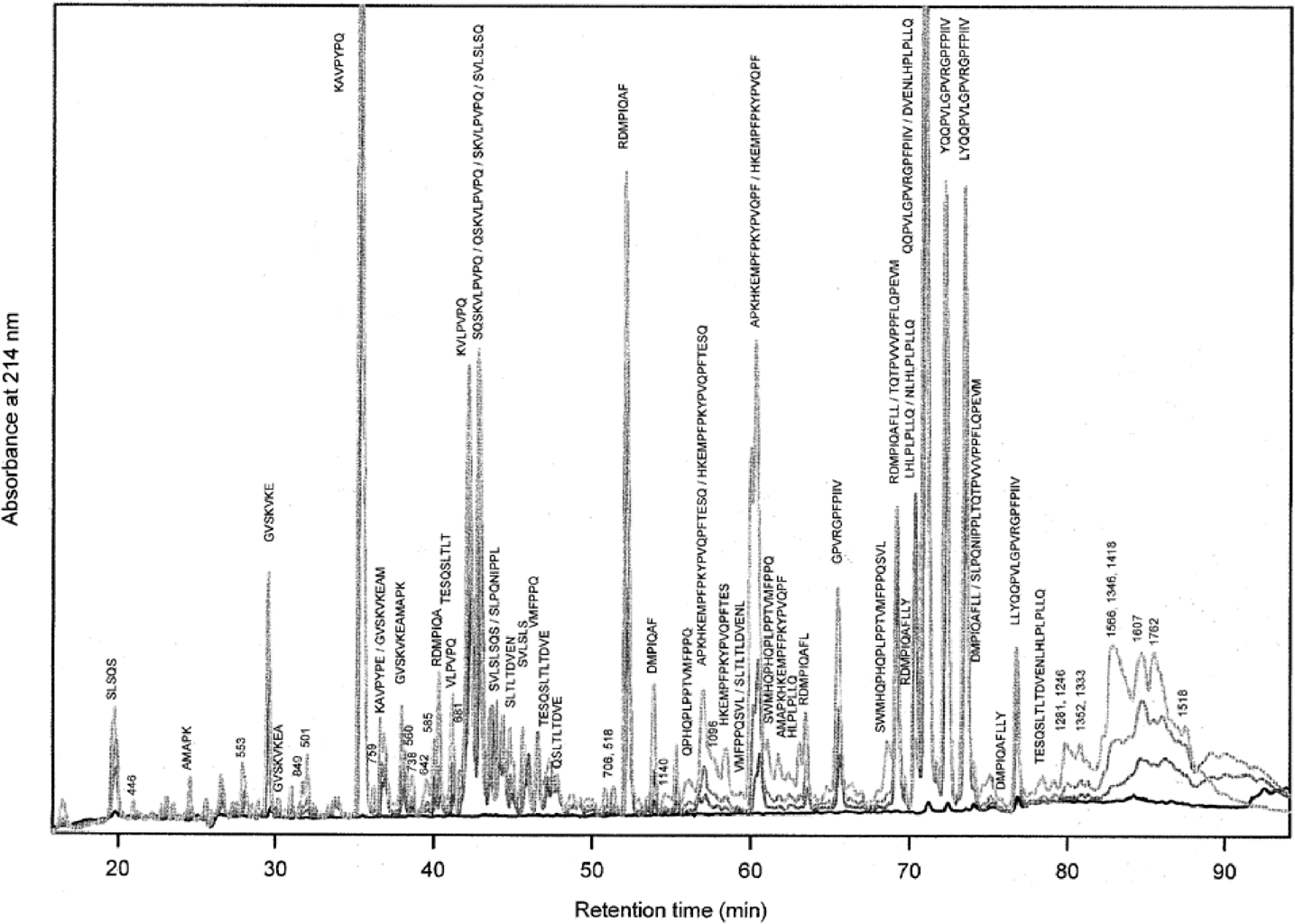
. Time-dependent increase in peptide formation from purified β-casein by L. lactis GF200. Purified β-casein (0.5% w/v) was added to washed cell suspensions of L. lactis GF200 (A660 of 20) in 100 mM MES-KOH containing 2 mM CaCl2, pH 6.5, and samples were taken immediately after addition (black line), after 0.5 h (dark grey line), 2 h (grey line) and 5 h (light grey line) of incubation. Cells were removed by centrifugation and whole β-casein was removed by ultracentrifugation. The samples were separated by high-performance liquid chromatography with a linear gradient from 0% to 60% acetonitrile/0.1% TFA in 90 min. The various peaks were identified by LC/MS as described under Experimental procedures. Unidentified peptides are labelled with the m/z-value(s) of the most abundant ion(s).
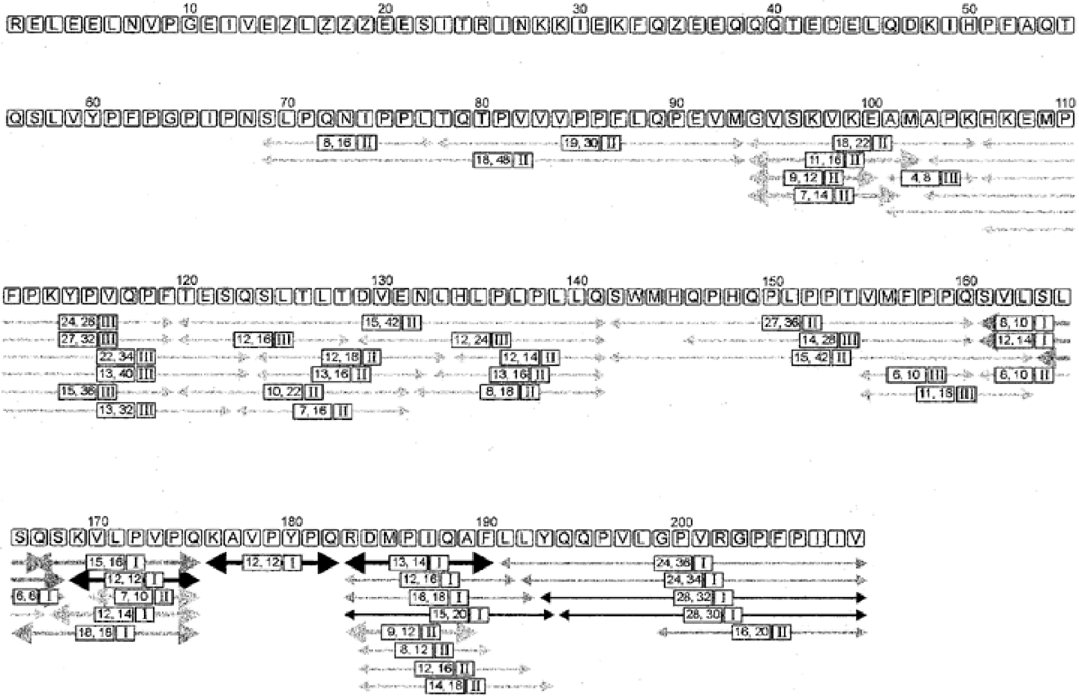
. Location of identified peptides in the primary sequence of β-casein. The lines are drawn in various shades of grey to indicate the relative amounts at which peptides were present in the hydrolysate after 0.5 h of incubation; the darker the line the higher the concentration. The numbers in the boxes (x, y) indicate the number of B and Y′′ fragments (Roepstorff and Fohlman, 1984) identified by LC/MS at a nozzle voltage of 270 (x) compared with the total number of expected fragments (y). The roman numbers in the smaller boxes indicate the kinetic class to which a peptide was assigned (see text; panel I, II and III of Fig. 3 correspond to these kinetic classes). Arrows in bold reflect peptides that were taken up via Opp (data from Fig. 4). Zs are phosphoserines.
In this way, we were able to identify 52 peptides in the hydrolysate of β-casein generated by the cell-wall-associated proteinase, which corresponds to about 66% of all peptides (absorbance at 214 nm) present in the hydrolysate. A number of ions could not be assigned with confidence to parts of β-casein and are indicated by their most predominant mass in Fig. 1. However, the majority of these ions correspond to minor constituents and varied considerably between the various hydrolysates studied. Some ions did not show a fragmentation pattern at all, pointing to the possibility that these were not derived from protein sources; they are not indicated in Fig. 1. From these studies it is evident that the cell-wall-attached PrtP can generate small-molecular-weight peptides from most of β-casein, with the exception of the N-terminal end (residues 1–68). No amino acids or di-, tri- and tetrapeptides could be detected in the hydrolysate.
The relative amounts of the peptides in the β-casein hydrolysate were estimated by measuring the absorbance at 214 nm and by comparing ion current signals obtained by mass spectrometry. Each method has disadvantages that make absolute quantification of the signals impossible. The absorbance at 214 nm reflects the number of peptide bonds and therefore depends on the size of the peptide. In addition, in many cases peptides elute at similar retention times. In the case of mass spectrometry, the ion current observed for a particular mass generally corresponds to the number of particles that have hit the detector. However, several factors influence the signal. First, the number of charged peptides liberated from the solvent by the electrical field will depend on the composition of the solvent, which varies throughout the gradient. Second, the charged state of the peptide in solution and its hydrophobicity influence the efficiency by which the peptide is released from the solvent and therefore depends on the composition of the peptide. Despite these limitations, the signals obtained by UV absorbance and mass spectrometric detection corresponded so well that the peptides could be divided into three categories (indicated by various shades of grey in Fig. 2). It is clear that the most abundant peptides were all derived from the C-terminal end of β-casein.
Inspection of the chromatographic data also revealed that PrtP-generated peptides accumulated in the external medium with different kinetics. All PrtP-generated peptides were detected throughout the degradation, but the relative amounts changed considerably with time. Three distinct kinetic classes could be discriminated, and representatives are depicted in Fig. 3. In those cases in which multiple peptides were present in single HPLC peaks, the mass spectrometric data were used to assign a peptide to a particular class (Roman numbers in Fig. 2). Peptides of the first class, which are exclusively derived from the C-terminal end of β-casein (Fig. 2), accumulated most rapidly in the initial phase of degradation, whereas their rates of production slowed down in later phases (Fig. 3; class I). The peptides belonging to the second class increased with lower overall rates and almost linearly with degradation times (Fig. 3; class II), whereas peptides of the third class had the highest accumulation rates in the final hours of degradation (Fig. 3; class III). Several unidentified peptides, which were estimated to be longer than 30 amino acid residues, increased rapidly in the initial hours of degradation and subsequently decreased at later times (see Fig. 1 between retention times 87 and 95 min).
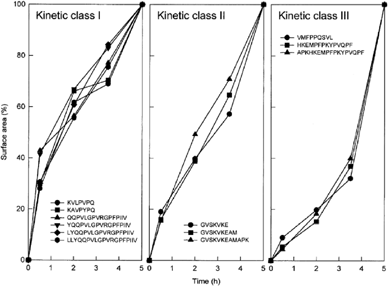
. The three kinetic classes by which peptides accumulate in the extracellular medium. Peptides that exemplify the different classes are depicted in panels I, II and III. The assignment is based on the time period in which the highest relative accumulation rate was observed. Data were taken from Fig. 1 and the accumulation level of each individual peptide after 5 h of incubation was taken as 100%.
Opp-dependent disappearance of peptides from the external medium
To identify the natural substrates of the oligopeptide transport system, we studied the Opp-dependent disappearance of peptides from the external medium. A peptide pool was generated using L. lactis GF200, and cells and large β-casein fragments were subsequently removed by centrifugation and ultrafiltration respectively. This peptide pool (obtained after 0.5 h of degradation) was subsequently offered to glucose-metabolizing L. lactis MG1363 cells (Gasson, 1983), which lack the proteinase but contain a functional Opp. Figure 4 shows the composition of the external medium immediately after the addition of cells and after 30 min of incubation as determined by LC/MS. The inset C of Fig. 4 shows the specific disappearance of four different peptides, of which three eluted at similar retention times. Also shown is an unidentified ion at m/z 681, which did not decrease after incubation with Opp-containing cells. inset D of Fig. 4 shows that the peptides RDMPIQAFLL and RDMPIQAFLLY, both eluting at similar retention times as QQPVLGPVRGPFPIIV, did not decrease significantly. In total, 14 peptides were found to decrease in an Opp-dependent manner (indicated as bold arrows in Fig. 2), i.e. KAVPYPQ, VLPVPQ, SVLSLSQS, SVLSLS (Fig. 4, main), SLSQS (inset A), GVSKVKE (inset B), KVLPVPQ, SKVLPVPQ, QSKVLPVPQ and the 10-mer SQSKVLPVPQ (inset C). Variability was observed in the disappearance of RDMPIQA, RDMPIQAF, GVSKVKEAM (Fig. 4, main) and GVSKVKEA (inset B). However, when present at relatively high concentrations, these peptides clearly decreased upon incubation with cells having an active Opp; without Opp there was no uptake of peptides whatsoever (data not shown). Apart from these and the above-mentioned peptides, none of the other peptides was taken up by the cells in an Opp-dependent manner, nor did the amounts decrease significantly in the mutant lacking both PrtP and Opp, i.e. L. lactis IM17 (data not shown).
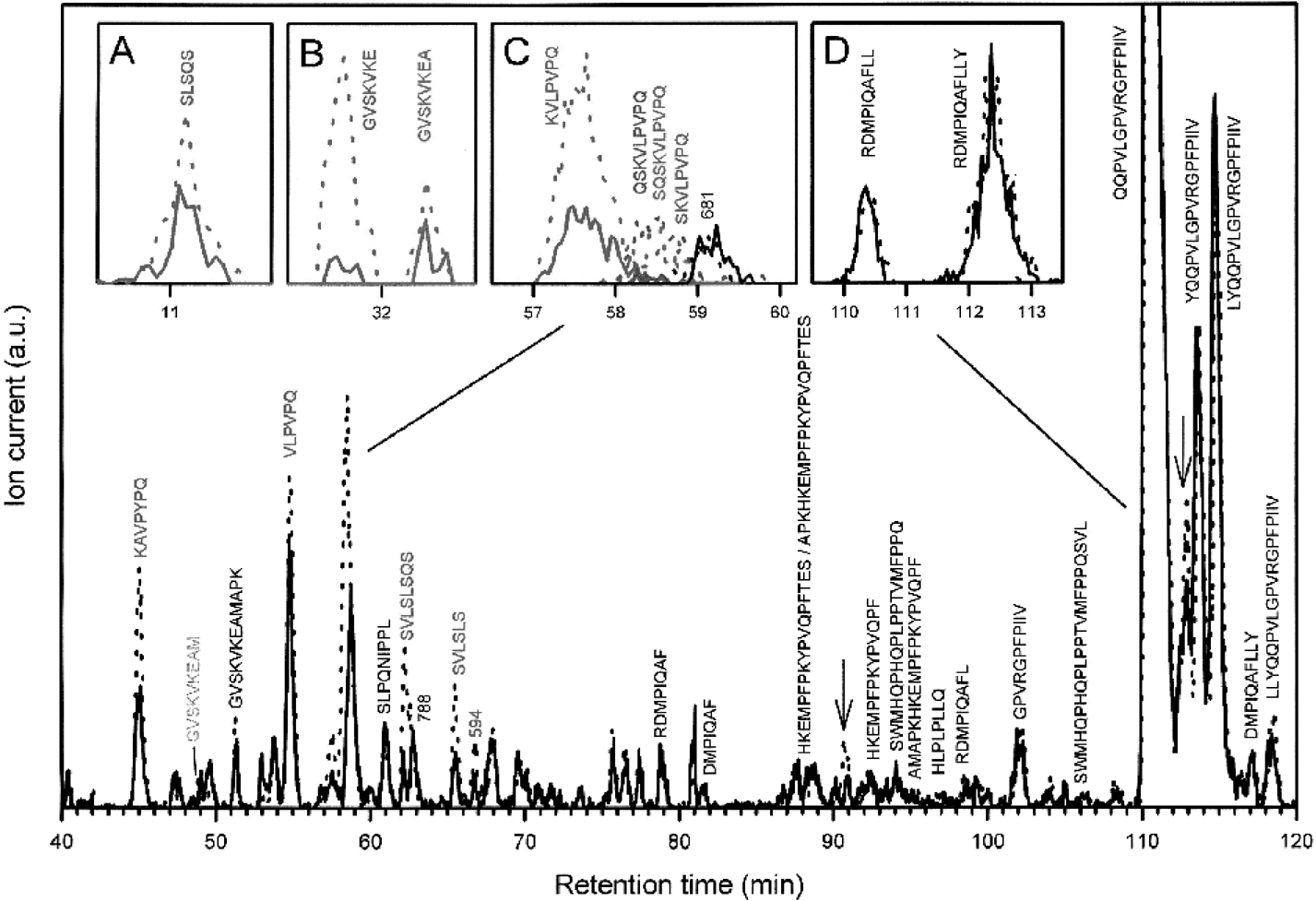
. Opp-dependent uptake of peptides from a β-casein-derived peptide pool. Cells lacking the proteinase, but containing Opp, were incubated in 100 mM MES-KOH, pH 6.5, with 0.5% glucose containing the cell-wall-attached PrtP-generated peptide pool derived from β-casein. Samples were taken immediately after addition of cells and after 30 min of incubation; thereafter, the cells were removed by centrifugation, and the samples were analysed by LC/MS. The total ion currents are depicted at a 70 V nozzle voltage after 0 min (dotted line) and after 30 min of incubation (straight line) in the range of 300–2000 m/z after removal of single-spike noise. Peptides that decrease significantly during the incubation period are depicted in colour, whereas peptides that did not decrease significantly are depicted in black. Peptides that show a minor decrease are depicted in grey. The criteria for actual uptake are a significant decrease in the mass signal compared with the noise level (which can be particularly high in certain parts of the mass chromatogram) and the reproducibility of the decrease when different uptake experiments are compared. The insets show the mass chromatograms of the individual peptides after 0 min (dotted line) and after 30 min of incubation (solid line). Buffer A consisted of 0.01% TFA/5% acetonitrile and buffer B of 0.096% TFA/60% acetonitrile in milliQ water. For separation of peptides the following gradient was used: 0–10 min 0% B, in 30 min to 15% B, in 50 min to 40% B, in 30 min to 55% B, in 15 min to 80% B (in total 135 min). The flow rate was 0.75 ml min−1.
Intracellular accumulation of transported peptides
To confirm that the peptides that were found to disappear from the extracellular medium had indeed been taken up, the intracellular accumulation of amino acids and peptides was studied in a fivefold peptidase-deficient mutant [XTOCN]−, which lacks PepX, PepN, PepT, PepO and PepC, and a fourfold peptidase-deficient mutant [XTCN]− (Mierau et al., 1996). Previous studies indicated that [XTOCN]− and [XTCN]− are unable to degrade a wide range of different peptides and, consequently, accumulate peptides intracellularly (Kunji et al., 1996b).
Accumulation of peptides was not observed in the wild-type and Opp mutant (Fig. 5A and B), but the intracellular fraction of [XTOCN]− cells showed a time-dependent increase in peptides (Fig. 5C). These peptides were subsequently identified by LC/MS. The peptides SLSQS, KAVPYPQ, KVLPVPQ, VLPVPQ, SKVLPVPQ, QSKVLPVPQ and SQSKVLPVPQ accumulated intracellularly, consistent with their extracellular disappearance. In addition to these peptides, a similar set was observed having Glu instead of Gln, which could have been the result of spontaneous deamination under the acidic conditions of the cell extraction (Juillard et al., 1995). A few putative hydrolytic products were observed that were not detected in the extracellular fractions. Some of these peptides may have been derived from the above-mentioned peptides, i.e. KAVP, KVLP, SKVLP and QSKVLP. Other accumulated hydrolytic products, such as GVSK and VKEAM, may have been derived from GVSKVKE, GVSKVKEA and GVSKVKEAM, but the identity of the complementary hydrolytic products (VPQ, VKE, VKEA and YPQ) could not be firmly established, because the levels of fragmentation at higher nozzle voltages are too low for complete identification. Only traces of the peptide RDMPIQA were found, and a mass of 518 present (at 28 min) in the chromatogram may correspond to RDMP or SVLSL. Several other compounds increased in a time-dependent manner in [XTOCN]− cells, but their identity could not be established, and they are therefore represented only by their predominant mass/charge in 5Fig. 5C. Similar observations were made for the [XTCN]− mutant.
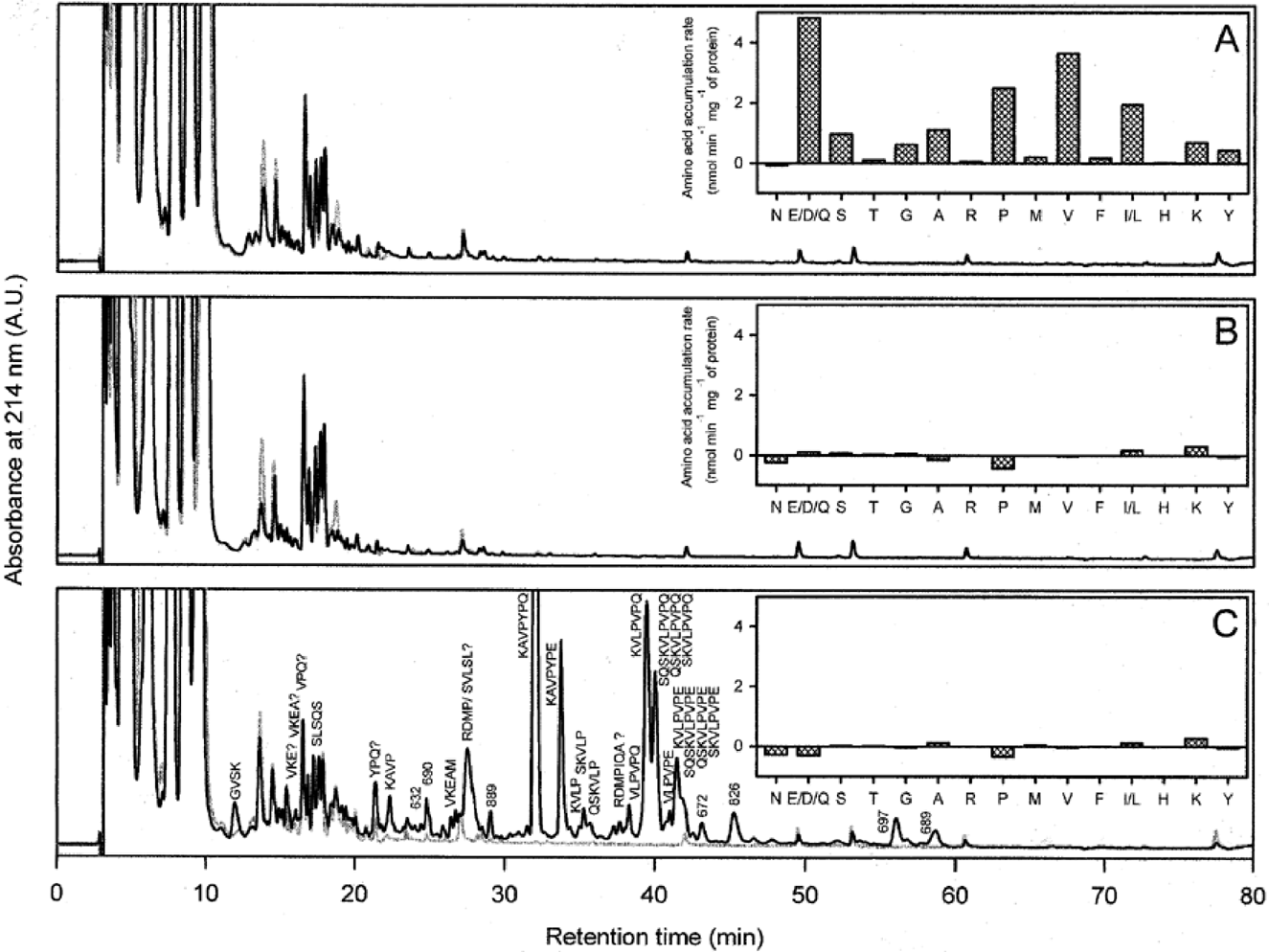
. HPLC analysis of the intracellular peptide and amino acid pools of wild type (A), Opp mutant (B) and five-fold peptidase mutant [XTOCN]− (C) upon incubation of glycolysing cells with PrtP-generated peptides. Samples were taken after 0 (grey line) and 30 min (black line) of incubation of cells in 100 mM MES-KOH, pH 6.5, containing 0.5% glucose and PrtP-generated peptides. Cells were collected on 0.45 μm cellulose acetate filters and washed three times with MES-KOH, pH 6.5. and extracted by 5% perchloric acid (PCA). The samples were subsequently analysed using HPLC with a linear gradient of 0–60% acetonitrile/acetate (0.1% w/v) and the peptides were detected at 214 nm. The PCA-extracted samples were also used to quantify the change in amino acid concentrations in the first 10 min of incubation as described previously (Kunji et al., 1993; Mierau et al., 1996).
In parallel with the analysis of the peptides, the changes in intracellular amino pools as a result of peptide uptake were followed (insets in Fig. 5). In both the Opp mutant and in [XTOCN]− cells, only minor changes in amino acid concentrations were observed (insets Fig. 5B and C). In wild-type cells, high initial accumulation rates of Glu/Asp/Gln, Pro, Val and Ile/Leu were observed, whereas the pools of Ser, Gly, Ala, Lys and Tyr increased more slowly (inset of Fig. 5A). Minor increases were found for Thr, Arg, Met and Phe, whereas Asn and His did not increase.
Discussion
The proteolytic pathway of L. lactis exemplifies a pathway used by several bacteria for the use of exogenous proteins. In this pathway, three separate steps can be discriminated: (i) exogenous proteins are degraded by an extracellular proteinase; (ii) part of the formed peptides are taken up by the cells via the oligopeptide transport system; and (iii) the translocated peptides are degraded to amino acids by a range of peptidases with different specificities. We have studied the first two steps of β-casein use in vivo by the targeted inactivation of the genes for one or more enzymes in the proteolytic pathway, using LC/MS to monitor the degradation and subsequent transport of peptides. These studies have generated detailed data that can be used to reconstruct the β-casein degradation pathway.
Proteinase activity
Several aspects of PrtP activity need to be assessed to evaluate its contribution to the growth of L. lactis. Identification of peptide product formation will determine which parts of β-casein become available for further metabolism. The rate of peptide appearance has to be studied to assess the kinetics of peptide accumulation, and the relative amounts of different peptides present in the hydrolysate will determine which peptides contribute most to the intracellular amino acid pools, if they are transported by the organism. In previous studies, the specificity of proteinase PrtP was studied after the enzyme was released from the cell wall by an autoproteolytic event. It was unclear whether this procedure influenced the properties of the enzyme. More than a hundred different peptides were identified in the hydrolysate of β-casein degraded by the purified proteinase for 24 h. Direct comparison of the HPLC profiles of products formed by the purified proteinase (Juillard et al., 1995) and the cell-wall-bound proteinase (this study) show that they are quite similar for the major and intermediate products formed; 25 out of the 52 peptides found in the current study were also observed in the product formation of the purified proteinase (Juillard et al., 1995). Important differences between the two studies relate to the nature and amounts of the minor products formed and the extent to which β-casein was degraded. In the case of the purified proteinase, more than 44% of all bonds in β-casein were hydrolysed, whereas in the present study only 16% of all bonds were found to be cleaved. It should be stressed, however, that the degradation times were considerably shorter in the present study, i.e. 5 h vs. 24 h.
Could extracellular peptidase activity have affected the peptide product formation? No peptidase activity could be detected with a chromogenic peptidase substrate (data not shown). Screening of mass spectra for putative hydrolytic products of the major peptides revealed a number of peptides that could have been the result of peptidolytic activity, i.e. VLPVPQ, DMPIQAF, DMPIQAFLLY and DMPIQAFLL. However, the bond between R183 and D184 is also hydrolysed by the purified proteinase (Juillard et al., 1995). In addition to the above-mentioned peptides, a number of peaks were detected that could potentially be hydrolytic products of KAVPYPQ, i.e. AVPYPQ (m/z 674) and VPYPQ (m/z 603), or of QQPVLGPVRGPFPIIV, i.e. QPVLGPVRGPFPIIV (m/z 796). The mass spectral signals were too low to show sufficient fragmentation at higher nozzle voltages to confirm their identity. Anyway, even if peptidolytic activity had occurred, the extent of degradation was insignificant relative to the product formation by PrtP.
The following picture of β-casein degradation emerges from these studies. The most noticeable event in the degradation of β-casein by PrtP is the rapid generation of peptides from its C-terminal end (161–208); they are most abundantly formed (Fig. 2) and the first to appear (Fig. 3; class I). This phenomenon is highly reproducible and is striking because β-casein is considered to be a molten-globular protein and that the specificity of PrtP is broad; after 24 h, 44% of all bonds in β-casein are hydrolysed by PrtP (Juillard et al., 1995). Although caseins in free solution behave as non-compact and largely flexible molecules with a high proportion of residues accessible to the solvent, there is evidence that the N-terminal end of β-casein has a significant secondary structure and is more compact than the C-terminal half (Holt and Sawyer, 1988). This may explain, at least partly, why the N-terminal half of the protein is less susceptible to breakdown. The initial cuts may produce large peptides that are subsequently degraded to smaller ones or lead to a further unfolding of the protein to expose other potential cleavage sites. As degradation rates are dependent on the concentration of the substrate, we postulate that the peptides with a relatively high accumulation rate at late degradation times (those of class III) are generated only after the initial cleavage events have taken place. We also speculate that peptides from class II are generated from β-casein directly by hydrolysis of less favourable cleavage sites. Finally, despite the broad specificity of the proteinase, peptides smaller than five residues are not formed in vivo.
Oligopeptide transport
Specific peptides were found to disappear from the PrtP-generated peptide pool in an Opp-dependent manner (Fig. 2; bold arrows), and these peptides and/or their degradation products appeared in the cytoplasm (SLSQS, KAVPYPQ, KVLPVPQ, VLPVPQ, SKVLPVPQ, QSKVLPVPQ and SQSKVLPVPQ). The two peptides SVLSLS and SVLSLSQS clearly disappeared from the extracellular fractions, but in the fivefold peptidase-deficient mutant accumulation of these peptides was not observed. These peptides are most likely degraded, but their degradation products could not be identified with certainty, e.g. the mass of about 518 could be either RDMP or SVLSL (or both). In general, those peptides that disappeared most rapidly from the medium accumulated to the highest levels in the cell.
The observed amino acid accumulations in wild-type cells do correspond fairly well with the composition of these peptides. Relatively high accumulations of Val, Pro and Glu/Gln (which can be interconverted) were observed, whereas more moderate increases in Ala, Leu/Ile, Lys and Ser concentrations were found. Gly, Arg, Ile, Phe and Met accumulated relatively slowly upon addition of β-casein and are most likely released from GVSKVKE, GVSKVKEA, GVSKVKEAM, RDMPIQA and RDMPIQAF. His is not present in the peptides that are taken up by L. lactis, which explains the inability of the cells to grow on β-casein as sole source of amino acid; the cells do grow when the medium is supplemented with β-casein plus histidine and leucine (Kunji et al., 1995).
Only a limited number of peptides (10–14) were transported by the oligopeptide transport system (Fig. 2; bold arrows). Several explanations can be given for this phenomenon. First, the transport system might only recognize particular peptides. Second, the observation that certain peptides are not transported might also reflect the upper size limit for the oligopeptide transport system. In our study, only peptides smaller than 11 amino acid residues were found to be transported. Third, this phenomenon could be the consequence of competition of peptides for the same binding site on the oligopeptide-binding protein; also ‘non-transported’ peptides may compete for binding. In our opinion, the competition of peptides for translocation by a single oligopeptide transport system, together with the large differences in concentration of peptides in the hydrolysates, is the major cause for the failure to detect transport of certain peptides. Nevertheless, this study clearly establishes that the use of oligopeptides of 5–10 residues by L. lactis is dependent on uptake via Opp. This finding strongly suggests that the observed size-exclusion limits of peptides of five to six residues for Opp in Gram-negative bacteria is an underestimate. Even although the crystal structure of OppA of S. typhimurium indicates that protein has a binding site for only five amino acids (Tame et al., 1994), it may be that the residues of longer peptides extend beyond the actual binding pocket.
Peptidase activity
The present study has also revealed the degree to which the multiple peptidase mutants are impaired in their ability to degrade β-casein-derived peptides. One surprising outcome of the experiments was that several intracellularly accumulated peptides were not degraded, or were only slowly degraded, by the remaining intracellular peptidases. The remaining activities include the dipeptidases PepV (X-X), PepR (Pro-X), and PepQ (X-Pro), the endopeptidases PepO2 and PepF, and the more specific enzymes such as PepA [Glu/Asp↓(X)n], PepI [Pro↓(X)n]and PepP [X↓Pro-(X)n] (for references see Kunji et al., 1996a). Only minor degradation was observed for KAVPYPQ, KVLPVPQ, SKVLPVPQ QSKVLPVPQ and SQSKVLPVPQ. Apparently, the peptide production rate of PrtP together with transport via Opp exceeds the degradation rate of the remaining peptidases.
In conclusion, the present study has established which parts of β-casein are used by L. lactis as a source of amino acids. Only two regions of β-casein were found to contribute significantly to the intracellular amino acid pools, i.e. the carboxy-terminal region 161–191 and to a lesser extent region 94–103 (Fig. 2). The larger part of β-casein remains unused because certain regions are poorly degraded by the extracellular proteinase. Owing to their presence in fairly small amounts, these peptides are easily out-competed for transport by more abundant peptides.
Experimental procedures
Bacterial strains and growth conditions
The bacterial strains used in this study are listed in Table 1. Lactococcal strains were grown at 29°C in M17 broth (Difco) supplemented with 0.5% (w/v) glucose or lactose. The strains were stored at −20°C in M17 broth with 10% glycerol.
Construction of an autolysin and oligopeptide transport-deficient strain expressing PrtP (L. lactis GF200) and general DNA techniques
The gene coding for the autolysin AcmA was deleted from the chromosome of the oligopeptide transport deficient L. lactis IM17 strain (Kunji et al., 1996a) as described previously (Buist et al., 1995). The genes for the use of lactose and expression of the proteinase (PrtP) were introduced into this strain by electroporation of plasmid pLP712 (L. lactis GF200) (Gasson, 1983). Plasmid DNA and chromosomal DNA from L. lactis were isolated by the methods of Anderson and McKay (1983). L. lactis was transformed by electroporation as described previously (Holo and Nes, 1989). DNA modification enzymes were obtained from Boehringer. Southern hybridizations were performed using the digoxygenin (DIG) DNA-labelling and detection kit according to the instructions of the manufacturer (Boehringer).
Proteinase activity
Proteinase activity was measured using the chromogenic peptide methoxy-succinyl-L-arginyl-L-prolyl-L-tyrosine-p-nitro-anilide (Chromogenix) as substrate, essentially as described (Mierau et al., 1996).
β-Casein degradation in vivo
Exponentially growing autolysin and oligopeptide transport-deficient cells, containing PrtP (L. lactis GF200), were washed twice with ice-cold 100 mM potassium-2-(N-morpholino)-ethanesulphonic acid (MES-KOH), pH 6.5, containing 2 mM CaCl2 to prevent autoproteolysis and release of the proteinase. Cells (A660 of 15) were subsequently incubated under gentle stirring in the same buffer at 30°C in the presence of 0.5% (w/v) β-casein. Samples were taken at appropriate time intervals, the cells were removed by centrifugation (5 min at 2500 × g ) and the supernatants were passed through Centriprep 30 ultrafiltration filters (Amicon) to remove undigested β-casein and high-molecular-weight products. The filtrates were subsequently analysed using HPLC as described below. To identify the products formed in low quantities, samples were passed through Centriprep 30 filters and the filtrate was lyophilized by freeze-drying, dissolved in milli Q to one-fifth of the original volume and analysed as described below.
HPLC analysis
HPLC analysis was performed with a Jasco HPLC system (Jasco). Peptides were separated on a reverse-phase HPLC column (250 mm × 4.6 mm, Hi-Pore 318, Bio-Rad). Solvent A was 0.11% TFA (v/v) in MilliQ water, and peptides were eluted at 30°C using a linear gradient (0–80%) of solvent B [0.1% TFA, 60% (v/v) acetonitrile in MilliQ water] within 100 min. For improved separation of particular peaks, other gradients were used as indicated in to the Figure legends. The flow rate was 1 ml min−1 unless otherwise indicated and peptides were detected by UV absorption at 214 nm. Analysis of intracellular fractions was performed essentially as described previously (Mierau et al., 1996). Peptide fractions were analysed as described above and amino acid fractions were quantified as described previously (Kunji et al., 1993).
LC/MS
LC/MS analyses were performed essentially as described previously (Juillard et al., 1995) using a Kratos Spectroflow 450 gradient controller, two Kratos Spectroflow 400 pumps and a Kratos Spectroflow 757 UV detector (Applied Biosystems) as HPLC unit, which was connected to a Nermag R 30–10 triple-quadrupole mass spectrometer. Peptides obtained from β-casein hydrolysis were loaded with a 100 μl loop in a Rheodyne 7125 injector (Rheodyne). Column and elution conditions were the same as described under HPLC analysis. By flow splitting of the column eluate, ≈1% of the sample was introduced into the ion spray LC/MS interface (Bruins et al., 1987). The mass spectrometer had a custom-built prototype atmospheric pressure ionization source (Bruins et al., 1988) and was used in positive ion mode. Multiply charged ions without fragmentation were generated at a low nozzle voltage (70 V), whereas fragment ions were generated by collision-induced dissociation (CID) at higher nozzle voltages (170 V, 270 V and 370 V) (Katta et al., 1991). Full-scan mass spectra were recorded from mass to charge ratios (m/z) of 50–1999. To correlate mass spectral data to β-casein sequence, the computer program MACPROMASS (version 1.05) was used (Lee and Vemuri, 1990). As an example, the identification of RDMPIQAFLL and RDMPIQAFLLY (Fig. 6) is described; these peptides elute from the column at similar retention times, as can be inferred from their mass chromatogram (solid and dotted black lines respectively). In agreement with the fact that the N-termini of the two peptides are identical, the ion currents of the N-terminal fragments (so-called B fragments; Roepstorff and Fohlman, 1984) were observed at retention times when both peptides were eluting (Fig. 6 A). The only exception is the B fragment that is unique for RDMPIQAFLLY (MH+ 1185), and, in agreement, the ion current of this fragment was confined to the retention times of this particular peptide. As the C-termini are different, the C-terminal fragments (so-called Y′′-fragments; Roepstorff and Fohlman, 1984) must be unique for each peptide. As expected, the Y′′ fragments of RDMPIQAFLL (Fig. 6B) and RDMPIQAFLLY (Fig. 6C) were confined to retention times of the peptide from which they were derived. The example given above also highlights some of the problems in the interpretation of the fragmentation patterns. As is evident from inspection of Fig. 6, the abundance of the ion current signals of the different fragments varied considerably. Some fragments were easily identified, whereas others were barely detectable above background. We only included fragments as positively identified when the signals were higher than the background and/or the frequency of appearance was considerably higher at the retention times at which the peptide was eluting compared with the background. Consequently, for peptides present in large quantities, an almost complete fragmentation pattern can be observed, whereas minor components of the peptide mixture yield incomplete fragmentation patterns. In addition, peptides longer than ≈20 amino acid residues have a number of fragments larger than the 2000 mass/charge limit of the apparatus and can consequently not be studied.
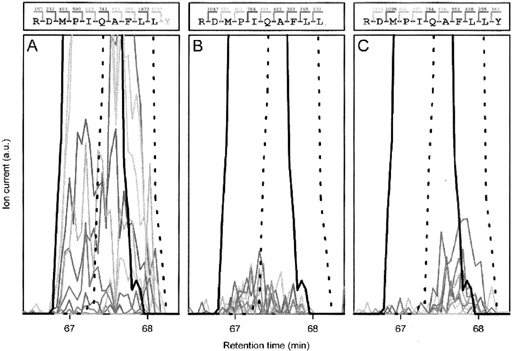
. Fragmentation analysis by LC/MS of the peptides RDMPIQAFLL and RDMPIQAFLLY at a nozzle voltage of 170. A shows N-terminal mass spectrometric fragments (B-type) of RDMPIQAFLL and RDMPIQAFLLY; B the C-terminal fragments (Y′′-type) of RDMPIQAFLL; and C the C-terminal fragments (Y′′-type) of RDMPIQAFLLY. The colours of the lines correspond to the ion currents of the different fragments depicted in the top panels (d, N-terminal fragment; e, C-terminal fragment). The solid black lines mark the abundance of the MH+ ion RDMPIQAFLL at m/z 1203 (off-scale at the magnification used in the mass chromatogram), whereas the dotted lines give the abundance of the MH+ ion of RDMPIQAFLLY at m/z 1367.
Transport of β-casein-derived peptides generated by PrtP
Before transport assays, cells were washed with 100 mM potassium-2-(N-morpholino)-ethanesulphonic acid (MES-KOH), pH 6.5. To inhibit protein synthesis, chloramphenicol (50 μg ml−1) was present in all further steps. Cells (A660 ± 25) were de-energized with 2-deoxyglucose (10 mM) for 20 min at 30°C, washed twice with 100 mM MES-KOH, pH 6.5, and resuspended in the same buffer (A660 of ≈ 50). Cells were subsequently diluted (A660 of 15) with MES-KOH containing the PrtP-generated peptide pool (see above) plus 25 mM glucose; samples were taken in time to determine the disappearance of peptides from the medium and accumulation of amino acid and peptides intracellularly as described (Kunji et al., 1993; Mierau et al., 1996).
Cell lysis determination
Samples were taken in parallel with the degradation and transport assays, cells were removed by filtration (0.45 μm cellulose nitrate) and the filtrate was incubated with lysyl-pNa (10 mM) for 24 h at 30°C. The aminopeptidase activities were related to those of sonicated cell samples (three times for15 s at an amplitude of 6 μm, on ice and under N2 atmosphere). As sonication does not result in 100% lysis it can be expected that quantification of lysis determined in this way is an overestimation.
Miscellaneous
Purified β-casein (A2 and A1 variant) was a generous gift from Wendy Levering (DMV-Campina, The Netherlands). The A2 and A1 variant differ at position 67 in the primary sequence, i.e. A1 has a His, whereas A2 a Pro residue. MilliQ water (Millipore) was used throughout the experiments. Protein concentrations were determined as described, using bovine serum albumin as standard (Lowry et al., 1951).
Acknowledgements
We would like to thank Girbe Buist for the generous gift of plasmid pINTAA for the construction of the autolysin negative mutants. We would also like to acknowledge Jan Knol for valuable suggestions throughout the work and Wendy Levering of DMV Campina for kindly providing purified β-casein. This study was supported by the BRIDGE T-project of the Biotechnology Programme of the European Community.





