Sperm length influences fertilization success during sperm competition in the snail Viviparus ater
Abstract
Sperm form and size is tremendously variable within and across species. However, a general explanation for this variation is lacking. It has been suggested that sperm size may influence sperm competition, and there is evidence for this in some taxa but not others. In addition to normal fertilizing sperm, a number of molluscs and insects produce nonfertile sperm that are also extremely morphologically variable, and distinct from fertilizing forms. There is evidence that nonfertile sperm play an indirect role in sperm competition by decreasing female remating propensity in Lepidopterans, but in most taxa the function of parasperm is unknown. We investigated the role of nonfertile (oligopyrene) sperm during sperm competition in the fresh water snail Viviparus ater. Previous studies found that the proportion of oligopyrene sperm increased with the risk of sperm competition, and hence it seems likely that these sperm influence fertilization success during competitive matings. In mating experiments in which females were sequentially housed with males, we examined a range of male characteristics which potentially influence fertilization success. We found that the size of oligopyrene sperm was the best predictor of fertilization success, with males having the longer sperm siring the highest proportion of offspring. Furthermore, we found a positive shell size and sperm concentration effect on paternity, and females with multiply sired families produced more offspring than females mating with only one male. This result suggests polyandry is beneficial for female snails.
Introduction
There is tremendous variation in sperm form across species (e.g. Cummins & Woodall 1985; Gage 1998). Sperm length varies by several orders of magnitude, from tiny porcupine sperm (28 µm) to the enormous sperm of Drosophila bifurca (5.8 cm) (Pitnick 1996; Gage 1998). There is also large variation in sperm length within species, and even within males (e.g. Ward & Hauschteck-Jungen 1993; Ward 1998; Morrow & Gage 2001a; Snook 2001). Never-theless, although general adaptive explanations for sperm number variation exist (e.g. Parker 1998), the selective advantages to different sperm sizes remain enigmatic. Because longer sperm may generate more power and swim faster (Katz & Drobnis 1990; Gomendio & Roldan 1991; but see Birkhead 1998), it has been suggested that longer sperm may be selectively favoured during sperm competition. Theory also indicates that sperm size can increase with sperm competition risk, although under some rather restrictive conditions (e.g. Parker 1993). There is some experimental evidence to support this idea in two species with aflagellate, amoeboid sperm (Radwan 1996; LaMunyon & Ward 1998), but in taxa producing flagellate sperm the evidence is more equivocal. Some comparative studies find that sperm size is positively associated with sperm competition risk (e.g. Gomendio & Roldan 1991; Gage 1994; Briskie et al. 1997), whereas others do not (e.g. Hosken 1997; Stockley et al. 1997). Also, three recent experimental studies found no evidence for sperm size influences on sperm competition outcomes (Hosken et al. 2001; Morrow & Gage 2001b; Pitnick et al. 2001). In addition to fertilizing sperm (eupyrene sperm), many taxa are sperm dimorphic and produce nonfertilizing parasperm (e.g. Silberglied et al. 1984; Buckland-Nicks 1998; Snook & Karr 1998). In most species the function of these gametes is unknown, but it has been suggested that they play a role in sperm competition, perhaps aiding transport of fertilizing sperm, or acting as cheap filler to deceive females about their sperm load. In support of this second hypothesis, in the butterfly Pieris napi, nonfertilizing apyrene sperm delay female remating, and hence reduce the risk of sperm competition faced by a male (Cook & Wedell 1999).
A large body of evidence also indicates that males may strategically alter ejaculate features relative to sperm competition risk and intensity. For example, males may alter ejaculate size based on female age, mating status or the presence of rival males (e.g. Gage 1991; Simmons et al. 1993; Martin & Hosken 2002). Also, in species producing two sperm morphs, males may alter the proportion of each sperm form transferred to females relative to sperm competition risk (e.g. Cook & Gage 1995; Cook & Wedell 1996; He & Miyata 1997). For example, male moths increase the number of eupyrene sperm in an ejaculate when mating with nonvirgin females (Cook & Gage 1995), and high larval rearing density increases the production of apyrene sperm (He & Miyata 1997).
In addition to confusion over the adaptive advantages of sperm size variation, polyandry in itself has been con-sidered rather enigmatic (Hosken & Stockley 2002). This is largely due to experiments with Drosophila that found little evidence for increased offspring production by females as a result of mating with multiple males (Bateman 1948), and to considerable mating costs documented in many species (e.g. Blanckenhorn et al. 2002 and references therein). However, females in most taxa are polyandrous and benefit from this behaviour in a variety of ways including in-creased fertility, fecundity and longevity (reviewed in Arnqvist & Nilsson 2000; Jennions & Petrie 2000; Hosken & Stockley 2002).
Like other dioceous prosobranchs, Viviparus ater females mate promiscuously and frequently (approximately every 3.5 days, range ≈ 1–7 days: Staub & Ribi 1995), and store fertile sperm for up to 2 years (Trüb 1990). In addition, males produce two types of sperm: normal eupyrene and nonfertilizing oligopyrene forms. Oligopyrene sperm contain a single chromosome and are thick, long and extremely motile compared with eupyrene sperm, which contain a full haploid chromosomal complement. Oligo-pyrene sperm are produced in large numbers in a highly organized developmental process (Buckland-Nicks 1998), which suggests an adaptive function. Recent work indicated that the presence of rival males affected the ratio of oligopyrene to eupyrene sperm produced (Oppliger et al. 1998). In experimental and natural populations, the proportion of oligopyrene sperm was significantly higher when the sex ratio was male biased. The change in the oligopyrene/eupyrene ratio with sperm competition risk suggests some role for the nonfertile sperm in sperm competition. The aim of this study was to determine how sperm characteristics influence fertilization success in this snail. We examined paternity patterns when virgin females were sequentially housed with two males (one previously kept in a male-biased and one kept in a female-biased environment) and investigated the potential influence of several sperm characteristics (oligopyrene/eupyrene ratio, sperm concentration, sperm length) on the proportion of offspring fathered by each of the two males.
Methods
Experimental design
Snails were collected during May 1998 from a natural population in Lake Zürich (Switzerland). In order to influence the oligopyrene/eupyrene ratio (OER), all males used in mating experiments were first kept for one month in a sex-biased environment (described in Oppliger et al. 1998). Briefly, snails were sexed (the right tentacle of the male is thickened and functions as a penis) and randomly assigned to one of the experimental treatments. The two treatments were: groups with a female-biased sex ratio (4 males and 20 females; 22 replicates) or with a male-biased sex ratio (20 males and 4 females; 5 replicates). All males of each treatment were older than 2 years, and although males were randomly allocated to treatments, there was a size difference between them with the male-biased sex ratio males tending to be larger (32 ± 0.6 vs. 30 ± 0.6 mm; t(69,63)= −3.32; P = 0.01). As a result, we included male size in our analyses (see below). Each experimental group was kept in rectangular cages (40 × 60 cm) in Lake Zürich from the beginning of May until the start of mating experiments in June (≈ 40 days). During early June 1998, the first set of 80 males was removed from experimental cages (40 males from the female-biased replicates and 40 males from the males-biased replicates), and housed with a virgin female aged 2 years (all the virgin females had been kept without males since birth) in a round cage (28 cm diameter). Six days later these males were removed and replaced by males that had been removed from the experimental cages the same day. In each of the mating cages, the first male was replaced by a second from the other experimental group (i.e. males from the female-biased treatment were replaced by males from the male-biased treatment). These second males were also housed with females for 6 days. Immediately after removal, males were killed by freezing and their sperm characteristics assessed (see below). The foot of each male was preserved in alcohol for paternity assignment. We were not able to observe mating behaviour directly in this study because of the obvious technical difficulties involved in observing large numbers of snails at the bottom of a lake. However, although we could not control for copula number, as females remate on average about every 3.5 days (range 1–7 days; Staub & Ribi 1995), we can be reasonably sure they copulated with each male, and variation in copula number should be randomized across treatments. We also note this is standard practice in most Drosophila sperm competition experiments (e.g. Hughes 1997). Nevertheless, in some analyses we restricted the data set to those families in which females definitely copu-lated with both males (i.e. mixed paternity families).
After the males had been removed, the females were housed alone for ≈ 2 years. During this period offspring were collected at the following times: 21 October 1998, 26 May 1999, 21 June 1999, 12 October 1999, 9 June 2000. During winter (November–March) the snails are inactive. On 9 June 2000 the females were collected, weighed, had their shell measured, and were then killed and stored in alcohol for genotyping. All offspring collected were also preserved in alcohol for paternity assignment.
Assessment of sperm characteristics
At the end of the 6-day mating periods, and after death, male shell size and body weight were measured. The shell was subsequently broken open and sperm collected directly from the testis, and always from the same place (just above the point at which the testis joins the ejaculatory duct), using a Pasteur pipette. Sperm extract (1.5 µL, which is approximately one-third to one-half of the total volume of sperm available for collection) was diluted in 100 µL of physiological serum, homogenized and 10-µL aliquots of this dilution were placed on two slides. One slide was fixed in methanol and stained with Colorap stain (Bioreac), and used to assess the numbers of the two sperm types and hence the OER. OER was estimated by counting the number of both types of sperm under a microscope (×400) using a eyepiece graticule. For each male, we counted spermatozoa in 10 of the grids. Sperm counts were made twice for a number of males to estimate the accuracy of our method. Regression analysis indicated high repeatability (n = 13, F = 82.8, P < 0.001, r2 = 0.88; see Oppliger et al. 1998). We also used sperm number in our 1.5-µL sample as an indicator of sperm concentration. The other slide was used to measure sperm length. Length was measured from microscope images conveyed to a PC running bioscan®optimas™ software. For each male, we measured the length of eight oligopyrene and eight eupyrene sperm.
Microsatellite analysis
To assign paternity we developed microsatellite markers using a modified version of Rassmann et al.'s (1991) protocol. The partial genomic library was constructed with genomic DNA purified from one snail. The genomic DNA was digested with Sau3A and the digestion product was resolved on agarose gel (3%) together with a size marker. Fragments between 400 and 900 bp were selected and purified for cloning in a pUC19/BamHI vector (Appli-gene). A mixture of synthetic oligonucleotides (TG)10, (CT)10 and (AAT)10 labelled with the DIG system (Boehringer) were used as probes to score screen the recombinant clones. Positive clones (n = 70) were then amplified using a polymerase chain reaction (PCR) using either M13FOR or M13REV universal primer, together with a mix of the dinucleotide and trinucleotide probes. Samples of the PCR products were resolved on agarose gel (3%) together with a size marker. This step allowed us to screen for additional positive clones prior to sequencing. Of the 37 amplified inserts, 25 were fully sequenced using the same two universal primers and following standard protocols, and primers flanking each repeat sequence were designed for 21 loci using the computer program primer 1.0 (Lincoln et al. 1993). Of these, four loci were variable and were used for paternity assignment (Table 1). One primer of each pair was fluorescent labelled (from Applied Biosystems) with HEX (locus F9), NED (loci E24, D370) or 6-FAM (locus F34). For paternity analysis, the whole high molecular mass DNA extraction was performed using the DNeasy mini Quiagen kit. The PCR were set up in a 10-µL reaction mixture containing: 1 µL 1× PCR buffer, 2 µL solu-tion Q (Quiagen), 0.6 µL MgCl2 (50 mm), 0.25 µL dCTP, dGTP dTTP and dATP (10 mm), 0.5 µL of each primer, 2 µL (≈ 50 ng) template DNA and 0.5 U of Taq DNA poly-merase (Quiagen). Samples were amplified on PTC-100 thermocyclers (MJ Research) using the cycling profile: initial denaturation step at 94 °C (3 min), followed by 30 cycles of denaturation (94 °C, 30 s), annealing (55 °C, 30 s) and elongation (72 °C, 45 s). We used a multiplex com-bination to simultaneously reveal all loci in a single lane. Prior to electrophoresis, 1.5 µL of the PCR products of each locus were then mixed and run on an ABI 373 XL sequencer and sized with internal lane standard (GeneScan 350-ROX; Applied Biosystems) using the genescan soft-ware (Applied Biosystems).
| Locus | Core repeat | Forward and reverse primers | Allele size range (bp) | No. alleles | GenBank Accession No. |
|---|---|---|---|---|---|
| E24 | (TC)18(AC)15 | F: TCCTTTTGCTTTTCGTCGGC R: GCAAAAAATTGCCCCAAAAGC | 154–162 | 4 | AF527033 |
| F34 | (CT)17 | F: CATTCATTAATCACTCTCACTTTTTG R: GAGTGATAGTTTGTCATAGAGTGACG | 178–194 | 9 | AF527034 |
| D370 | (TC)13(AC)21 | F: GTCAATCTCCTGTCCTGGAAAC R: CTTTATTTTTCTCGTTCTCCCTGTG | 140–144 | 3 | AF527035 |
| F9 | (CA)17 | F: GGAAGAAGTCAACTGTGCTGC R: CGCACTAACAGCAGGAAACA | 155–163 | 5 | AF527036 |
Statistical analyses
We compared P2 (the proportion of offspring within each brood sired by the second male to mate) across females in relation to several potential predictor variables. For each male we measured sperm concentration, oligopyrene/eupyrene sperm ratio, shell size, eupyrene and oligopyrene length, and assessed their effect on P2, using a generalized linear model (McCullagh & Nedler 1989). Because P2 data are proportional, they were analysed using logistic modelling with a logit link function and the size of each brood was taken into account. The final independent variable represented the difference between the explanatory variables of the first and second males (i.e. male 1 trait minus male 2 trait). Significance levels were determined from the changes in deviance of the null model after addition of the independent variables. The change in deviance was approximated by a χ2 distribution with corresponding degrees of freedom. Variables were entered with respect to their individual effect in the model (first the variable with the greatest individual effect and last the variable with the lowest individual effect). The effect of multiple mating on the number of offspring produced by a female was examined using an analysis of variance (anova, systat statistical package, Wilkinson 1989), and we used the Student's t-test to test the effect of the treatment on sperm characteristics. The relationship between the proportion of sperm and the size of the two types of sperm was analysed using a Pearson correlation coefficient. All data were screened to fit the assumptions of parametric analyses and transformed when required.
Of the 80 virgin females used in the mating experi-ments, our final sample size for the paternity analysis was reduced to 37 families. This was due to failure to produce offspring, the effects of a castrating parasite which meant that 24 of our males were infertile, and 18 cases in which paternity could not be assigned unequivocally because of amplification failure, or ambiguous banding patterns.
Results
Effects of treatment on sperm characteristics
The sex-biased treatment had no significant effect on the oligopyrene/eupyrene sperm ratio (male bias, OER = 0.65 ± 0.04; female bias, OER = 0.61 ± 0.4; t(69,63) = 0.761; P = 0.45), unlike a previous study (Oppliger et al. 1998), although the direction was the same. Treatment did influ-ence sperm concentration with males in the male-biased treatment producing more sperm of both types (e.g. sperm number/dilution (combining both types of sperm): male bias = 135.8 ± 7.8; female bias = 91.3 ± 4.7; t(69,63) = 4.79; P < 0.001; eupyrene sperm only, male bias = 82.5 ± 4.3; female bias = 58.1 ± 3.1; t(69,63) = 4.47, P < 0.001; oligo-pyrene sperm only, male bias = 53.4 ± 4.1; female bias = 33.2 ± 1.9; t(69,63) = 4.28, P < 0.001). There was also a pos-itive association between the number of the two sperm types within males (n = 132; r = 0.67; P = 0.001). Males kept in male-biased environments also had significantly longer oligopyrene sperm than males kept in female-biased environments (mean ± SE male bias = 127.5 ± 0.850 µm vs. female bias = 122.5 ± 0.803 µm; t(69,63) = 3.976, P = 0.0001) (Fig. 1), but eupyrene size did not differ between treatments (82.9 ± 0.2 vs. 82.9 ± 0.3; t(69,63) = 0.007; P = 0.99). There was also a positive correlation between number and length of oligopyrene sperm in the male-biased treatment (male biased: n= 69; r = 0.427; P = 0.002; female biased: n = 63, r = 0.184; P = 0.148), as well as between number of both sperm types and length of the oligopyrene sperm in the male-biased treatment (male biased: n = 69; r = 0.279; P = 0.019; female biased: n = 63; r = 0.193; P = 0.129). These results also differ from an earlier investigation which found evidence for a trade-off between oligopyrene size and number (Oppliger et al. 1998). Finally, because of body size differences between the treatment groups in this study, we looked at potential correlations between male size and sperm concentration and size (of both sperm types), but found no significant associations (all n = 132; all |r| < 0.14; all P > 0.1).
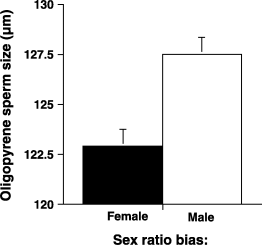
The size of the oligopyrene sperm from each of the sex ratio treatments. Sperm from the male bias cages were bigger.
To further investigate the apparent discrepancies between the two studies (Oppliger et al. 1998 and our study), we used standard meta-analysis techniques to combine the data sets and look at overall P-values (Rosenthal 1991). For the sperm ratio examination, we used test statistics from the June analysis in the first study (Oppliger et al. 1998) rather than those from the whole 4-month sampling period. This was done to ensure the two studies were directly comparable (i.e. we also sampled from June in the current investigation, and by June in both studies snails had been housed in the treatment groups for about the same time). This was also the most conservative approach, as later in our initial study the differences between sex ratio treatments became even more pronounced. First we calculated the one-tailed probabilities for the tests, converted these to a normal deviate Z-score and looked to see if the values differed significantly using two-tailed tests (Rosenthal 1991). For the sperm ratio comparison they did not differ significantly (Z = 1.237; P = 0.22), but for the size/number comparison across the studies they did (Z = 6.47; P < 0.0001), confirming that the two studies give contradictory results for the latter parameter. Z-scores were then combined and their significance tested (Rosenthal 1991). This analysis indicated that overall, sex ratio significantly changes the ratio of oligopyrene to eupyrene sperm (Z = 2.312; P = 0.021). As expected, however, there was no sign of an association between oligopyrene sperm size and number when the two data sets were combined (Z = 0.71; P = 0.48).
Pattern of P2
The proportion of offspring sired by the second male ranged from 0 to 100% (mean ± SD = 0.41 ± 0.38) and showed a bimodal distribution (Fig. 2). Twenty-one females produced multi-sired clutches and 16 produced single-sired clutches. Of the 16 single-sired clutches, 10 had been fathered by the first and 6 by the second male. In the single-sired group, there were no differences in male size determining who the successful male would be (P = 0.93).
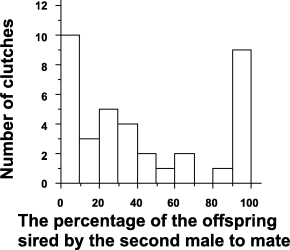
The pattern of P2 (the percentage of offspring sired by the second male to mate).
Predictors of siring success
This analysis initially included all females paired with two fertile males. Results from the logistic regression (Table 2) indicated that oligopyrene sperm length, sperm concentration and shell size were responsible for 24% of the variance in fertilization success. The length of oligopyrene sperm alone accounted for > 15% of the total variance. This means that, on average, the male with the longest oligopyrene sperm sired the most offspring. Sperm concentration (combined oligopyrene and eupy-rene sperm number/1.5-µL testis sample) and shell size also accounted for significant variation in fertilization suc-cess, but in a less important way (both were positively associated with fertilization success and accounted for 6 and 2.5% of the total variance in the model, respectively). When we restricted our analysis to only those females producing multiply sired clutches, oligopyrene sperm size explained > 38% of the models variance, and again the association was positive. Similarly, shell size and sperm concentration explained significant levels of variation in paternity, and the associations were also positive, but as before they explained less variance than oligopyrene size (Table 2).
| Source of deviance | df | Females housed with two fertile males (N = 37) | Females with a multi-sired clutch (N = 21) | ||
|---|---|---|---|---|---|
| Deviance (%) | Probability | Deviance (%) | Probability | ||
| Oligopyrene length | 1 | 15.25 | < 0.001 | 38.37 | < 0.001 |
| Total sperm# | 1 | 6.00 | < 0.001 | 10.59 | < 0.001 |
| Shell size | 1 | 2.47 | < 0.001 | 8.89 | 0.001 |
| Eupyrene length | 1 | 0.43 | 0.16 | 0.03 | 0.84 |
| OER | 1 | 0.39 | 0.18 | 1.67 | 0.15 |
| Residual | 30 | 75.65 | — | 40.43 | — |
- OER = the numbers of oligopyrene relative to eupyrene sperm, Total sperm = the number of sperm in 1.5 µL-testis sample. df = degree of freedom. Deviance is expressed as percentage of total.
Benefit of multiple mating
anova showed that females paired with two fertile males (noncastrated) produced significantly more offspring than females paired with only one fertile male (Mean ± SE, polyandrous 15.8 ± 0.8 vs. monandrous 12.2 ± 1.4; F(1,70) = 4.475; P = 0.038). This difference did not appear to be due to differences in female size because size did not differ between the two types of female (34.4 ± 0.4 vs. 34.1 ± 0.6 mm; t(21,16) = 0.38; P = 0.71). If we restricted the analysis to only those females which produced mixed clutches (i.e. we are certain they copulated with two males) and again compared number of offspring with females paired to a single fertile male, polyandrous females again produced more offspring (mean ± SE, polyandrous 17.4 ± 1.1 vs. monandrous 12.2 ± 1.4; F(1,37) = 7.12; P = 0.011).
Discussion
The main result of this study is that the size of the nonfertilizing oligopyrene sperm was the dominant correlate of male fertilizing success, with males that produced longer parasperm siring more offspring in competitive matings. This is true even when we conservatively restricted our analyses to those families in which we could be certain females mated with both males, and the effect was independent of mating order, all of which indicates oligopyrene sperm are likely to be important in sperm competition as previously suggested (Oppliger et al. 1998). However, OER was not significantly associated with fertilization success, even though previous work and the combined data suggested OER varies with sperm competition risk. It is possible that with the larger differences reported previously (Oppliger et al. 1998), a significant effect would be seen, and it is difficult to imagine why the ratio of the two sperm types would vary with sex ratio if OER had no influence on ejaculate competitiveness. Nevertheless, the exact role played by the oligopyrene sperm is unclear, and although they migrate to the females’ sperm stores with the eupyrene sperm, they do not persist there for long (≈ 7–15 days: AO personal observation). Because the association between oligopyrene size and paternity was independent of mating order, either the oligopyrene sperm have two functions, one defensive, the other offensive, or they may simply facilitate successful transfer of eupyrene sperm, and the efficiency of transfer is size dependent. Previously, both offensive and defensive functions have been suggested for parasperm (e.g. Buckland-Nicks 1998; Cook & Wedell 1999), and in some snails they secrete products which potentially create a hostile insemination site for subsequent males, and in some instances plug the female tract (Buckland-Nicks 1998), suggesting that offensive functions are possible. There are also data clearly indicating the size of amoeboid sperm influences fertilization success during sperm competition (Radwan 1996; LaMunyon & Ward 1998, 2002; see also Simmons et al. 1999). However, three recent evolutionary studies found no effect of sperm competition on sperm length in several sperm monomorphic insects (Hosken et al. 2001; Morrow & Gage 2001b; Pitnick et al. 2001).
It is also possible that females bias paternity toward males with longer oligopyrene sperm. There is ample evid-ence that sperm size correlates with aspects of female reproductive tract morphology across many taxa (e.g. Dybas & Dybas 1981; Morrow & Gage 2000; Minder 2002), and this is potentially due to female choice for longer sperm, but it may also be due to conflict over paternity. A final possible explanation for the sperm size effect is that snails producing larger sperm copulated more frequently or for longer giving them a numerical advantage during the sperm competition experiment. Although we cannot completely rule out this possibility, it seems unlikely because in the experimental manipulation of sex ratio, males from the male-biased treatment produced longer oligopyrene sperm, but because of the sex ratio bias, they were less likely to have copulated as frequently as males from the female-biased sex ratio. However, if they subsequently copulated more frequently because they had greater sperm reserves, the size/paternity association would be apparent. In any case, this line of reasoning points to a proximate explanation for the sperm concentration difference between treatments, males from the female-biased group copulated more frequently and were relatively sperm deleted.
There were two apparent discrepancies between this and a previous study of Viviparus ater that also varied sex ratio and looked at ejaculate characteristics. First, the ratio of the two sperm types did not differ statistically between sex ratio treatments here, but it did previously (Oppliger et al. 1998). However, the direction of change in our study was the same as that found previously, and using standard meta-analysis techniques, we found the studies’ results did not differ significantly and that overall, the effect was as reported previously: the OER increases with increasing number of competitors. The seeming difference between studies was probably due to the much shorter duration of our study. In any case, we found no effect of OER on fertil-ization success, although, as suggested above, there may be one once the OER becomes great enough. The second difference between the studies was that previously we found a negative association between oligopyrene size and number, but here we found the opposite. Theory predicts size/number trade-offs (Parker 1993), but they have rarely been found within species (but see Pitnick 1996). The results of this investigation cast serious doubt on the sig-nificance of our previous finding, and it is also difficult to interpret the positive association we found here in light of the trade-off reported earlier.
We also found that total sperm concentration significantly influenced fertilization success. This is in accordance with the most basic assumptions of sperm competition theory, that competition proceeds under the raffle principle, so males transferring more sperm fertilize more of a female's eggs (Parker 1984, 1998). It is difficult to say if the effect is driven by oligopyrene or eupyrene number as they correlate, but by using total sperm density rather than oligopyrene concentration alone, the effect does become stronger (e.g. across all females the variance explained increased from ≈ 5% to > 10%). This tentatively suggests that eupyrene numbers have a major effect. The sperm number and oligopyrene size effects are largely independent of each other as only males from the male-biased treatment showed an association, but it was fairly weak (r2 < 0.08). There was also a male size effect on paternity. This is not due to correlations between ejaculate or sperm size and male size, however. It may simply be that larger males are in better condition and are able to copulate for longer or more frequently, or as above, females may bias paternity in favour of larger males. We are also uncertain about why males were larger in the male-biased treatment, but it may relate to trade-offs between growth and mating. In addition, as with many studies of P2, we found a bimodal pattern of paternity. It has also been noted that high variance in P2 is often associated with low second male preced-ence (Simmons & Siva-Jothy 1998), which is precisely what we find here. In contrast to insects, however, where high P2 values are often associated with polyandry (Ridley 1989), we find relatively low P2 in our highly polyandrous snail.
Our data also indicate that females gain direct fitness benefits from polyandry. This result is in stark contrast to Bateman's (1948) study and does not appear to have been due to fecundity differences between females as fecundity typically increases with body size, but there were no size differences between monogamous and polyandrous females. However, in some insects elevated fecundity can occur via ejaculatory nutrients independently of body size (e.g. Gwynne 1984; reviewed in Hosken & Stockley 2002). As with the snails, a recent review of polyandry suggested direct fitness benefits to females is the major advantage to polyandry in insects (Arnqvist & Nilsson 2000; and see Ridley 1988). However, in our study it is not clear whether the increase in offspring number was due to increased fecundity, fertility or survival, or some combination of the three (also see Tregenza & Wedell 1998).
In conclusion, the size of the oligopyrene sperm was significantly correlated with fertilization success during sperm competition, although the exact reason for the association remains unclear. Nevertheless, this is one of few studies to find experimental evidence suggesting that sperm size is important during sperm competition, or that parasperm characteristics influence fertilization success. Male size and sperm concentration also influenced success during sperm competition, and as with a number of studies, females obtained direct benefits from polyandry.
Acknowledgements
We are grateful to R. Vitalis, V. Castella, N. Lugon-Moulin and C. Bouteiller for help in laboratory. We also thank Big Jérôme Goudet for statistical advise and he, Oliver Martin and two referees for kindly commenting on previous versions of the study. This work was supported by the Swiss National Science Foundation (grants to GR, YNG, AO & DJH).
References
This study is part of ongoing research on reproduction in V. ater. This work is headed by G. Ribi. A. Oppliger, Y. Naciri-Gracen & D. J. Hosken collaborate with the Ribi group and are broadly interested in evolutionary biology.




