Phylogeography and molecular rates of subterranean aquatic Stenasellid Isopods with a peri-Tyrrhenian distribution
Abstract
The subterranean Isopods belonging to the genus Stenasellus have an interesting disjunct distribution in the peri-Tyrrhenian area with morphologically closely related taxa occurring in Sardinia, Corsica, Tuscany and in the Pyrenees phreatic and interstitial waters. Because the dispersal capacities of these organisms are limited, their distribution has been associated traditionally with the tectonic events leading to the separation of the Sardinia–Corsica microplate from the Pyrenees and its subsequent movement towards the Italian peninsula. We sequenced a fragment of the mtDNA cytochrome oxidase I gene (COI) for multiple populations of the S. racovitzai species-group (Corsica, Sardinia, Tuscany) and S. virei (Pyrenees). We found that multiple phylogenetic analyses always gave the same topology, which is consistent with the genetic relations found using allozyme data, and with the palaeogeography of the area. The molecular data suggest that a combination of vicariance and dispersal events explain most effectively the present distribution pattern of these organisms. We also calculated COI rates and calibrated them against absolute time, taking advantage of the availability of two geologically based time estimates. Rates on all substitutions are similar to those published for other crustaceans for the same COI fragment, including taxonomically and ecologically distant groups. Rates on third codon positions or on transversions are generally lower than those found in other crustaceans.
Introduction
Subterranean Isopods belonging to the family Stenasellidae are among the most interesting palaeogeographical indicators. They are very rare and elusive inhabitants of phreatic waters discovered only in 1896. Until 1938 they were known only from subterranean waters of southern Europe, and knowledge of their actual distribution is still incomplete. They are highly adapted to subterranean life, spending their whole life in subsurface waters, such as porous ground water aquifers of river valleys and karst areas (stygobionts). Stenasellids have a discontinuous worldwide distribution. They are not related closely to any known surface taxa, and possess a suite of primitive morphological features. All the evidence suggests an extremely ancient separation from a presumed marine surface ancestor (Magniez 1974, 1981).
Because their ability to disperse through unsuitable ecological areas are very limited, it can be hypothesized reasonably that their present distribution largely reflects the palaeogeography of the occupied land masses rather than active dispersal. This is particularly evident for a group of species with a peri-Tyrrhenian distribution belonging to the genus Stenasellus. According to Magniez (1974) the S. racovitzai Razzauti, 1925 species-group, known for only six localities in Tuscany, Sardinia and Corsica (Ketmaier et al. 2000), is close morphologically to the Pyrenean species S. vireiDollfus (1897). The Pyrenean–Sardo–Corsican distribution is explained traditionally in vicariance terms following the tectonic events that led to the separation of the Sardinia–Corsica microplate from the Iberian Peninsula. Palaeomagnetic data indicate that the Sardinia–Corsica microplate split from the Pyrenean region 29 Myr ago. The separation of the two islands started as early as 20 Myr ago (Boccaletti et al. 1990) and was completed by 9 Myr ago (Fig. 1a, Alvarez 1972, 1974; Bellon et al. 1977; Bonin et al. 1979). The discontinuous distribution of these organisms in Sardinia is also explained in the context of the geological history of the area (Fig. 1b). Starting from the late Miocene cyclical sea introgressions separated the calcareous deposits of the island into isolated blocks (Fig. 1b; Cherchi & Montadert 1982). The presence of S. racovitzai in coastal Tuscany is likely to be associated with the interaction between the Corsica–Sardinia microplate and the Apennines, which were then being formed. This caused the emergence of the Tuscan Archipelago, including the islands that became incorporated later into the mainland (the so-called ‘fossil islands’, Fig. 1c; Lanza 1984). The presence of Stenasellus in Sardinia, Corsica and Tuscany could be the result of vicariance events dating back to some time in the Miocene (20 Myr ago) or up to the Messinian (5.5 Myr ago), when the land masses were reconnected during the Mediterranean salinity crisis (Esu & Kotsakis 1983; Boccaletti et al. 1990). Alternatively, the presence of Stenasellus in Tuscany could be due to active dispersal during the Quaternary (from 2 to 0.5 Myr ago), when lowering of sea levels led to the formation of a land bridge between Corsica, Sardinia and Tuscany (Lipparini 1976; Cherchi & Montadert 1982; Burgassi et al. 1983; La Greca 1990). These disjunct distributions are similar to those found in other groups of organisms with limited dispersal abilities and provide an opportunity to test the vicariance vs. dispersal scenarios in a wide variety of taxonomically unrelated groups (earthworms, stone flies, cave beetles and newts). Moreover, they allow calibratation of molecular rates against absolute time, as good estimates for the timing of several tectonic events in the Mediterranean are available (Caccone et al. 1994, 1997; Cobolli Sbordoni et al. 1992; Fochetti 1994; Caccone & Sbordoni 2001).

Schematic maps showing the location of the samples and the geological history of the region. (a) The movement of Corsica (C) and Sardinia (S) from the Iberian Peninsula. The square refers to the Tuscan area in enlarged in section c. (b) Map of Sardinia. Shaded areas are different Karst areas; double arrow indicates the Sardinian rift. (c) Fossil islands (stippled area) of Tuscany and Northern Latium (redrawn from Lanza 1984). The triangle indicates the only other Tuscan known locality for S. racovitzai.
The aims of this study are to produce a phylogeny for this group of rare organisms, to compare it with the relationships obtained using allozymes (Ketmaier et al. 1999, 2000), and to evaluate whether their distribution parallels the palaeogeography of the region. In addition, we want to compare molecular rates for the COI gene to rates for the same fragment in other Crustacea.
Materials and methods
Three individuals for each population were sequenced. Figure 1 shows the sampling localities and their three-letter codes. S. racovitzai was sampled in four of its six known locales. UCC is from a cave in Fontanile dei Cavalleggeri, Tuscany. The samples from southeastern (SE) Sardinia (QUI), northwestern (NW) Sardinia (PTO) and Corsica (COR) are from artificial wells. The samples of S. virei are from the subspecies hussoni from the Peyrot cave (Pyrenees, France). As outgroup we used Proasellus coxalis (Dollfus 1897) (River Aniene, Latium, PRO), a species within the family Asellidae, which is considered the closest relative to the family Stenasellidae (Magniez 1974). Population densities were similarly high for all the locations sampled.
Total DNA was extracted from frozen specimens using the Easy-DNA extraction kit from Invitrogen (Invitrogen). PCR amplifications were carried out using the protocols and the mitochondrial COI L6388 and H6951 primers reported in Theisen et al. (1995). The fragment of the COI we studied covers most of the range of variation found in the whole molecule (cf. Lunt et al. 1996). All individuals were sequenced in both directions using polymerase chain reaction (PCR) primers (420 base pairs (bp)). Sequences were edited using sequencher 3.1.1 (Gene Code Corporation) and aligned by eye. No indels were found. GenBank accession nos were AY028587–AY028592.
Aligned sequences were analysed by maximum parsimony (MP) (Farris 1970), maximum likelihood (ML) (Felsenstein 1981) and neighbour-joining (NJ) (Saitou & Nei 1987), as implemented in paup 4.0b8 (Swofford 2000). The robustness of the phylogenetic hypotheses were tested by 1000 bootstrap replicates (Felsenstein 1985). Bremer support index for the MP tree (Bremer 1988) was calculated using autodecay 4.0 (Eriksson 1999). Branch and bound searches (Hendy & Penny 1982) with the acctran character-state optimization were carried out with various weighting schemes: all substitution unweighted and down-weighting or excluding transitions. Maximum likelihood searches were run using the TVM + G (transversional model) with rates assumed to be variable and following a gamma distribution (shape parameter α = 0.2387). This model was the one that best fit our data according to the results obtained using modeltest (Posada & Crandall 1998). Heuristic searches were run using 100 random stepwise additions and the TBR branch-swapping algorithm. NJ analyses were carried out on Tamura & Nei (1993) distances (DT&N) calculated with the same empirically determined gamma parameter used for ML analyses. Competing phylogenetic hypotheses were tested using the Templeton (1983) test and the Shimodaira & Hasegawa (SH) test (Shimodaira & Hasegawa 1999), as implemented in paup* 4.0b8.
Rate homogeneity among species was tested using the 1D and 2D methods (Tajima 1993) and by using the maximum likelihood (ML) ratio test (Muse & Weir 1992).
Results and discussion
Levels and patterns of COI sequence variation
Different individuals of the same population have identical mtDNA sequences, thus we used a single sequence per population for our analyses. Percentages of variable sites range from 2.1% for the second codon positions to 85.7% for the third codon positions with an average value of 34.8% on all positions. The majority of parsimony informative sites are in third codon positions (45%), and in total 17.4% of changes were parsimony informative. As seen generally in mitochondrial DNA genes, this COI fragment is A + T rich, especially in third codon positions (65.2% all positions; 78.1% third codon positions). This pattern of nucleotide composition and sequence variation is similar to those reported in 33 other crustacean species analysed for the same COI fragment (Wetzer 2001).
In Table 1 the Tamura & Nei distances (DT&N) are reported together with the absolute number of substitutions (Tv + Ti) and the number of Tv changes between each species pair. Sequence divergence between the S. racovitzai and S. virei is remarkably high (0.720 ± 0.030 Tv + Ti; 0.170 ± 0.007 Tv only), and close to the levels found between these two species and P. coxalis (0.838 ± 0.028 Tv + Ti and 0.185 ± 0.047 Tv only). This is partly at odds with the close phyletic relationship between S. virei and S. racovitzai suggested by Magniez (1974) on the basis of the morphology of the exopod of the IV pleopod. The average sequence divergence within the S. racovitzai complex is also remarkably high for comparisons between populations assigned to the same nominal species (0.293 ± 0.061 Tv + Ti; 0.093 ± 0.017 Tv only). Indeed, they are of the same order of magnitude of the values obtained for interspecific comparisons among several Isopods species (Wetzer 2001). Granted that levels of sequence divergence should not be used as a direct indication of taxonomic status, the high divergence values found in this study raise the possibility of a taxonomic revision of the complex. A complete survey of all the species included in the genus, coupled with the analysis of a larger DNA data set, and the integration of the genetic and morphological data needs to be accomplished to properly address this aspect.
| Species | PEY | UCC | PTO | COR | QUI | PRO |
|---|---|---|---|---|---|---|
| PEY | — | 84/37 | 87/40 | 86/41 | 81/37 | 90/44 |
| UCC | 0.751 | — | 57/27 | 0/4 | 63/28 | 86/35 |
| PTO | 0.692 | 0.280 | — | 61/31 | 70/31 | 98/48 |
| COR | 0.735 | 0.009 | 0.310 | — | 67/32 | 89/39 |
| QUI | 0.765 | 0.342 | 0.444 | 0.377 | — | 88/35 |
| PRO | 0.789 | 0.819 | 0.946 | 0.846 | 0.793 | — |
Phylogenetic analyses and comparison with allozymes
The unweighted MP search produced a single most parsimonious tree 226 steps long (Fig. 2a). We obtained the same topology in all the MP analyses, regardless of the weighting scheme adopted. ML searches and NJ clustering produced trees with identical topologies. All three phylogenetic reconstructions have high bootstrap support values ranging from 72 to 100 over 1000 replicates. Similarly, Bremer support values for the MP tree are high (Fig. 2). Templeton's (1983) and Shimodaira & Hasegawa's (1999) tests indicate that the topology of the MP and ML trees (Fig. 2a) are significantly better than all other possible topologies (results available from the first author). Thus, the phylogenetic relationships obtained using this COI fragment are remarkably robust. The S. racovitzai complex is clearly a monophyletic group. This is in agreement with morphological analyses, which group these disjunct populations into the same phyletic lineage (Magniez 1974; Messana et al. 1995). Within S. racovitzai the two Sardinian populations are not each other's closest relatives. QUI, the population from SE Sardinia, is the sister taxon to a lineage which includes the NW Sardinian population (PTO) and the Corsican (COR) and Tuscan (UCC) populations.
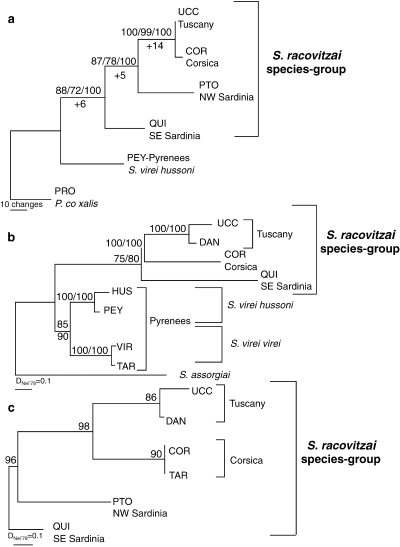
(a) Maximum parsimony (MP) tree based on mtDNA data (TL = 226; CI = 0.737). Values above and below branches indicate bootstrap percentages of 1000 replicates for MP, ML and NJ (first, second and third value) methods and the Bremer index (Bremer 1988). (b) Evolutionary relationships in the S. racovitzai and S. virei lineages based on allozymes. Values at nodes are bootstrap percentages of 1000 replications for ML (first value) and NJ (second value). (c) Unrooted NJ consensus tree based on Nei's D (Nei 1978) for all the known populations of the S. racovitzai species-group. Bootstrap percentages over 1000 replicates are reported.
Allozyme data are available for the same organisms, although not for exactly the same populations. Figure 2b (from Ketmaier et al. 1999) shows a dendrogram representing the genetic relationships among four populations of the S. racovitzai complex, and two other species within the same genus, S. virei (with two subspecies virei and hussoni) and S. assorgiai Argano, 1968. The tree in Fig. 2c summarizes the genetic relationships between all the known S. racovitzai populations, but does not include a outgroup (Ketmaier et al. 2000). Unfortunately, the three different data sets are not completely comparable because they do not share the same set of populations. However, it is interesting to note that within the S. racovitzai species-group the branching pattern recovered by the two data sets follows the same geographical scheme. The Corsican and Tuscan populations are always sister taxa (DNei’78 = 0.807 ± 0.139, Corsican vs. Tuscan populations; DNei’78 = 1.113 ± 0.134 Corsican and Tuscan vs. all Sardinian populations). The two Sardinian populations are not each other's closest relatives and the NW Sardinian population is the sister taxon of the Corsican–Tuscan clade (DNei’78 = 1.015 ± 0.055, NW Sardinia from Tuscany and Corsica; DNei’78 = 1.277 ± 0.024 SE Sardinia from the Corsican and Tuscan populations).
Molecular rates
Linearity of accumulation of substitutions over time was assessed by using two types of rate tests and and by taking advantage of the availability of absolute time estimates for two of the cladogenetic events in this group, the split of Corsica–Sardinia from the Pyrenees (29 Myr ago) and the separation of Sardinia from Corsica (20–9 Myr ago). A total of 96 Tajima (1993) tests was carried out, which included all the possible three taxa comparisons in the data set on all substitutions and on Tv only (in all positions and in third codon positions). We could not reject the null hypothesis of constant substitution rates at the P < 0.05 level (data available from first author). The ML ratio test produced similar results: the log likelihood from the ML analysis without enforcing a molecular clock was not significantly different from the log likelihood of the ML tree with a molecular clock enforced (χ2 = 5.270; d.f. = 5; 0.250 < P < 0.500).
We also checked the results of the Tajima and ML ratio tests against absolute times. We assume that the split of the Corsica–Sardinia plate from the Pyrenees is responsible for the separation of the S. racovitzai and S. virei lineages, and that the split between Corsica and Sardinia led to the isolation of the S. racovitzai populations on the two islands. If our mtDNA sequences were accumulating substitutions linearly over time we would expect to obtain similar sequence divergence estimates between Sardinian and Pyrenean and between Corsican and Pyrenean species. Indeed, levels of sequence divergence between the Pyrenean (PEY) and the two Sardinian populations (PTO/QUI) are quite close to each other and remarkably similar to the value between PEY and the Corsican population (DT&N= 0.692 and 0.765 for PEY vs. PTO and QUI; DT&N= 0.735 for PEY vs. COR, Table 1). It is important to emphasize that the time estimates obtained in this study are based on a biogeographical event and thus they must be considered as minimal estimates of divergence, because they do not have an upper and a lower bound as dating based on fossils might have. Thus, we cannot exclude the possibility that genetic divergence occurred some time before the geological events we are assuming were the causal factors behind the cladogenetic events. However, the fact that several taxa of organisms with limited dispersal abilities share a similar distribution points strongly to a causal relation between geological events and cladogenetic patterns. A similar logic has been used to justify biogeographical based datings in a diverse array of marine species across the Panama Isthmus (Knowlton et al. 1993; Schubart et al. 1998; Wares 2001).
Table 2 lists COI rates for the fragment studied here using 29 Myr as the dating of the divergence of the Pyrenean population (PYR) from the Corsican (COR) and Sardinian (PTO and QUI) populations and compares them with rates for the same gene fragment in other Crustacea. Interestingly, the rates on all substitutions for Stenasellus are remarkably similar to the rates in a variety of marine Crustacea and in two lineages of cave beetles inhabiting the same regions as the S. racovitzai–S. virei group. This similarity of rates holds among taxonomically (Cirripeda vs. Decapoda, Crustacea vs. Insects) and ecologically (marine vs. interstitial, aquatic vs. terrestrial) disparate groups. Rates based on a subset of substitutional changes vary by a factor of 2.7–5 and do not seem be associated with either taxonomy or ecological requirements.
| Lineage | Rate Tv + Ti | Rate Tv3rd + Ti3rd | Rate Tv3rd |
|---|---|---|---|
| Stenasellus | 1.25 | 0.91 | 0.46 |
| Cirripedia | |||
| Semibalanus spp.1 | — | 2.76 | 0.46 |
| Euraphia spp.1,2 | 1.5 | 3.8 | 1.37 |
| Decapoda | |||
| Sesarma spp.1,3 | 0.83 | 2.1 | 1.24 |
| Alpheus spp.1,4 | 1.2 | 1.9 | 0.74 |
| Cave beetles | |||
| Speonomus | 1.3 | 0.86 | 0.5 |
| Ovobathysciola | 1.2 | 0.78 | 0.7 |
- References: 1Wares & Cunningham (2001); 2Wares (2001); 3Schubart et al. (1998); 4Knowlton et al. (1993); 5Caccone & Sbordoni (2001).
Another way to take advantage of the absolute time estimates and to check for linearity of sequence evolution in our data set is to compare geological and molecular ratios. If substitutions accumulate in a linear fashion, then the ratio of the geological estimates for the separation of the land masses (dating of the split between Corsica–Sardinia from the Pyrenees/dating of the split between Corsica and Sardinia) should be similar to the ratio of the molecular distances (average distance between S. virei and all the S. racovitzai populations/average distances between the Corsican and Sardinian S. racovitzai populations). The ratios range from 1.5 to 3.2 (29/15–29/9) for the geological divergences and from 1.4 to 2.9 for the molecular distances, a result consistent with a linear accumulation of changes over time in this COI fragment. If the Pyrenean lineage of S. racovitzai (PEY) split from the Corsica–Sardinia–Tuscany clade (QUI–PTO–COR–UCC) 29 Myr ago, we can date the differentiation events between the Corsican and Sardinian taxa and see if they fall within the range of the geological estimates. Depending on the type of rate used (Table 2), time estimates for the split between the Sardinian (PTO) and Corsican (COR) populations range from 12 to 20 Myr ago. This result suggests that regardless of the type of rate used the differentiation between the Sardinian and Corsican/Tuscan clade occurred within the range of the geological estimates for the separation of the islands and that it predates the complete separation of the two islands (9 Myr ago). A similar time was obtained for the cladogenetic events leading to the separation of newts with the same distribution (Caccone et al. 1997). This result is in line with the consideration that geological datings are minimal estimates of genetic divergence and that separation of these taxa is likely to have started before the completion of the geological event.
We also used the mtDNA rates to provide times of divergences for the other cladogenetic events in the Stenasellus phylogeny that have been associated with geological events but have uncertain dating: the split between between Proasellus and Stenasellus, the separation of the two Sardinian taxa (QUI and PTO) and the split between the Corsican and Tuscan populations (COR and UCC).
Using the COI rates in Table 2 the divergence between the two genera occurred at least 33–29 Myr ago. We do not have the proper taxon sampling scheme to address this question, which is peripheral to the scope of this study. However, it is interesting to note that the Miocene time was an intense orogenetic period in Europe (Steininger & Rögl 1984), which might have played a role in the separation of the two genera. This implies that the separation of the Pyrenean and Sardinian–Corsican Stenasellus might have happened almost at the same time as the divergence of the whole genus from Proasellus.
Within Stenasellus the two Sardinian populations separated 17–22 Myr ago, according to the rates in Table 2. This timing is in agreement with the Miocene sea introgressions which formed a geographical barrier that split Sardinia into two distinct calcareous blocks (Fig. 1c; Cherchi & Montadert 1982; Boccaletti et al. 1990). All Stenasellus populations have always been found to be associated with limestone areas. These areas are surrounded by granite rocks, which isolate the different water systems in the area. Between the northern and southern Sardinian populations there is a deep and old break in the limestone deposits (Fig. 1b), which makes it impossible for this type of organisms to disperse actively.
Our estimates for the split between the Corsican (COR) and Tuscan (UCC) populations are more recent than those for the Sardinian populations, ranging from 2 to 0.4 Myr ago, between the Donau and the Mindel glaciations during the Quaternary period (La Greca 1998). This result supports the idea that the presence of Stenasellus in coastal Tuscany is not due to an old vicariance event but is rather the result of active dispersal. Enlarged fluvial basins (a condition ideal for dispersal of interstitial organisms) during interglacial periods could have facilitated dispersal through a continuous hydrographic system on the land bridge that connected these regions during the Quaternary (Giusti 1976; Lipparini 1976; Cherchi & Montadert 1982; Burgassi et al. 1983; La Greca 1990).
Phylogeographical scenario and biogeographical considerations
The phylogenetic relationships presented in this study are in remarkable agreement with the known geological history of the area. The Corsican and Sardinian populations are related more closely to each other than to the Pyrenean species, as expected on the basis of the history of the land masses they occupy. The deep divergence of the two populations from SE and NW Sardinia is also in good agreement with the history of sea regressions, that in Miocene–Pliocene times inundated the rift that crosses Sardinia from North to South producing a powerful geographical barrier for freshwater organisms with poor dispersal capacities (Fig. 1b). A comparable pattern of genetic divergence has also been found in other organisms from Sardinia with similar distributions and limited dispersal abilities, such as freshwater populations of the Proasellus coxalis species group (Ketmaier et al. 2001) and cave beetles (Caccone & Sbordoni 2001). The close phyletic relationships between the populations from NW Sardinia, Corsica and Tuscany also reflect the geology and the well-known connections between Sardinian, Corsican and coastal Tuscan faunas (Lanza 1984). A similar situation occurs in some other stygobiont taxa, such as the harpacticoid Parapseudoleptomesochra minoricae (Chappuis & Rouch, 1960) and the amphipod Bogidiella chappuisi Ruffo, 1952, which share the same disjunct distribution, and similar ecological requirements to Stenasellus. This parallel distribution pattern (Sardinian and Tuscan islands and coasts) has led to the recognition of a Sardinian stygofaunal province that also includes the insular and coastal areas of Tuscany (Pesce 1985).
In conclusion, we suggest that a combination of vicariance and dispersal events need to be invoked to explain the distribution of these taxa and their patterns of genetic differentiation. Vicariance can explain the separation of the Pyrenean populations from the Corsican and Sardinian ones, and can also be the main process responsible for the separation of the SE and NW Sardinian populations. However, we need to invoke active dispersal to explain the low levels of genetic divergence between the Corsican and Tuscan populations. However, is it reasonable to hypothesize active dispersal for organisms with very strict ecological requirements and very limited dispersal abilities? According to Magniez (1981), Stenasellids are adapted to live in all types of subterranean waters. Although they can be found in free water, they live primarily in interstitial waters. In such environments they can dig ramified galleries and, if continuous, migrate and extend their settlement. During the Pleistocene and later on in the Quaternary the lowering of sea levels determined water captures between rivers flowing into the Tyrrhenian Sea (Bartolini & Pranzini 1981). This created a favourable environment for dispersal of different phreatobic organisms, including a number of stygobionts such as copepods, ostracods, amphipods, and syncarids, which share the same disjunct distribution as Stenasellus, and have comparable ecological requirements. However, this still does not explain why Stenasellids arriving at the Tuscan Archipelago did not disperse any further and why Stenasellids are known for only two locations in Tuscany (see Fig. 1c). A possibility we cannot discount is that Stenasellids might have been more widespread at some time in the past and became extinct when the fossil islands were incorporated into the mainland because of the higher competitive pressures they had to face, moving from a continental habitat from an insular one (Lanza 1984). This could be the reason why their range is so small even in the fossil island, where these isopods are still present. At the same time, it should also be considered that these organisms are not common and easy to collect and it could be that further surveys in Tuscany could surprise us with new localities of these elusive organisms. However, it needs to be noted that in the past 10 years both the Sardinian and Corsican coast have been surveyed intensively for interstitial fauna (Messana & Argano, pers. com.) and no new locations for these organisms have been reported.
Acknowledgements
Dr Claude Bou and Dr G. Messana helped us with the sampling of Corsican and French specimens. Etsuko Moryama provided the MEA computer package to perform Tajima's tests. We are grateful to Jeff Powell for support and valuable suggestions on a draft of this paper. The authors are also grateful to Saverio Vicario for analytical suggestions. We also thank Cliff Cunningham for providing COI distances for the Crustacea species listed in Table 2. VK was supported by a Consiglio Nazionale delle Ricerche Fellowship. AC is supported by YIBS funds.
References
Valerio Ketmaier is a postdoctoral researcher working on molecular systematics and population genetics of a variety of organisms to infer current and historical processes at various temporal and spatial scales especially in small isolated populations. Roberto Argano is Professor of Zoology and his research focuses on systematics and biogeography of terrestrial and aquatic Isopods. Adalgisa Caccone heads the YIBS — Molecular Systematics and Conservation Genetics Laboratory and works on a variety of evolutionary genetic problems including island biogeography, population genetics of mosquitoes and conservation genetics of giant tortoises.




