Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone
Abstract
Chytridiomycosis is a recently identified fungal disease associated with global population declines of frogs. Although the fungus, Batrachochytrium dendrobatidis, is considered an emerging pathogen, little is known about its population genetics, including the origin of the current epidemic and how this relates to the dispersal ability of the fungus. In this study, we use multilocus sequence typing to examine genetic diversity and relationships among 35 fungal strains from North America, Africa and Australia. Only five variable nucleotide positions were detected among 10 loci (5918 bp). This low level of genetic variation is consistent with the description of B. dendrobatidis as a recently emerged disease agent. Fixed (i.e. 100%) or nearly fixed frequencies of heterozygous genotypes at two loci suggested that B. dendrobatidis is diploid and primarily reproduces clonally. In contrast to the lack of nucleotide polymorphism, electrophoretic karyotyping of multiple strains demonstrated a number of chromosome length polymorphisms.
Introduction
Emerging infectious diseases of wildlife are newly recognized or expanding diseases that threaten biodiversity and ecosystem function. Most emerging wildlife diseases are believed to result from anthropogenic disturbances such as ‘spill-over’ of infectious agents from humans or domestic animals, human-mediated host or parasite translocations, or climate change (Daszak et al. 2000). If their origins are related to disturbance, emerging pathogens may display nonequilibrium population genetics because their geographical or host range has expanded. For example, recent expansion has been invoked to explain low levels of detectable genetic variation in emerging diseases of humans (Sreevatsan et al. 1997; Achtman et al. 1999).
Chytridiomycosis, a disease of amphibians caused by the chytrid fungus Batrachochytrium dendrobatidis, has been implicated as the cause of rapid declines of amphibian populations in pristine and disturbed habitats in Central America, the western United States, Spain and Australia (Berger et al. 1998; Daszak et al. 1999; Lips 1999; Bosch et al. 2001; Bradley et al. 2002). The fungus, which infects keratinized epidermal cells, can cause irregular epidermal hyperplasia and hyperkeratosis, but reasons for death are uncertain (Berger et al. 1998; Pessier et al. 1999). The pathogen has been found in museum specimens collected as early as 1974 in the United States (Carey et al. 1999) and 1978 in Australia (Speare et al. 2001). The recent discovery of the disease (Berger et al. 1998; Pessier et al. 1999) and increasing recognition of its widespread occurrence qualifies chytridiomycosis as an emerging infectious disease (Daszak et al. 1999).
Batrachochytrium is a member of the fungal phylum Chytridiomycota in the order Chytridiales. Chytrids produce motile, aquatically distributed zoospores that typically possess a single posterior flagellum. Axenic culture of Batrachochytrium has made developmental (Longcore et al. 1999) and experimental studies possible, including fulfilling Koch's postulates (Nichols et al. 2001). Sexual reproduction in chytrid fungi is associated with the production of a resistant, thick-walled resting spore in ‘all well-authenticated occurrences’ (Sparrow 1960). For B. dendrobatidis, no sexual or asexual resting structures have been observed on the amphibian host or in culture. The possession of a resting stage could confer the ability to persist in the absence of amphibians and to be transported by wind, perhaps explaining the widespread distribution of the disease in relatively pristine areas (Berger et al. 1998; Daszak et al. 1999).
One hypothesis for the emergence of chytridiomycosis is recent human introduction of the fungus into naïve populations (Berger et al. 1999; Daszak et al. 1999). Alternatively, or in concert, climate change or pollutants may have altered a pre-existing host–parasite relationship (Daszak et al. 2000; Blaustein & Kiesecker 2002). In this study, we investigate the genetic diversity of this pathogen using multilocus sequence typing (MLST) to test these two hypotheses. The prediction for a disease that has emerged because of recent introduction or translocation is that of lower geographical population structure and lower overall genetic diversity than would be found if the disease has emerged because of extrinsic factors such as environmental change. We also used the MLST data to test whether B. dendrobatidis reproduces clonally or sexually.
Materials and methods
Strains and cultivation techniques
Our sample consisted of 35 strains isolated from 19 different species (Table 1). The majority of the amphibian hosts sampled displayed clinical signs of chytridiomycosis. Strains were isolated (Longcore et al. 1999) primarily from amphibians from North America, but include strains from Africa, Australia and Panama. Due to the difficulty in obtaining and isolating the fungus, a more robust population sample was unavailable. However, our sample included strains representing all areas from which the fungus has been cultured. Strains were maintained in glass culture tubes in tryptone–glucose (TG) broth (Longcore et al. 1999). Stock cultures were stored at 5–6 °C and transferred at 4–5-month intervals. Additional information about the strains is available from the authors upon request.
| Strain | Origin | Host | ctsyn1 | aprt13 | lsu35 | r6046 | MLST |
|---|---|---|---|---|---|---|---|
| JEL197 | National Zoological Park, DC, USA | Dendrobates azureus | (A/G)* | (A/G)† | (G)‡ | (CA/–)§ | 1 |
| JEL198 | National Zoological Park, DC, USA | Dendrobates auratus | A/G | A/G | G | CA/– | 1 |
| JEL213 | Mono Co., California, USA | Rana muscosa | A/G | A/G | G | CA/– | 1 |
| JEL214 | Mono Co., California, USA | Rana muscosa | A/G | A/G | G | CA/– | 1 |
| JEL215 | Mono Co., California, USA | Rana muscosa | A/G | A/G | G | CA/– | 1 |
| JEL226 | Yavapai Co., Arizona, USA | Rana yavapaiensis | A/G | A/G | G | CA/– | 1 |
| JEL229 | Montrose Canyon, Arizona, USA | Hyla arenicolor | A/G | A/G | G | CA/– | 1 |
| JEL230 | Montrose Canyon, Arizona, USA | Rana yavapaiensis | A/G | A/G | G | CA/– | 1 |
| JEL260 | Quebec, Canada | Rana catesbeiana | A/G | A/G | G | CA/– | 1 |
| JEL262 | Quebec, Canada | Rana catesbeiana | A/G | A/G | G | CA/– | 1 |
| JEL264 | Quebec, Canada | Rana catesbeiana | A/G | A/G | G | CA/– | 1 |
| JEL277 | Arizona, USA | Ambystoma tigrinum | A/G | A/G | G | CA/– | 1 |
| JEL284 | Wisconsin, USA (captive) | Rana pipiens | A/G | A/G | G | CA | 2 |
| JEL289 | Milford, Maine, USA | Rana pipiens | A/G | A/G | G | CA | 2 |
| JEL231 | Mesquite Wash, Arizona, USA | Rana yavapaiensis | A/G | A/G | A/G | CA | 3 |
| JEL254 | Orono, Maine, USA | Rana pipiens | A/G | A/G | A/G | CA/– | 4 |
| JEL258 | Orono, Maine, USA | Rana sylvatica | A/G | A/G | A/G | CA/– | 4 |
| JEL203 | Bronx Zoo, New York, USA | Dyscophus guineti | A/G | A/G | A | — | 5 |
| JEL270 | Point Reyes, California, USA | Rana catesbeiana | A/G | A/G | A | — | 5 |
| JEL271 | Point Reyes, California, USA | Rana catesbeiana | A/G | A/G | A | — | 5 |
| JEL282 | Toledo Zoo, Ohio, USA | Bufo americana | A/G | A/G | A | — | 5 |
| 00 545 | Melbourne, Victoria, Australia | Litoria lesueuri | A/G | A/G | A/G | — | 6 |
| 98 1810 3 | Tully, Queensland, Australia | Nyctimystes dayi | A/G | A/G | A/G | — | 6 |
| 99 1385 12 | Rockhampton, Queensland, Australia | Litoria caerulea | A/G | A/G | A/G | — | 6 |
| PM5 | Panama | Smilisca phaeota | A/G | A/G | A/G | — | 6 |
| PM7 | Panama | Smilisca phaeota | A/G | A/G | A/G | — | 6 |
| JEL253 | Melbourne, Victoria, Australia | Limnodynastes dumerilii | G | A/G | A/G | — | 7 |
| JEL225 | Wisconsin, USA (captive) | Silurana (Xenopus) tropicalis | G | A/G | G | — | 8 |
| JEL239 | Imported from Ghana | Silurana (Xenopus) tropicalis | A/G | A/G | G | — | 9 |
| JEL240 | Imported from Ghana | Silurana (Xenopus) tropicalis | A/G | A/G | G | — | 9 |
| JEL245 | Imported from Ghana | Silurana (Xenopus) tropicalis | A/G | A/G | G | — | 9 |
| JEL273 | Clear Creek Co., Colorado, USA | Bufo boreas | A/G | A/G | G | — | 9 |
| JEL275 | Clear Creek Co., Colorado, USA | Bufo boreas | A/G | A/G | G | — | 9 |
| PM1 | Panama | Eleuthodactylus caryophyllaceum | A/G | A/G | G | — | 9 |
| JEL274 | Clear Creek Co., Colorado, USA | Bufo boreas | A | A/G | G | — | 10 |
- * Genotype at base 400; A/G (adenine and guanine) indicates heterozygote at this position; GenBank accession no. = BH001044.
- † Genotype at base 679; GenBank accession no. = BH001045.
- ‡ Genotype at base 315; GenBank accession no. = BH001046.
- § Genotype between bases 499–500; CA = two basepair insertion, —= strains that lack the insertion, CA/– = heterozygote at this position; GenBank accession no. = BH001047.
DNA extraction
For DNA harvest, Petri-plates of TGhL agar medium (Longcore et al. 1999) were inoculated with 0.5–1 mL of stock culture in broth, allowed to dry in a laminar flow hood, sealed and incubated at 23 °C for ∼2 weeks. Sporangia and zoospores were harvested by scraping from the plate surface. Tissues were dehydrated, and DNA was extracted from ∼20 mg dry weight, following a CTAB miniprep procedure (Zolan & Pukkila 1986).
Library construction and sequencing
An incomplete genomic library of strain JEL197 (type strain) was created for the generation of molecular markers from random DNA sequences. DNA was partially digested with Sau3A and 500–1500 base pairs (bp) restriction fragments were isolated from an agarose gel and ligated into plasmid pZERO (Invitrogen). Plasmids were propagated in Escherichia coli strains XLI-Blue (Stratagene) and INVa1F (Invitrogen). Plasmid templates for DNA sequencing were prepared with the QIAprep Spin Miniprep Kit (Qiagen) and sequenced on both strands using universal forward and reverse M13 primers. Sequencing reactions were accomplished with the BigDye sequencing kit (Applied Biosystems) and analysed on an ABI3700 DNA sequencer.
Primer design
We sequenced 33 clones from the genomic library (average insert size ∼950 bp). Polymerase chain reaction (PCR) primer pairs were designed to amplify eight of the cloned DNA regions using the software package Primer3 (Rozen & Skaletsky 1997). We focused primarily on clones that had significant matches to GenBank, under the assumption that coding regions were more likely to amplify single, homologous gene regions in contrast to noncoding regions that might be redundant or repetitive. Putative gene regions and P-values from blast searches were: cysteinyl tRNA synthase (ctsyn1; P = 3 × 10−13), anthranilate phosphoribosyltransferase (aprt13; P = 5 × 10−6), and 60S ribosomal protein (r6046; P = 6 × 10−13). Other targeted gene regions matched unidentified genes (bdc42; P = 10−12, uorf48; P = 5 × 10−5) or did not show significant matches (bdc3, bdc33, rnap50). In addition, we examined sequences in GenBank to design primers to amplify translation elongation factor 1α (tef1) and the nuclear subunit ribosomal RNA gene (lsu35). The sequences for the 33 clones have been deposited in GenBank (accession nos BH001009-BH001047).
PCR conditions and DNA sequencing
We amplified gene regions with reaction conditions (Vilgalys & Hester 1990) that consisted of: ∼1 ng template DNA, 1.5 mm MgCl2, 0.2 mm each dNTP, 0.5 µm each primer and 0.625 U Taq polymerase (Applied Biosystems) in a 25-µL reaction. Thermal cycling parameters were an initial denaturation step at 94 °C for 3 min followed by 35 cycles of denaturing at 94 °C for 1 min, annealing at 50 °C for 30 s, extension at 72 °C for 1 min, and a 7-min final extension at 72 °C. For locus aprt13, the annealing temperature was raised to 55 °C. PCR amplicons were purified with ULTRAfree-MC centrifugal columns (Millipore Corp.), except for aprt13, which was purified from an agarose gel with a QIAquick Gel Extraction Kit (Qiagen). Amplicons were labelled with the BigDye kit and electrophoresed on an ABI3700. The following primers were used for PCR amplification (convention used is locus name followed by F and R designations for forward and reverse primers): ctsyn1F (5′-ACCAACTATAACATCATCAAG-3′), ctsyn1R (5′-CGAATATCAGTCAACGCAAGC-3′), aprt13F (5′-GTCAGGGTTGGCTATTGTTCT-3′), aprt13R (5′-TGCTACTATTGCTGCTGTTGC-3′), lsu35F (5′-ATCCCTGTGGTAACTTTTCTG-3′), lsu35R (5′-ACGGACATGGGGAATCTGACT-3′), r6046F (5′-CTATCTGCGCTCCCGTGTCAA-3′), r6046R (5′-AGGGCTGCAACAACTGGATTT-3′), uorf48F (5′-TCGAGGTGCAGACAAAACTTC-3′), uorf48R (5′-CAAACTGAGCCACAATAATGC-3′), rnap50F (5′-AATCCTATCCACCAGTTTCAG-3′), rnap50R (5′-TAACGATGAACGCCTTGTAGA-3′), bdc3F (5′-TTCTGCTGCAAGAATCATCG-3′), bdc3R (5′-AGTAGAAGCGGGTCGTTGAA-3′), bdc33F (5′-ATAGACCTTCGGGCTCTGGT-3′), bdc33R (5′-TTTCGTGTTAACCCAAAGGC-3′), bdc42F (5′-GGCCAACTTGTTGGATTTGT-3′), bdc42R (5′-TTGGAGCTCTGGTTCGACTT-3′), tef1F (5′-TACAARTGYGGTGGTATYGACA-3′), tef1R (5′-ACNGACTTGACYTCAGTRGT-3′).
Southern hybridization
Southern capillary transfers were carried out according to Ausubel et al. (1998). Probes were labelled with digoxigenin by PCR amplification (Roche). DNA hybridizations were performed in DIG Easy Hyb buffer (Roche) at 42 °C, with post-hybridization washes (65 °C) and chemiluminescent detection as per the manufacturer's instructions (Roche).
Pulsed field gel electrophoresis
Chromosomal DNA was prepared by a modification of the method of Iadonato & Gnirke (1996). Briefly, zoospores were harvested from plate cultures by flooding with water, centrifuged, washed in 50 mm EDTA (pH 8.0) and mixed with an equal volume of 2% LMP agarose to give 109 zoospores/mL. Plugs were cast and incubated in spheroplasting solution containing 1 m sorbitol, 20 mm EDTA (pH 8.0), 10 mm Tris-HCl (pH 7.5), 14 mmβ-mercaptoethanol, 2 mg/mL Zymolase (Seikogaku) and 5 mg/mL lysing enzyme (Sigma) at 37 °C for 5 h. Plugs were incubated in LDS buffer (100 mm EDTA, 10 mm Tris-HCl, 1% sodium dodecyl chloride, pH 8.0) at 37 °C overnight. Three washes for 30 min each at RT in NDS buffer (0.1 m EDTA, 2 mm Tris-HCl, 0.2% sarkosyl, pH 8.0) were followed by five 30-min washes in TE buffer. Chromosomes were separated with the CHEF DR-II pulsed field gel system (Bio-Rad) on a 1% agarose gel with the following parameters: 5.0 V/cm with a 100–200-s ramped switch time for 24 h followed by 2.9 V/cm with a 190–300-s ramped switch time for 24 h, all in 0.5 × TBE buffer at 10 °C.
Data analysis
A tree-like network connecting multilocus genotypes was calculated with statistical parsimony using the software package tcs v1.13 (Clement et al. 2000). The pairwise distance matrix between isolates was calculated by considering each locus as a separate character and the three possible genotypes at each locus as unique character states. The distance between the two homozygous genotypes at a locus was assumed to be two steps, and heterozygous genotypes were assumed to be one step from each homozygous type.
Results
Nucleotide variation
We screened for sequence variation in B. dendrobatidis by PCR/DNA sequencing of 10 gene regions (eight anonymous loci lsu35 and tef1). Direct sequencing of PCR amplicons from 35 strains revealed few polymorphic sites among the 10 loci (5 variable sites in 5918 nucleotides). Six of the 10 loci showed zero nucleotide substitutions among all 35 samples (bdc3, bdc33, bdc42, uorf48, rnap50, tef1; 3551 nucleotides). The four other loci contained one or two variable sites. More than one allele was present in many strains. A site with a double-peak in the sequence chromatogram indicated this heterogeneity within a PCR product. These double-peaks are referred to hereafter as ‘heterozygous sites’; the justification for this terminology is given below. We found five heterozygous sites: two at ctsyn1, and one each at aprt13, lsu35 and r6046. Four of the five nucleotide heterozygosities are A ↔ G transitions, none of which are predicted to cause a change in amino acid sequence in the most probable translations. The fifth heterozygosity, at locus r6046, is a 2-bp indel in a noncoding region. The five variable positions displayed both heterozygous and homozygous genotypes, and the combination of genotypes over the four variable loci formed 10 multilocus sequence types (MLSTs), shown in Table 1.
The frequency of heterozygous genotypes in the population sample varied but was as high as 100% (aprt13). At locus ctsyn1, 32 of the 35 strains were heterozygous (∼91%). Two individuals of one homozygous type and one individual of the other homozygous type were found (Fig. 1). At loci r6046 and lsu35, both types of homozygotes were detected and heterozygous genotypes were in the minority, 40% for r6046 and ∼26% for lsu35.
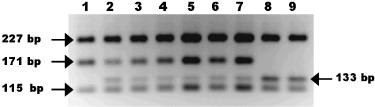
Digestion of ctsyn1 PCR amplicons with the restriction enzyme Fnu4HI reveals heterozygosity for most individuals at this locus. Fnu4HI cuts the 551-bp amplicon of one allele (homozygote in lane 1) into 227-bp, 171-bp, 115-bp and 38-bp fragments. Substitution of A for G in the other allele creates an additional Fnu4HI site in the 171-bp fragment, generating 133-bp and 38-bp fragments (homozygotes in lanes 8 and 9). Individuals in lanes 2–7 are heterozygous at this locus for the Fnu4HI RFLP. Lanes 1–9 are: JEL274, JEL197, JEL213, JEL230, JEL264, JEL284, JEL258, JEL225, JEL253. Reverse image of a 3% agarose gel stained with ethidium bromide is shown.
We confirmed that the two alleles at the putatively heterozygous loci were present in the amplified PCR products rather than being artefacts of the fluorescent DNA sequencing procedure by subcloning and sequencing PCR products from one or two strains for each of the four loci. Each subclone (n = 8–12) possessed separately one of the two possible alleles at each polymorphic site, and each PCR product showed segregation of the polymorphism among subclones, suggesting that the B. dendrobatidis strains possess two different alleles in their genomes. Additionally, restriction digests of some PCR amplicons demonstrated the presence of two alleles at the polymorphic loci. For example, locus ctsyn1 displayed a restriction fragment length polymorphism (RFLP) and digestion of these amplicons with the enzyme Fnu4HI showed that whereas most strains possessed two alleles, strains with only one of the two alleles were also present, confirming genotype assignments based on DNA sequencing (Fig. 1).
We confirmed that these loci are present in a single copy in the genome by hybridizing probes of the polymorphic loci to DNA digested with three different restriction endonucleases. With the exception of locus lsu35, only a single band hybridized to these probes under stringent conditions (Fig. 2). For locus lsu35, the large subunit rDNA gene, additional, weaker bonds were observed with most endonucleases. These data suggest that the presence of two alleles within a strain is probably a result of heterozygosity at a single locus and, therefore, B. dendrobatidis is likely of diploid or higher ploidy.
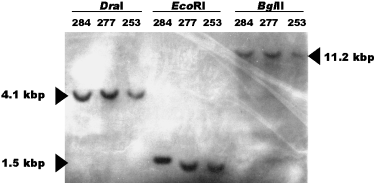
Southern hybridization of genomic DNA digests of three B. dendrobatidis strains to a probe for locus r6046. In each lane, a single hybridization signal is observed. Numbers above lane refer to strain number, and the restriction endonucleases used are indicated.
Population structure
We analysed genetic relationships among strains with statistical parsimony to determine whether the MLST data reveal any evidence for population substructure (Fig. 3). This network groups strains with related MLSTs such that each branch represents a change from a heterozygous state to a homozygous one at a single locus. In general, strains collected from the same locale displayed the same MLST (Table 1 and Fig. 3). For example, strains isolated from Rana muscosa from the Sierras in California are in MLST no.1 (JEL 213, 214 and 215). The other two California strains, which were isolated from R. catesbeiana collected from Point Reyes, are both in MLST no. 5. The strains from Africa, Australia, Panama and Colorado also demonstrate geographical relatedness. In each example, the strains within each geographical region are identical or differ from each other by the loss of heterozygosity at only a single locus (i.e. are connected by single branches in Fig. 3). Interestingly, MLST no. 6 is comprised of two Panamanian strains and three Australian strains, showing high genetic relatedness of certain strains from two areas for which chytridiomycosis has been implicated in amphibian declines (Berger et al. 1998). In general, the MLSTs show no clear global pattern of genotype distribution. However, the most frequently collected genotype in North America (MLST no. 1) was not recovered outside the continent. Moreover, the allele at locus r6046 containing the 2-bp insertion (‘CA’ in Table 1) has never been recorded outside North America, wherein its frequency is 58%. Genotype frequencies at r6046 are significantly different (P < 0.001; χ2 test) between North America isolates vs. isolates collected from outside North America. This result is also observed at lsu35 (P < 0.05).
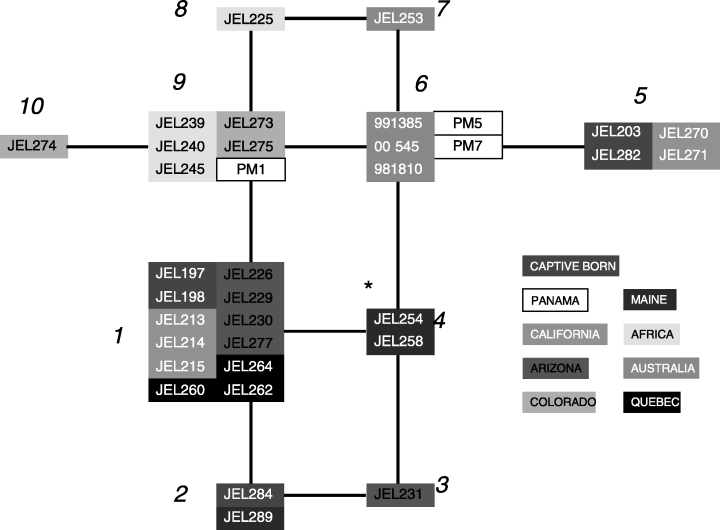
Depiction of MLSTs as a network of related genotypes. Isolates with the same MLST are grouped into abutting boxes and labelled according to MLST numbers in Table 1. Each branch interconnecting groups represents a change in genotype at a single locus. All changes can be regarded as the change from a heterozygous to a homozygous state. *Strains that are heterozygous at all four loci. Network estimated using the statistical parsimony algorithm as implemented in the TCS software package (Clement et al. 2000).
Chromosome polymorphism
We karyotyped several B. dendrobatidis strains with CHEF gel electrophoresis. Chromosomes ranged in size from ∼0.7–6.0 Mb, and numerous chromosome length polymorphisms existed among strains (Fig. 4). The estimated genome size is 35–40 Mb, although this estimate is difficult as the chromosomal bands stained at different intensities. If B. dendrobatidis is diploid, the intensely staining chromosomal bands may reflect comigrating homologous chromosomes, and length polymorphisms may reflect homologous chromosomes that vary in size, such as reported for Candida albicans (Iwaguchi et al. 1990). In this case, the genome size estimate would be between the true haploid and diploid genome size.
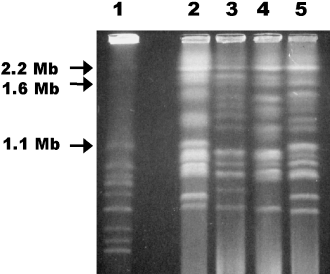
Separation of B. dendrobatidis chromosomes with CHEF gel electrophoresis shows multiple chromosome length polymorphisms. Lane 1 =Saccharomyces cerevisiae standard; lane 2 = JEL197; lane 3 = JEL245; lane 4 = JEL253; lane 5 = JEL289. Sizes of the largest S. cerevisiae chromosomes are indicated to the left of the photograph.
Discussion
This study demonstrated a low level of genetic variation within an intercontinental sample of B. dendrobatidis strains. Among the 10 loci surveyed only five variable sites were detected. For each of the loci that demonstrated variation heterozygous isolates were detected, and at two of the loci the majority of strains were heterozygous. From these data we conclude: our strains of B. dendrobatidis have the population genetic signature of a newly emerged pathogen, the fungus is probably diploid and reproduction is primarily clonal.
The low level of genetic variation among strains of B. dendrobatidis from North America, Central America, Africa and Australia implies that the coalescence time of the entire sample is relatively recent and that the fungus has become dispersed geographically over a time scale during which few mutations have occurred. Comparable data from other fungal pathogens are not extensive. Similar to B. dendrobatidis, other pathogenic fungal species such as Trichophyton rubrum (Gräser et al. 1999) and Fusarium oxysporum f. sp. ciceris (Jiménez-Gasco et al. 2002) have demonstrated an absence of nucleotide polymorphism among several nuclear loci. Nonetheless, population studies with other fungi generally reveal much greater genetic variation than that observed here (e.g. Forche et al. 1999; O’Donnell et al. 2000; Xu et al. 2000).
Low levels of polymorphism suggest at least four possible, but not mutually exclusive, explanations: (1) the global effective population size in B. dendrobatidis is small; (2) mutation rates in B. dendrobatidis are low; (3) there has been a recent bottleneck in population size; or (4) the species has recently emerged from a genotype(s) favoured by natural selection. In dismissal of point one, the generally broad distribution and host range of the fungus do not suggest a small effective population size. Additionally, with no a priori evidence to suggest that mutation rates in B. dendrobatidis are extraordinarily low (point 2), it seems reasonable to conclude that either a recent bottleneck occurred in the history of the fungus or that the population has recently emerged from a single, founder strain (points 3 and 4). In a clonal population, a recent population bottleneck would be expected to have a similar population genetic signature as the emergence of a virulent strain caused by strong selection, because such selective pressure should affect the whole genome of nonrecombining organisms through genetic hitchhiking.
An analysis of genetic relationships among strains showed low overall geographical structuring and host specificity of genotypes (Fig. 3). These data do not support the hypothesis that B. dendrobatidis has emerged from a pre-existing relationship between the fungus and amphibians via recent climatic change or other abiotic factors, and are more consistent with the recent introduction of the pathogen into naïve populations. If the relationship between frog and fungus had been established for many generations in the absence of gene flow, the MLST data would be predicted to show geographical population structure. Indeed, there does seem to be some geographical structuring of populations as evidenced by a significant genetic differentiation between North America and the rest of the world at loci r6046 and lsu35. These loci suggest the North American population may form a distinct gene pool. In contrast, the finding of identical MLSTs in Panama and Australia argues for recent dispersal or introduction of the pathogen into these two areas of amphibian population declines. B. dendrobatidis displays other characteristics of a virulent pathogen introduced into a naïve population, such as low host specificity and high mortality (Daszak et al. 1999). As with some human pathogens, [e.g. the agent of plague, Yersinia pestis (Achtman et al. 1999) and the fungal skin pathogen Trichophyton rubrum (Gräser et al. (1999)], low levels of intercontinental genetic variation in B. dendrobatidis could be due to recent dissemination of the disease by humans.
Despite the recent geographical expansion of the fungus, both asymptomatic and symptomatic frogs can share the same genotypes. Also, regions where populations are declining display nearly the same genotypes as regions where the fungus has not caused obvious population declines (e.g. eastern North America). This supports the argument that population declines are context-dependent (Blaustein & Kiesecker 2002), i.e. the disease interacts with both frog species and the environment in unique ways in different geographical areas.
The MLST data were also used to understand the mode of B. dendrobatidis reproduction. The detection of two loci for which frequencies of heterozygous individuals were either fixed or nearly fixed suggests that B. dendrobatidis has a predominately clonal population structure (Tibayrenc et al. 1990). Fixed heterozygosity arises from the absence of normal segregation of homologues at meiosis that breaks apart heterozygous genotypes.
Although we interpreted the double-peaked sites in the sequence chromatograms as heterozygous sites, we addressed alternative explanations for the presence of two sequence copies within a single strain. One possibility is that the strains, which are presumably single individuals, are actually a population of individuals that differ in alleles at the polymorphic loci (i.e. represent ‘mixed’ cultures). This can be dismissed because we sequenced the 10 loci from a strain derived from a single zoospore (JEL270), yet two of its 10 loci were heterozygous. Zoospores possess only a single nucleus (Longcore et al. 1999), therefore this strain derives from a single genetic individual. Double-peaked sites could also arise from the amplification of two paralogous loci that differ in sequence. This is obviously a possibility for ribosomal RNA gene lsu35. However, the hybridizing of probes of three polymorphic loci to single bands in Southern analyses suggests that these loci are present in a single copy in the B. dendrobatidis genome. Moreover, the low level of nucleotide variation between the two sequence types within a strain, approximately one silent polymorphism per 475-bp, suggests that the two sequence types are allelic rather than paralogous. These data strongly suggest that the observed double-peaked sites in the chromatograms are the result of heterozygosity; however, neither the chromosomal nor the sequence polymorphism data exclude the possibility that B. dendrobatidis is of higher than diploid ploidy. Hybridization of probes for the polymorphic loci to CHEF separated chromosomes or in situ chromosomal preparations are needed to more adequately determine ploidy.
In diploid organisms, all new point mutations arise in a heterozygous state. Homozygotes for the new mutation are created through sexuality or processes such as mitotic gene conversion or crossing over (collectively termed somatic recombination). A conflict arises because certain B. dendrobatidis loci suggest clonality through the extreme overrepresentation of heterozygous genotypes (ctsyn1 and aprt13), whereas others suggest segregation/recombination by displaying appreciable frequencies of both homozygous types (lsu35 and r6046). Such a pattern might emerge if the process of generating homozygous genotypes is a locus or chromosome-specific phenomenon and not a genome-wide phenomenon. Somatic recombination happens during vegetative growth and can affect single gene regions or chromosomes. Conversely, meiosis is a genome-wide recombination process that shuffles alleles as well as chromosomes. We suggest that the presence of both possible homozygous genotypes at a locus has arisen from somatic recombination within heterozygous progenitors. The difference between levels of heterozygosity at the four variable loci could be explained by differential rates of somatic recombination, perhaps as a result of the proximity to the centromere. For example loci ctsyn1 and aprt13, which display fixed heterozygosity, may be near the centromere where recombination is inhibited. That somatic recombination may play a significant role in generating novel multilocus genotypes in B. dendrobatidis is supported by evidence of recombination in other fungi that are believed to reproduce exclusively asexually, such as Coccidioides immitis and Aspergillus flavus (Burt et al. 1996; Geiser et al. 1998).
B. dendrobatidis displayed extensive chromosome length polymorphism among strains as has been observed in other fungal species (Zolan 1995). Chromosome length polymorphism in ancient asexual organisms is consistent with the expectation that large length differences between homologues could hinder proper pairing at meiosis or result in nondisjunction (Birky 1996; Welch & Meselson 1998). Despite this, because both sexual and asexual fungi frequently have chromosome length polymorphisms, our documentation of chromosome length polymorphism in B. dendrobatidis does not lead directly to the conclusion that the fungus is primarily asexual (Zolan 1995). The high level of genetic variation suggested by chromosome polymorphisms contrasts with the near absence nucleotide variation. The elevated level of chromosome rearrangement in B. dendrobatidis may be related to the processes governing somatic recombination.
The finding of a largely clonal population structure suggests that B. dendrobatidis may lack a sexual stage, therefore it is possible that a resting stage is absent. Predominately clonal reproduction in B. dendrobatidis would not be surprising because sexuality has not been observed in many chytridialean fungi, and has not been reported for any members of the Spizellomycetales or Neocallimastigales (Barr 2001). Although lack of sexuality does not preclude the generation of asexually produced resting spores, the only propagules that have been observed are zoospores, which cannot survive desiccation or marine travel. That drying can kill cultures of B. dendrobatidis (Berger et al. 1999) also supports the absence of a resting spore. An absence of a resting spore stage would suggest that natural causes such as airborne dispersal of spores are less likely to be the reason for the pathogen's entry into naïve populations than inadvertent dispersal by humans or perhaps other long distance travelers such as birds.
The MLST data are limiting because they do not provide high resolution of genetic relationships among the strains. In addition, our sampling is incomplete because it does not cover the entire range of the disease, and it is biased towards North American strains. Further work with more variable markers, such as microsatellite loci or amplified length fragment polymorphisms, and a more geographically diverse set of strains may be able to distinguish more genotypes than the MLST data and provide a better understanding of the origins of the disease.
Acknowledgements
This work would have not been possible without the gracious help of those who provided infected frogs from which fungal strains were isolated: Gregory A. Bradley, John Clarke, Elizabeth W. Davidson, Gary M. Fellers, Alastair Freeman, Mark S. Jones, Thomas R. Jones, Michael J. Linn, Jerry R. Longcore, Gerry Marantelli, Keith McDonald, Igor Mikaelian, Donald K. Nichols, Linda Northey, Martin Ouellet, Allan P. Pessier, Timothy Reichard, Michael J. Sredl, Benjamin Stubbs and Kathy Taylor. We thank Sudhir Kumar, Cynthia Riginos and two anonymous reviewers for reviews of earlier drafts, and Thomas G. Mitchell for generous sharing of laboratory space. This research was supported by the Howard Hughes Precollege Program, by IRCEB grant IBN-9977063 from the National Science Foundation (James P. Collins, PI), and a Clark Fellowship in Molecular Evolution to AG.
References
This investigation is part of the ongoing, multi-institutional effort at understanding the role of disease in global amphibian declines. E. A. Morehouse, T. Y. James, A. R. D. Ganley and R. Vilgalys are members of the Duke Mycology Research Unit and are dedicated to understanding the evolutionary genetics of pathogenic and saprobic fungi. P. J. Murphy's ecological interests include the responses of amphibians to variable environments, including unpredictable climate and novel pathogens. L. Berger is an amphibian pathologist who works on chytridiomycosis in Australia. J. E. Longcore is dedicated to all aspects of chytrid fungi.




