Phylogeny, paraphyly and ecological adaptation of the colour and pattern in the Anolis roquet complex on Martinique
Abstract
Martinique is an environmentally heterogeneous island with a complex geological history. It is occupied by a solitary anole, Anolis roquet, showing marked geographical variation in colour and other features. Phylogenetic analysis of a segment (1 kb) of the mitochondrial cytochrome b gene across the Anolis roquet series in the southern Lesser Antilles and at 63 localities of Anolis roquet in Martinique indicate that A. roquet is paraphyletic as A. extremus (Barbados) is nested within the Martinique populations. Moreover, divergent phylogenetic lineages exist within Martinique (max. 10.6% uncorrected pairwise), and these lineages are closely associated with the geological history of this complex island. However, objective quantification of the spectroradiometric analysis of hue by delta analysis, together with analysis of the colour pattern, indicate that they are primarily determined by adaptation to environmental conditions, irrespective of these phylogenetic lineages. There is remarkable convergence in hue and pattern in both extreme xeric (dark chevrons on a dull, generally grey/brown, background), and montane conditions (black reticulation and non-UV white spots on a bright, saturated green background). Moreover, parallel trends occur between Martinique and other Lesser Antillean anoles, which further argues for adaptation (increase in green saturation in montane areas and higher levels of UV on the dewlap of some Atlantic forms). As an exception, there are two specific situations where anoles from different lineages look different. These are (i) in the low-altitude regions of the northwest where the northwestern and central lineages make contact, and (ii) in the far south of the island where the southern and central lineages meet.
Introduction
The phylogenetic analysis of geographical populations within a species (Loudenslager & Gall 1980; Thorpe 1984) predates the widespread use of mitochondrial DNA gene trees in population studies. Nevertheless, it is the application of these molecular methods that has led to the term phylogeography, has provided a ‘clock’, and has had such a considerable impact (Avise 1994, 2000). These studies have contributed to understanding the impact of Pleistocene and Pliocene climate change (Joseph et al. 1995; Hewitt 1996; Avise & Walker 1998; Tarkhnishvili et al. 2000), and tectonic (Heads 1998) and volcanic events (Thorpe et al. 1996; Brown et al. 2000; Gübitz et al. 2000; Malhotra & Thorpe 2000a). Many such studies show deep phylogenetic divisions in species occupying small ranges (e.g. islands), but the extent to which these historical divisions impact on the microevolutionary trajectory (Gomez et al. 2002), or speciation process, is not well established (Orr & Smith 1998; Schneider et al. 1999). In some situations at least, historical divisions appear to have less impact on gene flow than current selection (Thorpe & Richard 2001).
The arboreal iguanid lizard, Anolis roquet, on the island of Martinique provides a good example of a system where both historical and current ecological factors may influence geographical variation. Martinique has a complex geological history, being composed of both outer-older, and inner-younger, Lesser Antillean geological arcs (Sigurdsson & Carey 1991), and like other Lesser Antillean islands, the anole populations may have distinct phylogenetic divisions (Malhotra & Thorpe 2000a; Thorpe 2002). Moreover, high-altitude Lesser Antillean islands have distinct ecological zonation. Substantial evidence from large-scale field experiments (Malhotra & Thorpe 1991; Thorpe & Malhotra 1996), correlational studies (Thorpe & Malhotra 1996; Malhotra & Thorpe 2000a; Thorpe 2002; Thorpe et al. 2002), parallel evolution (Thorpe et al. 2002) and common garden experiments (Thorpe et al. 2002) suggests that natural selection for these current ecological conditions on Lesser Antillean islands is capable of playing a key role in determining evolutionary patterns within these island anole species.
Anolis is the most species-rich amniote genus, with over 400 described species, nearly 150 of which are currently recognized in the Caribbean (Losos 2002). The anole communities of the large Greater Antillean islands are multispecies complexes, in which at least 70% of species on each island have arisen from in situ speciation (Losos 1996). In contrast, the Lesser Antillean islands contain depauperate Anolis communities, with the majority of islands having only a solitary species. Speciation in this group of Anolis is generally best explained by a succession of allopatric speciation events followed by overwater colonization (Thorpe et al. 2002). The Lesser Antilles are occupied by two adjacent, nonoverlapping, series of anoles (Underwood 1959). It is thought that the northern, bimaculatus, series colonized the chain from the north, extending southwards to Dominica, while the southern, roquet, series colonized the chain from the south, extending northwards to Martinique. Hence the Martinique anole represents the northernmost population of the roquet series. Anolis roquet is found at varying densities throughout Martinique except at altitudes over 920 m (Schwartz & Henderson 1991: personal observation). Lazell (1972) recognized six subspecies (with very large areas of intergradation), primarily on the basis of qualitative differences in a few characters (principally colour pattern). Subsequently, Gorman & Kim (1975) used allozymes to study the genetic variation of three populations of A. roquet, representing three morphological subspecies, but found little variation within or between populations.
Colour (and pattern) is one of the character systems that may be strongly influenced by both historical processes and natural selection (Malhotra & Thorpe 2000a; Thorpe 2002). Moreover, it may also play an important role in sexual selection (Andersson & Amundsen 1997; Andersson et al. 1998; LeBas & Marshall 2000; Thorpe & Richard 2001), thereby potentially influencing the evolutionary trajectory (Thorpe & Richard 2001). However, the analysis of colour is fraught with difficulty as human perception of colour is subjective and limited. For example, humans (and other primates), lack an amino acid in the SWS1 (short wavelength sensitive type 1) pigment that allows perception in the UV segment of the spectrum (Yokoyama & Shi 2000), whereas UV vision may be ancestral and widespread in vertebrates. Consequently, to record colour effectively one needs a spectroradiometric recording (per cent reflectance per wavelength), which in turn requires appropriate statistical treatment for evolutionary comparisons (Thorpe 2002).
This study aims to establish the molecular phylogenetic relationships of the Anolis roquet populations on Martinique and their relatives in the southern Lesser Antilles. Recently developed procedures for quantifying colour are employed (Thorpe 2002) and the dynamic interaction between selection and historical processes is evaluated to elucidate the causes for the variation in colour and pattern.
Materials and methods
Molecular phylogenetics
Specimens from 63 localities on Martinique (Fig. 1) were sampled by nonintrusive tail-tipping (tissue preserved in 80% ethanol). In addition samples were available from the other species in the Anolis roquet series: A. trinitatis (St Vincent, three localities), A. luciae (St Lucia, two localities), A. bonarensis (Bonaire), A. griseus (St Vincent), A. richardi (Grenada/Grenadines), A. aeneus (Grenada/Grenadines), A. extremus (Barbados), together with A. oculatus (Dominica) from the bimaculatus series. Only A. blanquillanus from the roquet group was not represented.
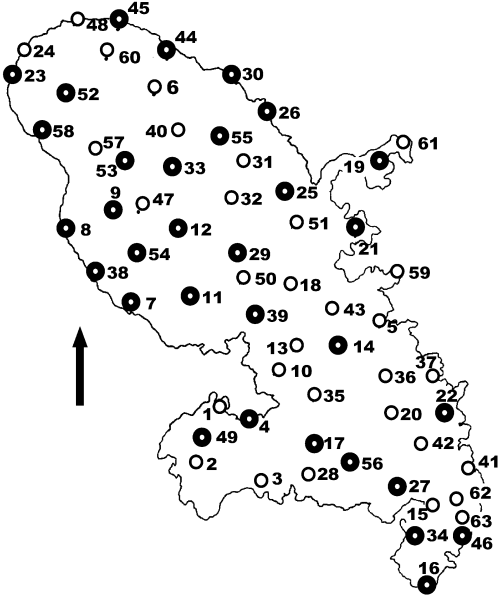
Martinique (north indicated by arrow) with study localities numbered. Bold circles are those localities that were also used for the spectrophotometry study.
Whole genome DNA was extracted following Sambrook et al. (1989) and a 1139 base pair (bp) segment of the cytochrome b gene was amplified with modified (Malhotra & Thorpe 2000b) Mt-A (Lenk & Wink 1997) and Mt-F (Wink 1995) primers. The primer sequences were Mt-A (5′-CTCCCAGCCCCATCCAACATCTCAGCATGATGAAACTTCG-3′), and Mt-F (5′-AGGGTGGAGTCTTCTGTTTTTGGTTTACAAGACCAATG-3′). Polymerase chain reactions (PCR) were carried out in 50-µL volumes containing 100–150 ng template DNA. The final reaction volumes consisted of 1 × reaction buffer (50 mm KCl, 20 mm Tris–HCl pH 8.4), magnesium chloride at 3 mm, Mt-A and Mt-F primers at 400 nm, each dNTP at 400 µm and Taq DNA polymerase (Gibco BRL) at 0.04 U/µL. After denaturation for 3 min at 94 °C, five cycles of 30 s at 94 °C, 1 min at 45 °C and 1 min at 72 °C were followed by 30 cycles with the annealing temperature increased to 51 °C and a final elongation step of 5 min at 72 °C. Electrophoresis through 1% agarose gels was employed to remove excess primers, dNTPs and nonspecific amplification products. The slice of gel containing the amplification product was excised under UV illumination and the DNA was recovered using Concert Rapid Gel Extraction System (Gibco BRL). The concentration of the recovered DNA was increased by ethanol precipitation. Big-Dye terminator (PE Biosystems) cycle sequencing reactions were carried out following the manufacturer's protocol. The resultant reaction products were analysed on an ABI 377 automatic sequencer.
mega v1.02 (Kumar et al. 1993) was used to translate base pair sequences into amino acid sequences to test for stop codons, insertions and deletions in order to reveal pseudogenes (Sorenson & Fleischer 1996; Zhang & Hewitt 1996). For some critical Martinique localities, additional specimens were sequenced. Both maximum likelihood (ML) and maximum parsimony (MP) methods were used to reconstruct the haplotype trees using paup*4.0b8 (Swofford 1901). modeltest 3.0 (Posada & Crandall 1998) was employed to test for the most appropriate model of sequence evolution for the particular data set under investigation. The parameters indicated by modeltest were then used to reconstruct ML haplotype trees (heuristic search, random addition of sequences, tree bisection–reconnection with 100 replications). MP haplotype trees were also reconstructed based on a heuristic search as above with 5000 replications. Bootstrap support for the nodes of both ML and MP trees were assessed using the above procedures with 100 replications (paup*4.0b8), but Bremer (1994) support indices are only given for critical nodes of the MP trees.
The phylogenetic analyses were iterative, and are presented here in two steps. First (between species), the relationships between the Martinique populations (a representative subset of 13 localities covering the geographical range and all clades) and other members of the roquet series were investigated with the modeltest model optimized for this data set, and with A. oculatus (bimaculatus series) as the outgroup. Second (within the roquet/extremus complex) the relationships among the 63 Martinique populations and extremus were investigated with the modeltest parameters optimized for this ingroup, with the sister species elucidated by the first step (above) as the outgroup, i.e. A. aeneus. The uncorrected p-distances among clades (maximum, mean and standard error) are given using phyltest version 2.0 (Kumar 1996).
Colour and pattern
The diffuse reflectance of a surface, as a percentage of a Spectralon (Labsphere UK) white tile standard was measured using an S2000 spectrometer (Ocean Optics Europe) with dual deuterium and halogen light sources and spectrawin 4.1 software (Top Sensor Systems). A standard (Ocean Optics) reflection probe with a 200 µ receptor fibre was presented to the surface at 45° with purpose-made matt-black head screwed to the probe.
Five adult males were studied from each of 32 sites across Martinique (Fig. 1) selected to represent each clade and habitat type. For each specimen, the reflectance spectrum was measured from seven regions of the body: posterior dewlap, anterior dewlap (chin), the temporal area (mid-point between the ear and eye), the lighter marking above the fore-arm pit (oxter), and the dorsal surface of the trunk. The spectra were averaged across four independent recordings for each body region. All recordings were taken at the same temperature, and as anoles may darken under stress (metachrosis), care was taken to avoid this during recording. All specimens were released unharmed at the site of capture.
The procedures for the quantitative analysis of hue follow Thorpe (2002). Using purpose-written software (Thorpe 2002), the percentage reflectance from 330 to 710 nm was excised from the spectrometer output file, standardized against the area under the curve, averaged across the repeat recordings, cut into 10-nm segments, and arranged in files by character and locality, ready for statistical analysis by other programs. For each of the five body regions, the standardized reflectance of each 10-nm segment was subjected to an anova where each of the 32 localities represents a group and each individual specimen represents a replicate. The F-values are then plotted against the wavelength for each body region to visualize wavelength regions that are not informative. One should not use these 10-nm segments directly as characters in comparative evolutionary studies as regions of the spectrum are strongly autocorrelated. Subjecting the data for each body region to a multiple-group principal component matrix with each 10-nm segment as a ‘character’ and each locality as a group can reveal these regions. The difference between adjacent eigenvector coefficients, δ (Thorpe 2002), was then summed over the first five vectors. These can be averaged across body regions and plotted against wavelength to reveal peaks where the spectrum can be cut to produce a few, relatively independent, characters that can be used in a comparative evolutionary study such as this. For each body region this yielded a few characters representing hues (such as ‘UV’ or ‘green’) that are treated separately, or generalized across a body region using canonical variate analysis, with Mahalanobis D2 indicating the dissimilarity among localities.
Qualitative comparisons provided supporting information. Spectrometry recordings were taken from the bright ‘white’ spots of a representative proportion of montane males. Also the occurrence and distribution of UV reflectance on the anoles was recorded using high-resolution digital macrophotography from the 32 localities for which hue was investigated (Fig. 1). A prefocused 100-mm Canon macro lens on a D30 body (mounted on a tripod) was fitted with a 360-nm UV pass filter, and the anole was illuminated against a UV reflective background (that included a non-UV reflective standard) by a Canon flash modified for UV output. The camera was set to the equivalent of 1600 asa, and the digital image was manipulated in a graphics package to eliminate the red and green channels and convert to a grey-scale. This method was validated by photographing a known natural spectrum (rainbow).
For the colour pattern, the number of chevrons (Fig. 2a) and the development of light (Fig. 2c) and ‘white’ (Fig. 2e) dorsal spots were recorded from Kodachrome photographs of five males from localities 1–60. In addition, the extent of the black on the head and dorsum of five mature males was recorded for specific subsets of localities (see Analyses E and G below). The development of light/white spots, black on dorsum and black on the head are coded as three state ordinal characters.
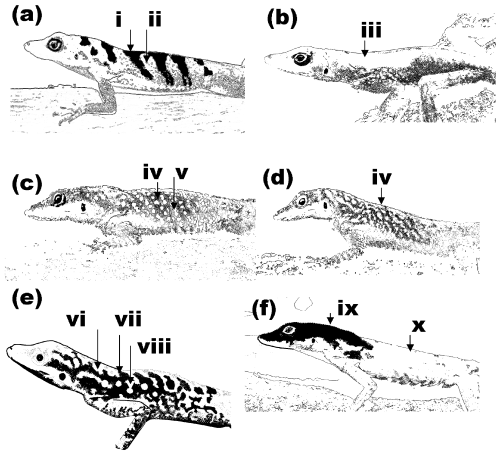
Geographic variation in colour and pattern of mature males. (a) In the southern lineage and extreme xeric environments of the western (north Caribbean coast 7, 8, 38) and central lineages (tip of Caravelle peninsular Atlantic coast, 61) the males have chevrons (i) on a dull grey/brown background with low-contrast marbling (ii) and no light or white spots. (b) On the northern Atlantic coast the less xeric littoral habitat occupied by the central lineage (localities 19, 26, 30) has males with a dull grey/brown/green background (iii) with low-contrast marbling. Slightly lighter spots may be present at the humid extreme and a hint of chevrons at the xeric extreme. (c, d) In the low-altitude mesic sections of the island both southwestern lineage and central lineage forms have a mid-green background with marbling (iv) and may have lighter spots in rows (v). (e) Irrespective of lineage, the saturation of the green background, the extent of black on the dorsum, and size and whiteness of the spots increases with altitude such that montane (e.g. 53, 33) forms have a bright saturated green background (vi), with areas of irregular or reticulate black (vii) and large, non-UV white spots (viii). (f) On the north, Atlantic coast, just north of the central lineage (localities 19, 26, 30), there is an abrupt change to northwestern lineage forms (localities 44, 45, 48) in a wetter area where the males tend to have black heads and shoulders (ix) and green or even blue-green dorsum (x), often with white spots edged in black.
Hypothesis testing
The analyses are organized into eight sets (A to H) with analyses A and H having just one analysis per set and analyses B to G being repeated across several facets of the hue or pattern.
Analysis set A. The association between the main lineages and main geological regions is tested by pairwise matrix correspondence (Mantel test) with 10 000 randomizations. The geological regions are derived primarily from Maury et al. (1990), but see also Andreieff et al. (1976), Westercamp & Tomblin (1979), Bouysse et al. (1983) and Sigurdsson & Carey (1991). The phylogeny (dependent variable) is represented by the mean patristic, or tree, distances (Page & Holmes 1998) among the Martinique localities and ingroups based on the maximum likelihood gene tree for the roquet/extremus analysis. For the independent matrix a locality is given a binary code (1 for presence, 0 for absence) for allocation to one of the six geological regions and the dissimilarity among the localities is computed as the Euclidean distance in this six-dimensional space. The six geological regions are Barbados, Caravelle peninsula, St Anne peninsula, Central region, southwest (Trois Ilets) peninsula and the northwest (Fig. 3). These may have been separate ‘islands’ at the approximate time suggested by pairwise genetic divergence (Table 1).

Geology regions and DNA lineages. The symbols indicate the modal haplotypes at a locality with empty circles representing the central lineage, solid circles the southern lineage, and triangles the western lineage, with equilateral triangles for the southwestern lineage and acute triangles for the northwestern lineage. These show a relationship with the geological regions: Caravelle (A), St Anne (B), central (C); northwest (D), and southwest (E); see text for details. The areas where there is a sharp transition between morphotypes associated with haplotype lineages are indicated (st).
| (A) | Central | South | West |
| Central | 2.0 | ||
| South | 9.5 (7.2 ± 0.7) | 1.8 | |
| West | 10.6 (8.5 ± 0.7) | 10.1 (8.6 ± 0.8) | 4.1 |
| (B) | Northwest | Southwest | |
| Northwest | 2.7 | ||
| Southwest | 6.7 (5.5 ± 0.6) | 2.1 | |
| (C) | North-NW | West-NW | |
| North-northwest | 1.2 | ||
| West-northwest | 3.7 (3.2 ± 0.5) | 1.0 | |
| (D) | far-west-SW | near-west-SW | |
| Far-west-SW | 1.1 | ||
| Near-west-SW | 3.8 (3.0 ± 0.5) | 1.0 |
- NW, northwest; SW, southwest.
Analysis set B. In analyses B to F the association between various facets of the hue/pattern and several putative causal hypotheses is tested across various sets of localities using locality means for hue/pattern computed across the five specimens per locality. This is achieved by pairwise matrix correspondence where the facet of the hue/pattern is used to construct the dependent matrix and the hypothesis is used to construct the independent matrix (the order of the matrices being the number of included localities). Occasionally a given dependent matrix has a significant pairwise association with more than one dependent matrix, and in this circumstance all the significant dependent matrices are entered into a partial matrix correspondence test (Thorpe et al. 1996). The five hypotheses available to be tested in analyses B to F are as follows:
- (i)
Isolation by distance. This predicts an association between geographical proximity among selected localities (used to construct the independent matrix) and the similarity in the facet of the hue/pattern being considered.
- (ii)
Adaptation to altitude. Altitude, measured in metres above sea level, is an objective, unambiguous measure, but is a simplification of several physical and biotic factors that may be important in the selection regime of these anoles. High-altitude localities are wetter and colder than low-altitude localities and have different vegetation (transitional, and then montane, rainforest). This hypothesis predicts adaptation by natural selection to these altitudinal differences (Thorpe & Baez 1993; Malhotra & Thorpe 2000a; Thorpe 2002).
- (iii)
Adaptation to xeric habitat. Generally the Caribbean coastal region in the rain shadow of mountains has very low rainfall and a specific xeric woodland biotope (Beard 1948; Malhotra & Thorpe 2000a). In northern Martinique Caribbean coastal localities in the rain shadow of the Pitons du Carbet (locations 7, 8, 38) have less than 1250 mm of rain a year (Meteo France; Lassere 1977). However, the far south of the island is also very dry (locations 34, 16, 46) as is the extreme end of the Atlantic Caravelle peninsula (location 61). Extreme xeric localities are coded as 2. Less xeric localities (rainfall less than 1500 mm per year) extend down much of the Caribbean coast, around the St Anne Peninsula and along some of the southern Atlantic coast. These are coded as 1, and nonxeric localities are coded as 0. The dissimilarity matrix employed for matrix correspondence is a simple Euclidean distance based on this ordinal score. This hypothesis predicts natural selection for these xeric conditions (Malhotra & Thorpe 2000a; Thorpe 2002).
- (iv)
Adaptation to northern Atlantic coastal habitat. In many Lesser Antillean islands the Atlantic coast is exposed to the prevailing winds and has damper, littoral woodland (Beard 1948). The northern Atlantic coastal localities (26, 30, 44, 45) that meet the prevailing weather before the Pitons du Carbet are wetter than those localities further south. These are coded as 1, while other localities are coded as zero, and the dissimilarity among localities is computed. This hypothesis predicts adaptation by natural selection to the Atlantic (littoral) conditions (Malhotra & Thorpe 2000a; Thorpe 2002).
- (v)
Historical processes. The historical processes (such as divergence during geographical isolation) are represented by the roquet/extremus phylogeny. This is coded as the mean patristic distances among the selected Martinique localities based on the maximum likelihood gene tree for the roquet/extremus analysis as in analysis A above. This hypothesis predicts that past ancestral–descendant relationships, rather than current selection pressures are associated with the hue/pattern features.
In analysis set B the hue is characterized as a series of spectral segments for each body region, and generalized across all spectral segments for that body region using the Mahalanobis D2 among localities. These are tested against all five hypotheses above for the 32 localities for which hue was recorded (Fig. 1).
Analysis set C. This is the same as analysis set B, except only the northern 19 localities are used for which hue is recorded (locations 7, 8, 9, 11, 12, 23, 25, 26, 29, 30, 33, 38, 44, 45, 52, 53, 54, 55, 58). The northern localities are analysed as a subset because the north represents a simpler, high-altitude, system more comparable with other lesser Antillean island such as St Vincent (Thorpe 2002) and Dominica (Malhotra & Thorpe 2000a).
Analysis set D. Colour-pattern characters (chevrons and light spots) from island-wide localities 1–60 (Fig. 1) are tested against all five above hypotheses explained under analysis set B.
Analysis set E. This is similar to analysis set D, but further focuses on the relationship between chevrons, xeric adaptation and phylogenetic lineage in the south of the island. The number of chevrons is tested against isolation by distance (proximity), xeric habitat and patristic distance (significant in analysis D) for the 23 low-altitude localities of the south/central lineage in the southern sector of the island (locations 5, 10, 13, 14, 15, 16, 18, 19, 20, 21, 22, 27, 34, 35, 36, 37, 39, 41, 42, 43, 46, 51, 59).
Analysis set F. This is similar to analysis set C but allows further focus on altitudinal adaptation in the north of the island. The extent of light spots and black on the head and dorsum are tested against isolation by proximity, altitude and lineage for the 15 localities (coastal to montane rainforest at 665 m) in a broad transect across the northern part of the island with the central mountain spine (Chutes du Carbet) (locations in north–northwest lineage 7, 8, 9, 38, 53, 54, 57; locations in central lineage 12, 25, 26, 30, 31, 32, 33, 55). There is no pronounced phylogeographic structure within either lineage for these localities, but maximum altitudinal difference, thus enabling this analysis to further test altitude adaptation. In addition to the matrix correspondence tests a histogram is plotted of the relative amount (i.e. saturation) of green on the dorsal background of lizards from these localities to illustrate the effect of altitude vs. lineage.
Analysis set G. Contrast analyses of variance (anovas) were used to test for a phylogenetic effect on the dorsal green hue, the extent of black on the head and dorsum, and the extent of light spotting by contrasting north–northwestern (locations 44, 45) and central (locations 26, 30) lineage localities where these lineages meet on the Atlantic coast.
Analysis set H. Data from 87 specimens from a suite of four colour-pattern characters, six body dimensions (adjusted against snout-vent length) and four scalation characters were taken from Dawes (2001). They were from three localities; one either side of, but close to, the contact zone for the southern and central lineages, and one on the contact zone. The localities (north of the zone, on the zone, and south of the zone) are located 1250 m north, 150 m north, and 1500 m south of locality 15, respectively. A canonical variate analysis was performed and the scores (first canonical variate) of the 43 individuals from the locality on the zone are plotted as a histogram to elucidate uni- or bimodality.
Results
Molecular phylogeny: between species
Almost a kilobase (989 bp) of sequence was available for all samples. modeltest identified the general time reversible plus gamma (GTR + G + I) model for the ML phylogenetic reconstruction (– ln L = 6354.5) with the rate matrix as [A-C] 1.0, [A-G] 5.89, [A-T] 1.0, [C-G] 1.0, [C-T] 9.84, [G-T] 1.0, the ACGT base frequencies as 0.332, 0.251, 0.098 and 0.320, gamma shape as 1.899, and the proportion of invariable sites as 0.505. For the MP reconstruction (1174 steps) 324 of the 989 sites were parsimony informative. A strict consensus (Fig. 4) of both MP and ML trees shows that Martinique roquet is not a monophyletic group, but is rendered paraphyletic by Anolis extremus (Barbados) being nested well within the Martinique groups. This has high bootstrap support for both ML and MP trees and the MP node showing the roquet/extremus clade as monophyletic also has high Bremer support. The sister species of the roquet/extremus complex is shown to be A. aeneus (Grenada/Grenadines), once again with strong bootstrap support. Deeper than this, the other Grenada/St Vincent species (griseus, richardi, trinitatus), together with aeneus, extremus and roquet, form a well-supported clade, from which luciae (St Lucia) and bonairensis (Bonaire) are excluded. However, the deeper relationships are generally poorly resolved while the finer relationships are very well supported and show a distinct, well-supported, structure within the roquet/extremus complex.

Molecular phylogeny: between species. Strict consensus of ML and MP haplotype trees, with bootstrap support for the ML and MP trees, respectively (outgroup rooted with Anolis oculatus). Bremer support is given for the node showing the monophyly of the roquet/extremus complex. M followed by a number represents a Martinique sample with locality number. Other terminal nodes are labelled with species names, together with approximate localities for luciae and trinitatus (NW, northwest; NE, northwest; SW, southwest). Note that the roquet/extremus complex is monophyletic but that roquet is paraphyletic as extremus is nested within it.
Molecular phylogeny: within the roquet/extremus complex
modeltest identified the HKY85 + G + I model for the ML phylogenetic reconstruction (– ln L = 5579.1) with the ACGT base frequencies as 0.324, 0.221, 0.126 and 0.329, the gamma parameter as 0.956 and the proportion of invariable sites as 0.490 (representative sequences GenBank accessions AF543049 to AF543071). For the MP reconstruction (729 steps) 210 of the 989 sites are parsimony informative. The above between-species study showed aeneus to be the sister taxa of the roquet/extremus complex, so this was used as the outgroup for this roquet/extremus phylogenetic analyses. Figure 5 illustrates the ML tree with branch lengths. The ML and MP trees are identical in all important aspects, with all the main nodes having extremely strong bootstrap support on the ML and MP trees and Bremer support on the MP tree. As with the between-species analyses, extremus is nested inside the Martinique haplotypes as a sister taxa to the central locality haplotypes. There is a very strong geographical structure in Martinique; the western Martinique haplotypes form a group, as do the southern/central haplotypes, which subsequently split. There is also further geographical structure in the western haplotype with northwestern and southwestern clades. Both of these in turn have further geographical structure (3, 5) with the northwestern haplotypes dividing into north-northwestern (locations 6, 23, 24, 40, 44, 45, 48, 60) and west-northwestern (locations 7, 8, 9, 38, 47, 52, 53, 54, 57, 58) clades, and the southwestern haplotypes dividing into far-west-southwestern (locations 1, 2, 3, 4, 49) and near-west-southwestern (locations 15, 17, 28, 41, 56) clades. There is little geographical interdigitation of these haplotypes but some localities have representatives of more than one lineage: locality 15 is modally southern but with both southwestern and central haplotypes, and locality 41 is modally southern but with a southwestern haplotype. Specimens from localities 22 and 55 (three from each) all belong to central lineage. The maximum uncorrected pairwise genetic divergence among the Martinique haplotypes is 10.6% (central and western) with the maximum and mean (with standard error) divergence among clades, and the mean divergence within clades, given in Table 1.

Molecular phylogeny: roquet/extremus complex. Maximum likelihood gene tree with ML and MP bootstrap support, together with Bremer support (respectively) for key nodes (Anolis aeneus as the outgroup). The ML and MP trees are identical for key nodes, and nodes at which both trees have < 50% bootstrap support are illustrated with an asterisk. As with Fig. 4, A. extremus is the sister taxa to the central lineage, rendering A. roquet paraphyletic. The main lineages referred to in the text are indicated although both the northwestern and southwestern lineages are further subdivided into geographically coherent lineages. The locality numbers of northwestern and central region extreme xeric populations with chevrons are marked. These show convergence with the chevroned xeric populations from St Anne (occupied by the southern lineage).
Hence, there are several contact zones between haplotype lineages, representing a wide range of divergence. The three main contact zones are between the central and western lineages in the north (10.6% maximum divergence) and south (9.8% maximum divergence), and the central and southern lineage (9.5% maximum divergence). Contact between the north-northwest and west-northwest lineages in the northwest, and between far-west-southwest and near-west-southwest lineages in the southwest exemplify lower levels of divergence (3.7 and 3.8% max divergence, respectively). The lineages are significantly associated with the geological regions (analysis A, Fig. 3) at r = 0.67, P < 0.0001. Where the central and southern lineages meet, a multivariate analysis of the general morphology does not suggest a unimodal distribution (Fig. 6).

Frequency of southern and central lineage multivariate morphological types at a single locality where these haplotype lineages meet (text for details). There is no clear evidence of an intermediate unimodal distribution as predicted from complete introgression between these lineages.
Tests for colour and pattern
The anovas of the spectrometry data for each body region showed that the 320 +, 490 +, 500 + and 510 + segments tended to have lower F ratios than other segments. The delta statistic (Thorpe 2002) averaged across all body regions (Fig. 7) indicates peaks that, together with the above anovas, allow divisions into five relatively independent regions: 330–420, 420–490, 520–590, 590–640, and 640–710. These are, respectively, and arbitrarily, referred to as ultraviolet (UV), blue (BL), green (GR), yellow–orange (YO) and red (RE), for convenience.

Analysis of colour spectra using δ. The distribution of δ (averaged across all body regions) against wavelengths shows low areas where spectra are interdependent and can be combined within a segment. The peaks at which adjacent spectra trend to be independent are illustrated giving segments 330–420 (‘UV’), 420–490 (blue), 520–590 (green), 590–640 (‘yellow’), and 640–710 (red). The spectra < 330 nm and 490–520 nm are not included in the segments as they show high independence and low levels of geographical variation.
Several generalizations are readily apparent from the matrix correspondence tests in Table 2. There is a clear difference in trend between the signalling area (dewlap) and the rest of the body, both island-wide (Analysis B) and in the north (Analysis C). The hue of the dewlap is predominantly influenced by whether the population is located on the Atlantic coastal (littoral) habitat, but the hue of other body regions is primarily determined by altitude, and secondarily by xeric Caribbean coastal habitat. It is notable that the distinct phylogenetic lineages do not generally tend to have different hues. Altitude and xeric habitats are also an important determinant of some colour pattern characters, i.e. the development of light spots (Analyses D, F) and amount of black on head and dorsum (Analysis F). Moreover, the number of chevrons is also closely associated with extreme xeric conditions, irrespective of lineage (Analysis D), but is one of the few characteristics with a strong phylogenetic component, as the southern lineage populations all have chevrons (Analysis E). Although the matrix correspondence tests show that overall, the amount of green (e.g. on dorsal), degree of white spotting, black on the head and dorsum are strongly associated with an increase in altitude, a contrast anova of the four Atlantic littoral coastal populations (Analysis G) shows that when the two lineages are contrasted there is more dorsal green (F = 30.4, P < 0.0001), more black on the head (F = 102.4, P < 0.0001) and dorsum (F = 32.0, P < 0.0001) and greater white spot development (F = 20.0, P < 0.0005) in the northwestern lineage compared to the central lineage.
| An. | Char. | Prox | Alt | Xer | Atl | Phyl | |
|---|---|---|---|---|---|---|---|
| B | Chin | UV | — | 0.48 | — | — | — |
| C | — | 0.49 | — | — | — | ||
| B | BL | — | 0.56 | — | — | — | |
| C | — | 0.49 | — | — | — | ||
| B | GR | — | 0.53 | — | — | — | |
| C | — | 0.52 P | 0.20 P | — | — | ||
| B | YO | — | 0.52 | — | — | — | |
| C | — | 0.50 | — | — | — | ||
| B | RE | 0.23 | — | — | — | — | |
| C | 0.22 | — | — | — | — | ||
| B | GEN | — | 0.52 | — | — | — | |
| C | — | 0.46 P | 0.32 P | — | — | ||
| B | Dewlap | UV | — | — | — | 0.44 | — |
| C | — | — | — | 0.40 | — | ||
| B | BL | — | — | — | 0.50 | — | |
| C | — | — | — | 0.44 | — | ||
| B | GR | — | 0.43 P | — | 0.23 P | — | |
| C | — | 0.30 | — | 0.25 | — | ||
| B | YO | — | — | — | 0.47 | — | |
| C | — | — | — | 0.41 | — | ||
| B | RE | — | — | — | 0.48 | — | |
| C | 0.23 | — | — | 0.42 | 0.32 P | ||
| B | GEN | — | 0.31 P | — | 0.34 P | — | |
| C | — | — | — | 0.30 | — | ||
| B | Dorsal | UV | — | 0.51 | — | — | — |
| C | — | 0.52 | — | — | — | ||
| B | BL | — | 0.55 | — | — | — | |
| C | — | 0.46 P | 0.22 P | — | — | ||
| B | GR | — | 0.48 P | 0.34 P | — | — | |
| C | — | 0.40 P | 0.46 P | — | — | ||
| B | YO | — | 0.37 | — | — | — | |
| C | — | 0.38 | — | — | — | ||
| B | RE | — | — | 0.68 | — | — | |
| C | — | — | 0.84 | — | — | ||
| B | GEN | — | 0.38 P | 0.48 P | — | — | |
| C | — | 0.31 P | 0.62 P | — | — | ||
| B | Temp | UV | 0.16 | — | — | — | — |
| C | — | — | — | — | — | ||
| B | BL | — | 0.19 | — | — | — | |
| C | — | 0.23 | — | — | — | ||
| B | GR | — | 0.17 | — | — | — | |
| C | — | 0.30 | — | — | — | ||
| B | YO | — | — | — | — | — | |
| C | — | 0.24 | — | — | — | ||
| B | RE | — | — | 0.31 | — | — | |
| C | — | — | 0.20 | — | 0.24 P | ||
| B | GEN | — | 0.20 P | 0.25 P | — | — | |
| C | — | 0.30 | — | — | — | ||
| B | Oxter | UV | — | 0.62 | — | — | — |
| C | — | 0.57 | — | — | — | ||
| B | BL | — | 0.61 | — | — | — | |
| C | — | 0.57 | — | — | — | ||
| B | GR | — | 0.57 | — | — | — | |
| C | — | 0.53 | — | — | — | ||
| B | YO | — | 0.63 | — | — | — | |
| C | — | 0.61 | — | — | — | ||
| B | RE | — | 0.16 | 0.36 P | — | — | |
| C | — | — | 0.57 | — | — | ||
| B | GEN | — | 0.56 | – | — | — | |
| C | — | 0.53 P | 0.30 P | — | — | ||
| D | Colour | CH | 0.24 | — | 0.72 P | — | 0.22 |
| E | pattern | 0.20 | na | 0.59 P | na | 0.53 P | |
| D | LS | 0.19 P | 0.23 P | 0.21 P | — | 0.12 | |
| F | — | 0.73 | na | na | — | ||
| F | BH | — | 0.23 | na | na | — | |
| F | BD | — | 0.66 | na | na | — |
- An., analysis; Char., character.
- Codes for hypotheses are: Prox, proximity; Alt, altitude; Xer, xeric woodland; Atl, northern Atlantic coastal habitat; Phyl, phylogeny. Codes for analyses are A to F (see text for description). Codes for hue characters are UV, BL, GR, YO and RE with GEN being the multivariate generalization across all five colour segments for that body region the (see text for definition), and codes for pattern characters are LS, light spots; CH, chevron; BH, black head; BD, black dorsal. P, partial regression significant after sequential Bonferoni correction; and na, not applicable.
Geographic variation in colour and pattern of mature males
The southern lineage occupies a small area of xeric, or extreme xeric, habitat and the males generally have chevrons on a dull grey/brown background (green reduced and other hues, particularly red, increased) with low contrast marbling and no light or white spots (Fig. 2a). Apart from the southern lineage, the main lineages (central and western) occupy a wide range of habitats with generally little difference among lineages where they meet geographically. The western lineage (Caribbean coast localities 7, 8, 38), and even the central lineage population at the tip of the Atlantic Caravelle peninsula (location 61) have extreme xeric populations similar to the southern lineage (chevrons, etc. as in Fig. 2a). These three extreme xeric regions, occupied by populations representing each of the three different lineages, therefore show remarkable convergence in colour and pattern.
Opposite the extreme xeric Caribbean habitat is the Atlantic coastal littoral habitat. Most notable is that their dewlap is high in low-frequency reflectance (UV, blue) and low in other hues. The low-altitude populations of the central lineage that occupy the northern Atlantic coast also tend to have a dull background (relative low in green hues and higher in red) with low-contrast marbling and few light spots (Fig. 2b).
However, it is adaptation to the correlates of altitude that dominates colour and pattern. For both the central and western lineages the saturation of the green background, the extent of black on the dorsum, and size and whiteness of the spots increases with altitude such that montane (e.g. 53, 33) forms have a bright saturated green background with areas of irregular or reticulate black markings and large, non-UV-white bright spots. The north has the highest altitude and the northwestern and central lineages meet along the central spine (Fig. 3). Where these lineages meet in these montane rainforest regions they are indistinguishable, both having individuals appearing as in Fig. 2(e), thus revealing another example of remarkable convergence. The convergence in hue is illustrated in Fig. 8. Both montane forms have a saturated green background irrespective of lineage, and both coastal forms have a relatively low amount of green irrespective of lineage.

A transect (from the Caribbean to Atlantic across the Pitons du Carbet region) show remarkable convergence in the extent to which the dorsum is green in the two (northwest and central) lineages at high altitude. Percentage green on dorsum is the proportion of the reflected spectrum in the 520–590 range, the bars (locality numbers underneath) are given in a west–east sequence, with high altitude c. > 400 m and low altitude (Lo alt) < 100 m above sea level (see text for details of Analysis F.
Further south, the bulk of the island tends to be relatively low altitude with moderate rainfall. In these areas of the island both southwestern lineage and central lineage forms appear broadly similar and have a mid-green background with marbling (Fig. 2d) and may have small light (not large bright white) spots in rows (Fig. 2c). There are still altitudinal differences, with associated changes in colour and pattern, in this region; higher altitude populations being somewhat ‘montane’ (Fig. 2e) in appearance.
Although the western lineage forms appear similar to the central lineage forms where they meet in the south (southwest to central contact) and in the high-altitude north (northwest to central contact), there is an abrupt change between low-altitude northwestern lineage (localities 44, 45) and central lineage (localities 26, 30) forms where they meet on the Atlantic coast. In this region the north-northwest lineage (Fig. 2f) males tend to have black heads and shoulders and green (or even blue-green in some individuals) dorsum, often with white spots edged in black. Hence, this, together with the south-central lineage contact, provides an exception to the rule that phylogeny appears to have little impact on colour or pattern. These two limited regions, where lineage contact is obvious in the colour and pattern, are indicated on Fig. 3.
Discussion
Phylogeny
There have been several recent attempts to reconstruct an interspecific molecular phylogeny for the roquet series (Giannasi et al. 2000; Creer et al. 2001) in order to understand size evolution and speciation of anoles, but not all aspects of the trees have been reliably resolved and they have not been designed to reveal paraphyly of an island population. It can be important to reveal paraphyly in populations in an archipelago, because it can occur when one island is ‘recently’ colonized from another. For example the La Palma lizard (Gallotia galloti) is derived from within Tenerife (Thorpe et al. 1994), and the anole on Saba (Anolis sabanus) is recently derived from Guadeloupe rendering the Guadeloupe species (A. marmoratus) paraphyletic (Thorpe et al. 2002). Hence, it is not the aim of this study to focus on interspecific relationships as such, but to establish the relationships among taxa sufficiently to reveal whether the focal populations (Martinique) are paraphyletic and select an appropriate outgroup. Here, the kilobase of cytochrome b sequence, although not resolving all interspecific relationships, reliably resolves the critically important terminal nodes to indicate two key points. First, A. aeneus (Grenada and Grenadines) is the sister taxa of the extremus/roquet group. This conforms to the analysis of the joint mitochondrial DNA data of Giannasi et al. (2000) and Creer et al. (2001) in the latter study. Moreover, it is compatible with the aeneus/extremus/roquet group being defined by a specific karyotype (Gorman & Atkins 1969). Second, A. extremus (Barbados and introduced to St Lucia) is nested within the Martinique populations rendering Martinique roquet paraphyletic. Hence, A. extremus is part of the ingroup for the analysis of Martinique populations and A. aeneus is the outgroup. Moreover, it is not possible to interpret the origin (Gorman & Atkins 1969) and timing (Creer et al. 2001) of the colonization of Barbados without appreciating this.
At a lower level of divergence the kilobase of cytochrome b sequence proved capable of providing a very reliable phylogenetic reconstruction of the main lineages in the roquet/extremus group, all of which had very high bootstrap support. The extent of cytochrome b divergence within Martinique is, as found in some other island lizards, very high. The maximum divergence (i.e. between west and central lineages) within Martinique is 10.6% (uncorrected). This is the highest recorded within the Lesser Antillean anoles (9.3% corrected divergence in A. oculatus within Dominica, 9.2% uncorrected divergence in A. marmoratus in main-island Guadeloupe, and 6.2% uncorrected divergence of A. trinitatus within St Vincent), but is lower than the 12.8% divergence in the gecko Tarentola delalandii within Tenerife (Gübitz et al. 2000; Malhotra & Thorpe 2000a; Thorpe 2002; Stenson unpublished data).
Martinique is composed of two ancient precursors (Caravelle, St Anne peninsulas), and two younger (late Miocene/Pliocene) regions (southwest Trois Ilets Peninsular and the northwest) all positioned around the periphery of an intermediate aged central region, some of which has recently emerged (Andreieff et al. 1976; Westercamp & Tomblin 1979; Bouysse et al. 1983; Maury et al. 1990; Sigurdsson & Carey 1991). Barbados is ancient, but is thought to have recently emerged (Speed 1994). The divergent lineages occupy coherent, largely mutually exclusive, geographical regions, which significantly relate (giving very high values for matrix correspondence) to the above geological regions; i.e. the southern and northern branches of the western lineages, respectively, on the young southwest peninsula and northwest regions, the southern lineage on the ancient St Anne region in the south, the central lineage on both the Caravelle peninsula and the predominantly low-altitude, intermediate-aged central region, and A. extremus on Barbados. It is possible to construct aspects of the geological hypothesis employed in the matrix correspondence tests with some objectivity, for example, there is little ambiguity over the ranges of the ancient Caravelle and St Anne peninsulas. Other aspects are more ambiguous, for example, when young rocks are erupting beneath or adjacent to submarine deposits. If the southwest peninsula region is coded to include the adjacent and older Ducos–St Lucie volcanism (Maury et al. 1990) then the matrix correspondence between geological regions and the phylogeny is even higher (r = 0.82, P < 0.00001).
The maximum divergence is between the western and central/southern/extremus lineages and is 10.6% uncorrected [or ≈ 7.6 million years ago (Ma) at 1.4% divergence per million years], which is a divergence rate that appears suitable for these lizards (Macey et al. 1998; Malhotra & Thorpe 2000a; Creer et al. 2001; Thorpe 2002). Hence, even the younger inner arc in the west was active at the time of divergence of this species (Andreieff et al. 1976; Maury et al. 1990) so that generally the geological regions involved (except Barbados) were available for colonization at the estimated time of divergence.
The estimated time of divergence of the first split (western to central/south/extremus) 7.6 Ma corresponds with the late Miocene origin, ≈ 8 Ma (Maury et al. 1990) of the older parts of the younger arc in west (i.e. the southwest peninsular excluding the Ducos–St Lucie volcanism). The subsequent split between the southern and central/extremus lineages at an estimated 6.8 Ma substantially postdates the geological origin of the southern (St Anne) peninsula (≈ 19 Ma in Sigurdsson & Carey 1991) suggesting that the current population has not occupied the island since its origin. In the west, the subsequent split of the northwestern and southwestern lineages at an estimated 4.8 Ma is compatible with the Pliocene origin of the northwest (Maury et al. 1990), although younger volcanic deposits now cover much of this region.
While both the western lineages show spatial phylogenetic substructuring, the central lineage shows relatively little internal divergence and no clear spatial substructuring, even though it occupies a large geographical area. This suggests that the central population has recently expanded from a small region. This is compatible with the geology, as the central region is substantially composed of submarine deposits that have recently emerged.
Anolis extremus is the sister lineage of the central lineage. Creer et al. (2001) estimate the divergence between extremus and roquet at 7.8%, but as Martinique was represented by only one locality this comparison may not have been between sister lineages. Although Barbados is ancient it is thought to have only re-emerged ≈ 1 Ma (Speed 1994); too late for this level of divergence. Given the comparison between sister lineages allowed in this study even the minimum divergence between the central and extremus lineage is still 5.9% or ≈ 4 Ma. This is still greater than the approximate one million year emergence time suggested for Barbados (Speed 1994), and given the exhaustive sampling of Martinique in this study, is unlikely to be due to failure to sample extant lineages that diverged on Martinique prior to the colonization of Barbados. Moreover, the discrepancy here is too large to be explained by the difficulties involved in assessing clock rates. Two explanations remain. First, that part of Barbados has been emergent for ≈ 4 million years even if most was submerged. Second, that the island lineage (nested in the Martinique populations) that gave rise to Barbados within the last million years has subsequently become extinct. The small Caravelle peninsula is the best candidate for the location of this lineage as it is adjacent to the central area and would have been an island before it was attached to the rest of Martinique by the emergence of the central region.
These considerations suggest the following phylogeographic events. The Martinique precursor islands (Fig. 3), Caravelle, St Anne (south), northwest, southwest and central (much smaller than present) all existed (perhaps as separate islands), at or close to the time of original colonization (from the south). The first split ≈ 7.6 Ma was between the geographically adjacent western lineage (perhaps located in the southwest) and central/south/extremus lineage, perhaps located in the higher altitude southern section of the central region. The southern region was then colonized from the adjacent central region ≈ 6.8 Ma. In the west the northwest was colonized from the southwest ≈ 4.8 Ma. At about the same time a population, an island (possibly Caravelle) was colonized from the central region. This population subsequently colonized Barbados less than 1 Ma and then became extinct. Alternatively, Barbados has been emergent longer than suggested as was colonized directly from the central region of Martinique. Finally, the greater part of the central region emerged (i) joining all the peripheral regions (St Anne, southwest, northwest, Caravelle), (ii) allowing an expansion of the central lineage from its small geographical base into the rest of the central region and into the now connected Caravelle peninsula, and (iii) resulting in secondary contact between the central region lineage and each of the peripheral lineages (southwest, northwest and southern lineages).
Although matrix correspondence allowed a test of association between the geological regions and the mitochondrial DNA lineages, the lineages are too divergent for nested clade analysis (Templeton 1988) as the absence of intermediate haplotypes does not give a significant parsimonious connection among these divergent lineages. The full evolutionary/taxonomic status of these divergent lineages is beyond the scope of this study, but in the one case where a multivariate analysis of the phenotype at a contact zone has been undertaken (southern and central lineages) the transition is very sharp, and at a pinpoint locality where they meet, there appears to be a bimodal distribution (Fig. 6). This argues against complete introgression of the genome at this point and perhaps argues for full species status for this southern form (Thorpe et al. 2002). However, the degree of gene flow among populations is under investigation (Ogden & Thorpe 2002) and taxonomic revision awaits further clarification of the ambiguous situation in this species complex.
In spite of the divergence of these lineages, neither the previous allozyme work (Gorman & Kim 1975), nor subspecies (Lazell 1972), indicates compatible divergence. The allozymes were sampled from populations that can be ascribed to lineages with estimated diverge of 7.6 Ma, yet they failed to detect divergence. This does not recommend them for this type of work and raises the issue of whether it is sound to combine past allozyme and current molecular data as in Creer et al. (2001). The six subspecies give a tangential, qualitative, reflection of biotope adaptation and do not relate to the lineages, except the southern lineage, which coincides with a dry habitat. A similar situation occurs with the subspecies in the Dominican anole (Malhotra & Thorpe 2000a), and the practice of sampling in molecular evolutionary studies according to conventional subspecies has nothing to recommend it, neither has the practice of mindlessly upgrading subspecies to species status.
Colour and pattern
Overall, the hue and colour pattern variation are dominated by adaptation to biotope rather than reflecting the lineages, this is shown in the matrix correspondence tests (Table 2) and Fig. 8. There are two notable exceptions to this. First, the southern lineage has chevrons (Fig. 2a) even though it occupies both ‘less xeric’ as well as ‘extreme xeric’ habitats and the approximate contact between southern and central lineages is marked by a change in chevron frequency (2, 3). This association with phylogeny is reflected in the matrix correspondence tests. Given the limited size and xeric nature of the St Anne peninsular, chevrons may have become genetically fixed during a past bottleneck. Second, the only other exception is the association between a small section of a contact zone between the north-northwestern (Fig. 2f) and central lineages (Fig. 2b) at its low-altitude eastern limit (Fig. 3). In this limited region there is a marked change in pattern and hue. However, the contact zones among lineages are generally not reflected in the hue or colour pattern changes, even though the lineages are so divergent. Consequently, although colour and pattern may have some association with intraspecific phylogeny in Lesser Antillean anoles, including A. trinitatis on St Vincent (Thorpe 2002) and A. oculatus on Dominica (Malhotra & Thorpe 2000a), it tends to be less influential than adaptation to the biotope.
There are clear generalizations in adaptation to biotope, including two outstanding cases of convergence within Martinique, and parallels with colour evolution of anoles in other Lesser Antillean islands. Irrespective of the association between chevrons and phylogeny discussed above, each of the three main lineages on Martinique (west, central and southern) all have populations exposed to extreme xeric conditions and these tend to develop chevrons. These regions are the southern lineage on the St Anne peninsula, the extreme Atlantic tip of the Caravelle peninsula, which is part of the area occupied by the central lineage, and the driest part of the north Caribbean coast, which is part of the area occupied by the western lineage. This convergence of phylogenetically unrelated forms is illustrated on the phylogeny (Fig. 5). The other case of convergence involves a complex of features that gradually change with altitude until the forms in montane rainforests (e.g. localities 33 and 53) have an intense saturated green background hue (Fig. 8), on which is black marbling and several large non-UV white spots. This constellation of character states occurs in montane forms in the northern rainforest irrespective of whether they are of the northwestern or central lineage, producing effectively identical forms (2, 8) in the lineages with an estimated divergence of 7.6 Ma: a remarkable case of convergence. Such a trend (although not necessarily to its extreme endpoint), occurs wherever there is appropriate habitat, such as in the wetter, higher altitude areas occupied by the southwestern and central lineages. These trends were tested against altitude as it can be confidently and objectively measured. A range of ecological attributes are related to an increase in altitude such as increased rainfall, lower temperature and lush vegetation. These factors are not differentiated here, but the tendency for some of the populations from the lower altitude, high rainfall areas of the far northern part of Martinique to also have a tendency to posses these colour features, suggests rainfall and associated lush vegetation may be important.
In so far as hue is concerned, there is a trend for lower altitude xeric populations to have a higher proportion of red giving a dull brownish background, while wetter higher altitude populations have relatively more green. This trend is paralleled, not only in all appropriate Martinique lineages (Fig. 8), but also in the St Vincent anole A. trinitatus (Thorpe 2002). Moreover, bright green anoles (A. marmoratus) are also seen in similar montane habitats in Basse Terre, Guadeloupe. While other body regions have hue variation primarily associated with altitude and xeric habitats, the dewlap (primarily used for signalling) hue variation is primarily associated with the northern Atlantic coastal habitat. In this habitat the dewlap has notably higher short wavelength hues (UV, blue) in parallel to the situation found with A. trinitatus in St Vincent. Thorpe (2002) suggests this may be linked with ambient light conditions, but more work is required on this topic.
While adaptation by natural or sexual selection is suggested by these parallels, phenotypic plasticity is difficult to test and reject for these, or other, morphological features. Nevertheless, substantial common garden experiments on the anole from the adjacent island of Dominica strongly indicate that where geographically varying morphological features can be tested, one can reject plasticity, but not adaptation by natural selection, as the cause of the variation (Thorpe et al. 2002).
Acknowledgements
R.S.T. wishes to thank the authorities for permission to work in Martinique (particularly J.-L. Millo, A. Gourbeyre and M. Tanasi at the latter stages of this work), P. Jarne and M. Breuil for their assistance, Dr N. Giannasi for the majority of samples, Cathy Pook for computing assistance, Jacqueline Thorpe and Rob Ogden for field assistance, Wolfgang Wüster for his comments, the Leverhulme Trust (RF and G/2/9900012) and Linnean Society for funding fieldwork, and the Wellcome Trust for equipment and support (057257/Z/99/Z, 060384/Z/00/Z).
References
This paper forms part of a long-term study of the causal factors influencing geographical variation and speciation of lizards in the Lesser Antilles and Canary Islands led by Roger S. Thorpe. Dr Andrew G. Stenson's research has been on the population genetics and molecular phylogeny of anoles.




