Serum pharmacokinetics and tissue and milk residues of oxytetracycline in goats following a single intramuscular injection of a long-acting preparation and milk residues following a single subcutaneous injection
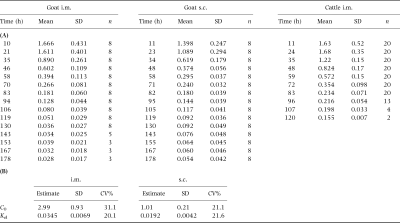
Abstract
Separate groups of goats were used to determine drug depletion patterns in serum (n=10), tissue (n=20) and milk (n=8) following a single intramuscular (i.m.) dose of 20 mg/kg of a long-acting oxytetracycline (OTC) formulation (Liquamycin® LA-200®). Milk residues were also determined following a subcutaneous (s.c.) administration of the same product at the same dose. Serum samples were taken for 24 h post-treatment and tissues (fat, liver, kidney, muscle and injection site) collected at 4, 7, 14, 21 and 28 days following injection. Milk from lactating goats was collected every 12 h for 8 days following both the i.m. and s.c. treatments utilizing an intervening 5-week washout period. Residues in serum and tissue were measured using a microbial inhibition assay, while milk residues were measured using both a microbial inhibition assay and a validated HPLC method. The serum pharmacokinetic parameters of OTC in goats were determined, with a mean AUC=67.4 μg h/mL, mean terminal half-life=14.4 h, and apparent clearance=0.33 L/kg h. Tissue half-lives could not be determined with confidence because the collection times provided only two points at which residues could be measured for most tissues. Oxytetracycline residues in all goat tissue samples measured less then cattle tissue tolerance by 96 h postdosing. One-compartment model describing milk depletion data for i.m. and s.c. dosing had terminal slope half-lives of 20.1 and 36.1 h, respectively. By 96 h post-treatment none of the milk samples contained OTC residues in excess of the cattle milk tolerance (0.3 p.p.m.). For both milk and tissue, the upper-bound 99% confidence intervals for the samples taken from goats 96 h postdosing were lower than approved cow milk and tissue tolerances.
INTRODUCTION
Oxytetracycline (OTC) is a broad-spectrum antibiotic that inhibits the growth of many pathogenic organisms, including bacteria, mycoplasma, and some protozoa (Prescott & Baggot, 1993). Oxytetracycline formulations are approved for use in the US in a number of food-producing species. Label indications for cattle include bacterial infections of the respiratory and GI tracts, eye, hoof, and uterus, as well as leptospirosis and wound infections. There are numerous published studies examining the pharmacokinetics of OTC in cattle and the effects of formulation, animal age, and disease-state on kinetic parameters. While there has been some limited data published on OTC pharmacokinetics in goats (Table 4), no papers have reported tissue depletion in that species. There has been one short communication regarding milk residues in goats following intramuscular (i.m.) administration of an unspecified formulation (Reja et al., 1994) and no published milk studies addressing milk residues following subcutaneous (s.c.) treatment in goats.
There is no OTC product approved in the US for use in goats. The tissue and milk residue data presented here were collected to support a drug approval application for goats of a sustained-release formulation of OTC (Liquamycin® LA-200®, Pfizer Animal Health, Exton, PA, USA). This food safety data represents only a portion of the information collected for applications made under the auspices of United States Department of Agriculture (USDA)'s National Research Support Project Number 7 (NRSP-7), the Minor Species Drug Approval Program.
MATERIALS AND METHODS
Animals
For the serum pharmacokinetic study, 10 female mixed breed goats weighing between 30 and 36 kg were used. For the tissue residue study, 21 mixed breed goats weighing at least 32 kg at the time of initial injection were used. Both groups of animals were housed at the Caldwell Veterinary Teaching Center for 7 days before drug treatment. The goats were examined by a veterinarian prior to inclusion in the study, and only healthy animals were used. Water was available ad libitum. For the milk residue study, 10 lactating goats weighing an average of 59 ± 7 kg (range=51–68 kg) were used. The goats were part of the herd at the University of California, Davis (UCD) Dairy Goat Research Center and were housed in an outside pen with access to shelter from rain and sun. Water was available ad libitum. This study was performed in accordance with all local, state and federal laws, ordinances, rules and regulations applicable to animal welfare.
Drug treatment
For both the serum pharmacokinetic and tissue residue studies a single i.m. injection into the semimembranosus/semitendinosus muscle of a long-acting OTC product (Liquamycin® LA-200®) was given at 20 mg/kg body weight. For the milk studies, treated goats received the same product and dose as above, first i.m. and then s.c. (in the shoulder/neck region) with an intervening 5 week wash-out period. One animal for the tissue study and two animals for the milk studies remained untreated as controls.
Sampling
Serum.
Ten millilitre blood samples were collected via jugular venipuncture from each animal pretreatment and at 5, 10, 15, 30 and 45 min, and 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12 and 24 h postdosing.
Tissue.
Four goats were slaughtered on each of the following days postinjection: 4, 7, 14, 21 and 28. Samples of liver, kidney, fat, skeletal muscle and injection site were taken and frozen immediately. The control animal was slaughtered and samples of the same tissues taken for use in assay validation and as spiked storage samples.
Milk.
Goats were milked twice a day and samples collected for a total of 9 days (1 day before treatment and 8 days following treatment). Each goat was milked individually by an automated milking machine giving a composite sample from which subsamples were taken. All samples were stored below –75°C until assayed.
Sample handling
Blood samples were allowed to clot for at least 1 h at room temperature and then centrifuged at 1000 × g for 10 min. The serum was separated into two aliquots which were frozen immediately at –70°C. All samples were kept frozen at < –20°C until assayed. One aliquot of each serum sample was shipped to the University of California NRSP-7 laboratory for assay, and all samples arrived frozen and in good condition. Tissue samples were immediately frozen in separate containers. All samples were kept frozen at < –20°C until assayed. All tissue samples were shipped to the University of California NRSP-7 laboratory for assay, and all samples arrived frozen and in good condition. Standards were created using control serum and tissues, and frozen to provide stability data.
Serum analysis
Quantitation of OTC in serum samples was accomplished using a modification of the microbial inhibition assay of Bennett et al. (1966). Bioassay plates were prepared by placing 9.5 g Mueller Hinton Medium (Difco Laboratories, Detroit, MI, USA) and 250 mL H2O into 500 mL Erlenmeyer flask which was autoclaved for 20 min solution then cooled to 50°C in a water-bath. One millilitre of a commercially available Bacillus cereus spore suspension (Difco Laboratories) was diluted in 50 mL of sterile saline and 0.4 mL of the diluted suspension was added to the media. After pouring and solidification of the media, 500-μL wells were cut into the solidified bioassay plates. Serum samples (500-μL) were placed directly into the wells without a cleanup step. Standards prepared using control serum were also added to each plate, and the plates were incubated overnight at room temperature (23°C). Zones of inhibition were measured using micrometers and the results from the standards used to calculate the OTC concentration in each sample. The standard curves were linear between 0.125 and 16 μg/mL, the average recovery was 105%, the overall precision (between days, reported as relative standard deviation) was 23%, and the limit of detection for the assay for serum samples was 0.01 μg/mL.
Tissue analysis
The microbial inhibition assay for tissues differed from that used for serum only in extraction method. After solidification of the media, 500-μL wells were cut into the bioassay plates. Twenty gram tissue samples were homogenized in 80 mL of a potassium phosphate buffer (0.1 M, pH 4.5) and centrifuged. Triplicate 500-μL aliquots of the supernatant from each sample were pipetted into individual wells. Standards prepared in control tissue were also added to each plate, and the plates were incubated overnight at room temperature (23°C). Zones of inhibition were measured using micrometers and the results from the standards were used to calculate the OTC concentration in each of the samples. The limit of detection of OTC in all tissues was less than 0.1 μg/mL. The standard curves were linear between 0.1 and 2 μg/mL and overall precision for all tissues was 8.5%. Recoveries of OTC, as measured by comparing extracted spiked tissue sample results to buffer-only standards averaged 100% for all tissues.
Milk analysis
Oxytetracycline milk residues were measured by both a microbial inhibition assay and a HPLC assay. The microbial inhibition assay used is described in the FDA document Antibiotic Residues in Milk, Dairy Products, and Animal Tissues: Methods, Reports and Protocols (Anonymous, 1968), with modifications described in Bennett et al. (1966). Bioassay plates were prepared by placing 6.38 g Antibiotic Medium no. 8 and 250 mL H2O into a 500-mL Erlernmeyer flask and autoclaving for 20 min. The resulting solution was cooled to 50°C in a water-bath, and 0.8 mL of spore suspension (same as for serum assay) added. After solidification of the media, 500-μL wells were cut into the solidified bioassay plates. Milk samples were prepared for the wells by adding 1.5 mL 0.1 M phosphate buffer to 0.75 mL milk. Triplicate 500-μL aliquots of each sample were pipetted into individual wells. Standards prepared with control milk were also added to each plate and the plates were incubated overnight at room temperature. Zones of inhibition were measured using micrometers and the results from the standards were used to calculate the OTC concentration in each of the samples. The assay was linear between 0.15 and 2.4 μg/mL, the limit of detection for the assay was 0.15 μg/mL and the recovery was 98% with an overall precision of 11%.
The method of Carson and Breslyn (1996) was also used to quantify OTC residues in milk. Defatted milk was precipitated with sodium succinate and the supernatant applied to a minicolumn containing Sepharose 6B fast flow and copper sulphate. The minicolumns were eluted with McIlvaine buffer containing 0.1 M EDTA and 0.5 M sodium chloride. The eluate was then ultra-filtered before injection into the HPLC. Detection was by UV at 355 nm using a tertiary gradient of 10 mM oxalic acid:methanol:acetonitrile. The column was a PLRP-S 100 A, 5 μm, 150 × 4.6 mm with a PLRP-S guard column cartridge at 30°C. The assay was linear between 5 and 500 ng/mL, the LOD and LOQ were 7 and 10 ng/mL, respectively, and the mean recovery was 77% with an overall precision of 11.3%.
A comparison of the microbial inhibition and HPLC assays was performed using aliquots of identically incurred residue and spiked residue milk samples. Ratios of the microbial inhibition assay results to the HPLC method results were calculated after adjustment for recovery.
Pharmacokinetic data and statistical analysis
The data for the serum concentrations of OTC in goats were analysed using WinNONLIN™ (Pharsight Corporation, 299 California Avenue, Palo Alto, CA, USA). A two-compartment open model with first order absorption provided the best fit for all of the individual animal data based on the Akaike information criterion. The parameters calculated were the AUC0→∞ (area under the curve to infinity), Kab (absorption rate constant), λ1 (primary disposition phase rate constant), λ2 (secondary disposition phase rate constant), A (primary disposition phase intercept), and B (secondary phase disposition phase intercept). The observed Cmax (maximum serum concentration) and Tmax (time of maximum serum concentration) were taken directly from the data and half-lives for the model rate constants were calculated as t1/2=0.693/rate constant. To obtain estimates of the elimination rate of OTC in milk, the milk time-concentration data from 21 to 178 h postdosing were modelled with WinNONLIN™ using a one-compartment open model with i.v. input and first order elimination. The HPLC milk assay, having a lower limit of detection, resulted in more data points than the microbial inhibition assay. Because the HPLC assays provided a richer data set, all pharmacokinetic modelling for milk was performed using the HPLC data only.
RESULTS
Table 1 shows the derived serum pharmacokinetic parameters for OTC in goats. The rate of elimination variation for the Tmax and Cmax values were large indicating considerable subject variability in the absorption of the drug. Figure 1 shows the time/concentration plot of the data points and the line predicted by the mean parameter values using a two-compartment model with first-order absorption and elimination.
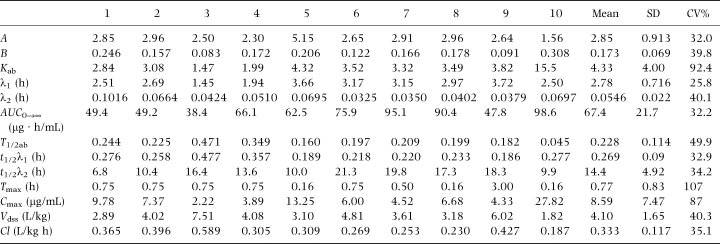
. Oxytetracycline concentrations in goat serum following a single i.m. injection of 20 mg oxytetracycline (Liquamycin® LA 200®) per kilogram body weight (n=10). The line represents the concentrations predicted by the mean parameter values for a two-compartment open model.
The results of the goat tissue residue determinations are presented in Table 2. The sampling times chosen for the study did not yield sufficient data points to calculate residue half-lives. Nevertheless, the data are useful in demonstrating that average OTC residue in tissue samples measured less than cattle tissue tolerances by 96 h postdosing.
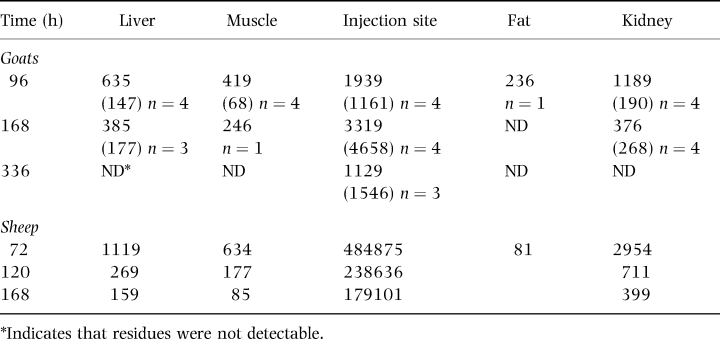
Table 3 shows the mean milk concentrations of OTC in goat milk, compared with those reported for dairy cattle dosed with the same product at 20 mg/kg (Anonymous, 1998b). Terminal slope half-lives of OTC were 20.1 and 36.1 h for i.m. and s.c. dosing, respectively. Figure 2 shows a plot of the milk data with the line predicted from the s.c. and i.m. paramaters.
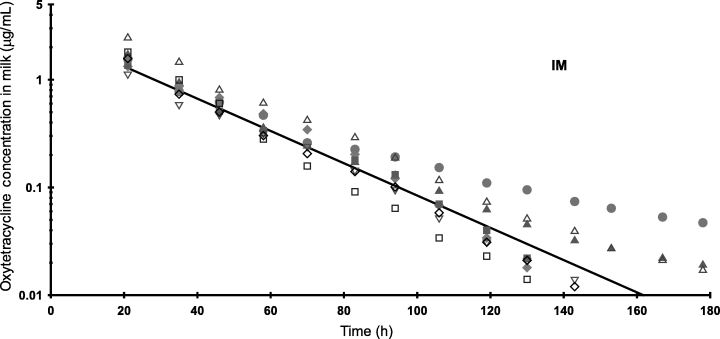
. Oxytetracycline concentrations in goat milk (HPLC method) following i.m. and s.c. dosing with 20 mg/kg (n=8). The lines represent the predicted concentrations for each dosing route using the mean parameter values for a one-compartment open model.
The results of the comparison of the microbiological assay with the HPLC assay for OTC incurred residues in milk showed that the mean ± standard deviation of the bioassay/HPLC assay ratio for all incurred residue samples was 1.33 ± 0.24. The mean ± standard deviation of the ratios for fortified samples was 1.19 ± 0.21.
DISCUSSION
A variety of OTC parenteral products in the US are available for cattle and swine, but none are approved for use in sheep or goats. Commercial cattle products are typically classified as either `conventional' or `long-acting', depending on their OTC concentration and type of vehicle. Conventional formulations having 50–100 mg/mL are labelled for daily i.v., i.m. or s.c. administration at 3–5 mg/lb (6.6–11 mg/kg). Long-acting preparations uniformly contain 200 mg/mL and are labelled for a single i.m. or s.c. administration of 9 mg/lb (19.8 mg/kg). Manufacturers promote long-acting products as providing therapeutic OTC blood concentrations for 72 h.
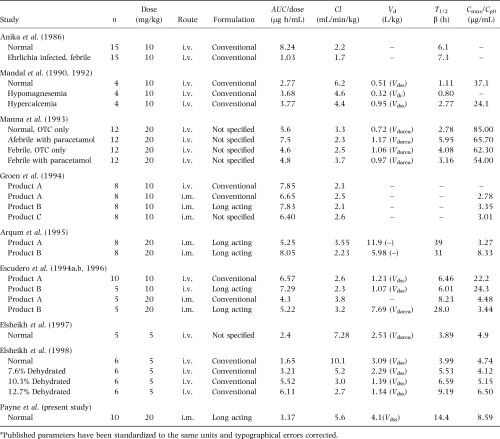
Differences in product formulation, patient species and health can result in variations in pharmacokinetic parameters. Published parameters following OTC administration to goats are summarized in Table 4. Only two of these citation studies (Escudero et al., 1994a; Arqum et al., 1995) reported complete parameters in goats following i.m. administration of long-acting formulations. Notable differences between those studies and the present one, respectively, are AUC/dose (5.22–8.05 vs. 3.37 μg h/mL, respectively), Cl (2.23–3.55 vs. 5.6 mL/min/kg), and T½β (28–39 vs. 14.4 h). These differences are likely accounted for by the fact that serum samples in the present study were not collected beyond 24 h postdosing and the prolonged elimination phase of OTC from the long-acting preparation was not observed.
There have been no previously published studies of OTC residues in goat tissues and only limited tissue data regarding OTC residues in other small ruminants. Nouws et al. (1990) reported concentrations of OTC in tissues taken from sheep following i.m. injection of three sustained-release formulations at 20 mg/kg. Ten days following treatment all muscle samples were below the limit of detection of 0.08 p.p.m. and average kidney concentrations ranged from 0.17 to 0.27 p.p.m.
Table 2 compares the present study's goat tissue results with that of earlier work by the same authors in sheep using the same drug formulation and dose. By 168 h post-treatment kidney concentrations of both species were virtually identical. At that time liver and muscle concentrations in goats were at least twice as high as in sheep but the injection site residues were considerably lower. The question of whether these observed differences in tissue depletion is real or experimental artefact remains unanswered. Different analytical methods (bioassay for goat and HPLC for sheep) could account for much of the variation. Elsheikh et al. (1997) and Escudero et al. (1996) however, reported statistically significant differences in OTC blood pharmacokinetics in direct comparisons of sheep and goats, possibly signalling real differences in drug disposition between the two species.
In 1998 the Food and Drug Administration established new tissue tolerances for the tetracycline family of drugs based on their effect on intestinal microflora (Anonymous, 1998a, b). These tolerances represent the permitted sum of tetracycline residues to include tetracycline, chlortetracycline and OTC. Cattle tolerances are 2 p.p.m. for muscle, 6 p.p.m. for liver, and 12 p.p.m. for both kidney and fat. These revised tolerances are considerably larger than the previous US tolerance of 0.1 p.p.m. for all edible tissues of cattle.
In the present study all OTC residues in tissue samples measured less than cattle tolerances by the first tissue sampling, 96 h (4 days) postdosing. In addition, the 99% upper bound confidence interval for tissue concentrations measured at day 4 were less than the cattle tissue tolerance. Conventional cattle products have slaughter withdrawal times ranging from 18 to 22 days. When used at their labels' higher dose, long-acting products have a 28-day slaughter withdrawal time. Most commercial OTC cattle products had withdrawal times established prior to the creation of the new, higher tolerances.
Data describing depletion of OTC in goat milk in the published literature are scarce. Two studies (Hill et al., 1984; Long et al., 1984) involve intramammary administration and are not relevant to the present study. A brief communication by Reja et al. (1994) reported OTC milk residues of 0.459 p.p.m. 100 h following the last of four daily i.m. treatments at 15 mg/kg of an unspecified OTC product.
The product used in the present study received a label addition for dairy cattle in 1998 (Anonymous, 1998b). That label change permits milk from treated cows to be marketed 96 h following a single i.m. or s.c. dose of 20 mg/kg. With that approval the Food and Drug Administration concurrently established the US first tolerance for tetracyclines in milk (Anonymous, 1998b). Like the revised tissue tolerances, the new cow-milk tolerance of 0.3 p.p.m. is based on the sum of all tetracycline family residues detected in a sample.
In the present study all milk samples from goats treated either i.m. or s.c. contained less OTC than cow milk tolerance by 96 h. The 99% upper bound confidence interval on concentrations measured 94 and 95 h after i.m. and s.c. dosing are 0.17 and 0.18 p.p.m., respectively, both lower than the cattle milk tolerance of 0.3 p.p.m. These data suggest that the milk withholding period established for cattle (4 days) will be adequate to ensure that goats treated with 20 mg/kg long-acting OTC preparation will not have residues in milk at a concentration relevant to human health concern.
ACKNOWLEDGMENTS
This study was funded primarily by the USDA CSREES National Research Support Project Number 7 (NRSP-7), the Minor Use Animal Drug Program. The authors are grateful and indebted to Ms. Sandra Ogletree, the Western Region NRSP-7 Administrative Assistant, for her outstanding technical assistance in the conduct and coordination of the research and editorial assistance in the preparation of this manuscript.