The circulating renin–angiotensin system during treatment with metoprolol or captopril in patients with heart failure due to non-ischaemic dilated cardiomyopathy
Abstract
Abstract. Jansson K, Dahlström U, Karlberg BE, Karlsson E, Nylander E, Nyquist O, Karlberg K-E (Linköping University Hospital and Huddinge University Hospital, Sweden). The circulating renin–angiotensin system during treatment with metoprolol or captopril in patients with heart failure due to non-ischaemic dilated cardiomyopathy. J Intern Med 1999; 245: 435–443.
Objective. To investigate the effects of beta-blocker (metoprolol) or angiotensin-converting enzyme inhibitor (captopril) treatment on neurohormonal function in a randomized prospective study on patients with heart failure due to dilated cardiomyopathy.
Patients. Fifty-four patients (42 men and 12 women, mean age 50 years) were studied. There were three patients in NYHA (New York Heart Association) functional class I, 32 patients in class II and 19 patients in class III.
Methods. Measurements of plasma renin activity (PRA), plasma angiotensin II (A II) concentration and plasma atrial natriuretic peptide (ANP) concentration were made at rest and also in a subgroup (n = 32) during exercise. The urinary excretion of aldosterone was also determined. Investigations were performed at baseline, and after 3 and 6 months. Therapy was then stopped and the patients were re-investigated 1 month thereafter.
Results. The mean level of PRA was normal at baseline, reduced during therapy with metoprolol, and increased during therapy with captopril. The mean plasma concentration of A II was reduced during exercise and there was a trend towards a reduction even at rest in the metoprolol group, but not in the captopril group. The urinary excretion of aldosterone decreased in both groups. The mean plasma concentration of ANP was elevated at baseline and declined during exercise in the metoprolol group.
Conclusion. In patients with dilated cardiomyopathy and only a partly activated renin–angiotensin system, both metoprolol and captopril reduced urinary excretion of aldosterone. Furthermore, metoprolol suppressed the exercise-induced increase in ANP, suggesting a favourable effect on ventricular performance.
Introduction
Congestive heart failure (CHF) is characterized by depressed cardiac function and abnormalities in neurohormonal regulation [1]. Several neurohormonal systems are involved in the regulation of fluid and electrolyte balance. One of the most important is the renin–angiotensin–aldosterone system (RAAS). There are also neurohormonal systems within the heart itself. One of these is the atrial natriuretic peptide (ANP) system [2–4]. Several studies have shown a good correlation between circulating ANP levels and cardiac filling pressures and have also demonstrated the effect of exercise on ANP levels [5–8].
Disturbances in the neurohormonal systems as well as an activated sympathetic nervous system are related to the prognosis in CHF [9, 10].
Many papers [11–14] have discussed the use of beta-blockers in patients with heart failure due to idiopathic dilated cardiomyopathy (DCM). The theoretical background is that, in this situation, the heart could benefit from protection against adrenergic overstimulation. There are reports showing beneficial effects on left ventricular (LV) function [12–15], as well as reduced mortality [16]. On the other hand, there are also reports that do not confirm these results [17–18].
The aim of the present study was therefore to investigate the effects of a beta-adrenergic receptor blocker (metoprolol) and an angiotensin-converting enzyme (ACE) inhibitor (captopril) on neurohormonal regulation in patients with heart failure due to idiopathic DCM. In our study, components of the RAA and ANP systems were investigated both at rest and during supine exercise. The circulating concentrations of these hormones were studied before, during and after therapy with either metoprolol or captopril.
Patients and methods
Inclusion/exclusion criteria
The inclusion criteria were: patients fulfilling the WHO/ISFC task force criteria for the definition and classification of dilated cardiomyopathy [19]; and age between 18 and 65 years.
The exclusion criteria were: coronary artery disease (> 50% reduction in diameter of a major epicardial vessel) based on coronary angiography performed in all patients over the age of 30 years; clinical signs or a history of myocarditis; obstructive lung disease requiring treatment with beta-2 agonists; excessive alcohol consumption (> 700 g week–1); diabetes mellitus; history of hypothyroidism or thyrotoxicosis; hypertension or other serious disease; heart valve disease; AV-block II or III; severely depressed renal function (serum creatinine > 200 µmol L–1); previous or ongoing treatment with beta-blockers or ACE inhibitors; and, finally, patients in need of heart transplantation.
Patients
Fifty-four patients (42 men and 12 women), with a mean age of 50 (range 26–62) years, were included. The patients were classified according to the recommendations of the New York Heart Association (NYHA). As a result of the inclusion and exclusion criteria, no patient with a history of ischaemic heart disease or diabetes mellitus was included in the study. The diagnosis of DCM was based on echocardiography. Measurements were carried out according to the recommendations of the American Society of Echocardiography [20]. LV dilatation was defined as LV end-diastolic dimension greater than 2 mm above the upper limit of normal for age and body weight [21]. Systolic dysfunction was defined as a fractional shortening less than 24%. To balance the more severely diseased patients between the two groups, an exercise test was performed before inclusion. The test was symptom-limited and maximal work performance was related to reference values for age, sex and body weight [22]. Patients were stratified according to exercise capacity above or below 50% of the reference value.
Medical therapy
Only patients with a history of atrial fibrillation were treated with digoxin (n = 21). Amiodarone was used in two patients (both in the captopril group) because of previous ventricular dysrhythmias. Almost all patients (n = 51) suffered from dyspnoea to some extent and 49 patients were treated with diuretics (furosemide). The dosage could be changed during the study, if necessary. No patient was treated with vasodilating drugs.
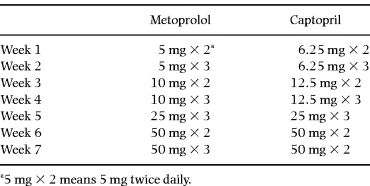
Treatment was started with either metoprolol 5 mg twice daily or captopril 6.25 mg twice daily. The daily dose was increased over 6 weeks according to the schedule in Table 1. The target dose for metoprolol was 150 mg daily and for captopril 100 mg daily. Drugs were administered on a double-dummy basis.
Study design and methods of patient evaluation
The study was designed as a prospective, double-blind, controlled parallel-group investigation. After stratification, the patients were randomized to active treatment with either captopril or metoprolol. All patients were investigated four times over 7 months. The neurohormonal systems were studied at rest and also, in a subgroup of patients (n = 32), during supine exercise in the middle of the day when the patients had fasted for 4 h. The investigations were always performed at the same hour of the day for each individual patient and about 4 h after intake of the medication.
All blood samples were drawn from a previously inserted catheter in a forearm vein after the patients had been resting on a couch in the supine position for at least half an hour. An electrically braked bicycle ergometer was then attached to the lower end of the couch. The patients exercised for 6 min at an initial workload of 20 W (watts), which was the lowest possible. This was immediately followed by a workload individually chosen as 40% of the patient’s maximal workload capacity at steady state during a seated exercise test. This corresponded to an absolute workload of 20–70 W with a mean of 40 W. Three patients performed at the lowest workload only (20 W) due to low work capacity. The blood samples during exercise were taken when the patients had exercised for 11 min, except for the three patients who could only perform at a workload of 20 W, in which case the duration of exercise was only 6 min.
After the baseline investigation (I), therapy was started and new investigations were performed 3 (II) and 6 (III) months later. The treatment was then single-blindly stopped and a new investigation was performed approximately 1 month later (IV).
Urine was collected over 24 h in these 32 patients, who also had their neurohormones investigated during exercise.
All patients gave their informed consent to the trial, which was approved by the local committee of ethics at the University Hospital of Linköping (L) and the University Hospital of Huddinge (H). The study was planned and performed before the beneficial effects of ACE inhibitor treatment in patients with mild-to-moderate heart failure were known.
Hormone assays
Plasma renin activity (PRA) was determined as angiotensin I generated as measured by radioimmunoassay (RIA) [23]. Intra- and interassay coefficients of variation were 4 and 11%, respectively. Plasma concentration of A II was also measured by RIA [24]. Here intra- and interassay coefficients of variation were 10 and 6.6%, respectively. Urinary excretion of aldosterone was analysed using RIA and the methods and antibodies described by McKenzie & Clements [25] and Brown et al. [26], utilizing an anti-aldosterone antibody with unique specificity. Plasma concentrations of ANP were also determined by RIA, using a commercially available kit [27]. Our reference range with this method in a reference population (n = 219) was 2.3–10.5 pmol L–1.
Statistical methods
All data are given as means. Calculations were performed using the software Statview 4.5 for the Macintosh computer. The interaction effect was determined by analysis of variance ( anova) with repeated measurements including values at baseline (I), and after 3 (II) and 6 (III) months of therapy. These analyses were also performed with log-transformed values, but this did not change the conclusions. Comparison between the treatment effects on ANP was calculated using a two-sided t-test for unpaired groups.
Results
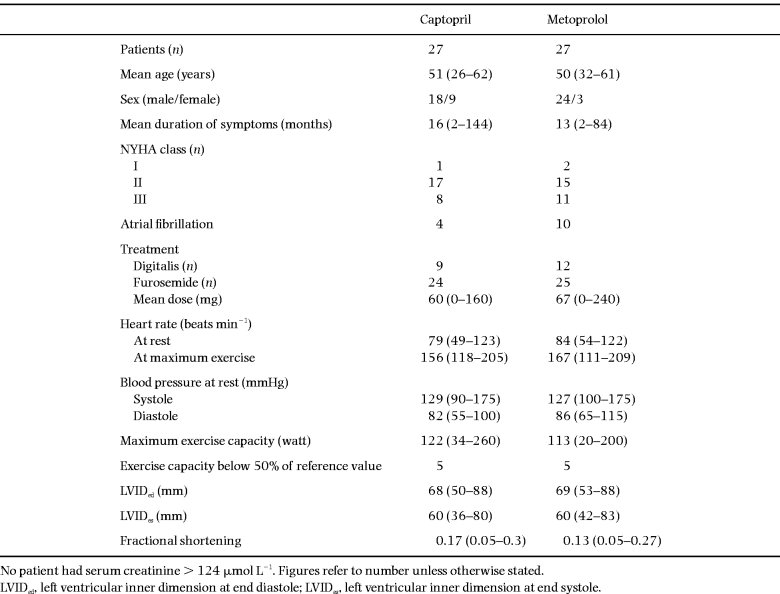
The clinical characteristics at baseline are shown in Table 2. There were only minor differences regarding NYHA function class, duration of symptoms, exercise capacity and treatment between the captopril and the metoprolol groups at baseline. In the captopril group there were more female patients, and in the metoprolol group there were more patients with atrial fibrillation. There were no differences regarding baseline clinical characteristics between the subgroup (n = 32), which was also investigated during exercise, and the patients who did not perform this investigation (n = 22).
Drug tolerance
Both drugs were well-tolerated. The mean daily dose of metoprolol was 135 mg and that of captopril was 98 mg [28]. The dosage of furosemide was increased in two patients in each group during the study, and in one patient in each group the dosage was reduced. All patients, with one exception, performed both investigations I and II, but thereafter two patients (one in the captopril and one in the metoprolol group) did not perform investigations III and IV, both belonging to the group not investigated during exercise (one of them was lost and the other withdrawn).
Renin–angiotensin–aldosterone system and ANP
The neurohormonal data regarding the RAAS and the ANP values are shown in 1-4.
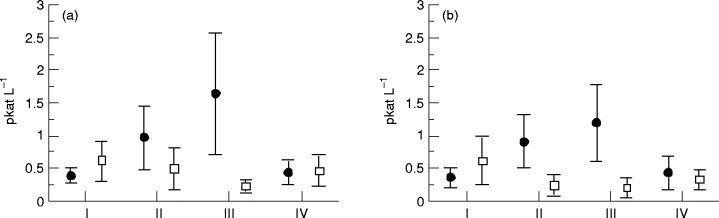
PRA at baseline (I), after 3 and 6 months of therapy (II and III), and 1 month after therapy was stopped (IV). Values are means and 95% confidence intervals. (a) At rest: ●, captopril (n = 26); □, metoprolol (n = 25). P < 0.001 in the captopril group; P < 0.05 in the metoprolol group ( anova). (b) During exercise: ●, captopril (n = 15); □, metoprolol (n = 16). P < 0.001 in both the captopril and metoprolol groups ( anova).
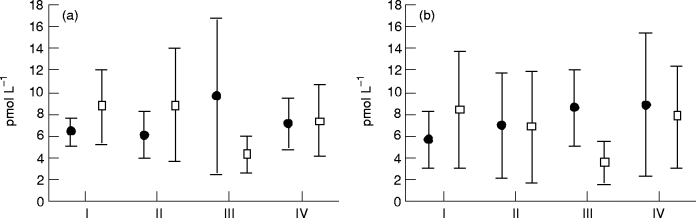
Angiotensin II at baseline (I), after 3 and 6 months of therapy (II and III), and 1 month after therapy was stopped (IV). Values are means and 95% confidence intervals. (a) At rest: ●, captopril (n = 26); □, metoprolol (n = 25). P = 0.058 in the metoprolol group ( anova including I, II and III). (b) During exercise: ●, captopril (n = 15); □, metoprolol (n = 16).
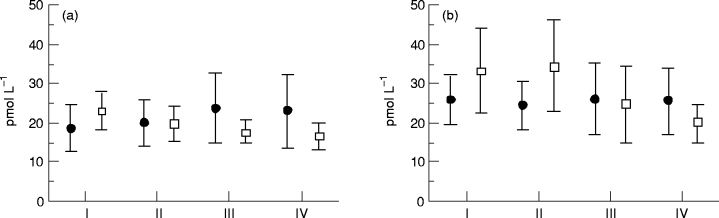
ANP at baseline (I), after 3 and 6 months of therapy (II and III), and 1 month after therapy was stopped (IV). Values are means and 95% confidence intervals. (a) At rest: ●, captopril (n = 26); □, metoprolol (n = 25). (b) During exercise: ●, captopril (n = 15); □, metoprolol (n = 16). P < 0.05 in the metoprolol group ( anova).
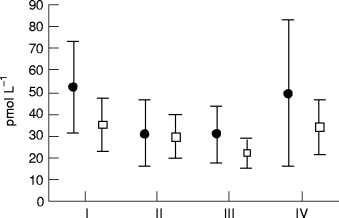
Twenty-four hour urinary excretion at baseline (I), after 3 and 6 months of therapy (II and III), and 1 month after therapy was stopped (IV). Values are means and 95% confidence intervals. ●, captopril (n = 15); □, metoprolol (n = 16).
Renin. The PRA levels were within our normal range at baseline. There were no significant differences between the values at rest and those during exercise in both the metoprolol and captopril groups. During treatment with metoprolol, there was a reduction in PRA levels both at rest (P < 0.05) and during exercise (P < 0.001). During treatment with captopril, the expected increase in mean PRA level was observed both at rest (P < 0.001) and during exercise (P < 0.001). After stopping treatment, the PRA level returned to near baseline values in both groups (see Figs. 1a and b).
Angiotensin II. The plasma angiotensin II concentrations were within the normal range in both the metoprolol and captopril groups at baseline. There was, however, a reduction in the plasma angiotensin II concentration both at rest and during exercise in the metoprolol group. The reduction at rest reached only borderline significance (P = 0.058). In the captopril group, there were no differences in concentration either at rest or during exercise (see Figs. 2a and b).
ANP. At baseline, the mean plasma concentration of ANP in both the metoprolol and captopril groups was at least twice that of our reference population (see ‘Methods’). At rest, the plasma ANP levels in the metoprolol group did not change during the study. However, during exercise, plasma ANP was reduced in the metoprolol group (P < 0.05) compared with the baseline level. Physical exercise usually stimulates the secretion of ANP. At baseline, an increase in the level of ANP during exercise compared with resting values (P < 0.01) was observed, but after therapy with metoprolol, resting and exercise ANP values were comparable (see Figs. 3a and b). In the captopril group, there were no differences in concentration at rest and during exercise compared with baseline. However, the difference between these two pharmacological strategies on the level of ANP did not reach statistical significance.
The circulating levels of ANP were the same in the different NYHA classes (in pmol L–1): 23.8 in NYHA I (only three patients), 18.8 in NYHA II and 23.0 in NYHA III. The ventricular filling pressures were investigated at baseline and after 3 months of therapy [28] and there were no correlations between the left or right ventricular filling pressures (at rest and during exercise) and the level of ANP in either the metoprolol or the captopril group.
Aldosterone in urine. Twenty-four hour urinary aldosterone excretions are shown in Fig. 4. There was no difference between the two groups at baseline. In both the metoprolol and captopril groups, there was a significant reduction in urinary aldosterone excretion (P < 0.05). After therapy was stopped, urinary excretion of aldosterone returned to baseline levels in both groups ( Fig. 4).
Discussion
At rest
This report is part of another study concerning the haemodynamic changes during treatment with either a beta-blocker or an ACE-inhibitor in patients with dilated cardiomyopathy [28]. In that study, metoprolol was shown to reduce the LV filling pressure and to increase LV stroke volume to a greater extent than did captopril. In the present study on patients with heart failure due to dilated cardiomyopathy (DCM), the baseline PRA and plasma A II concentration were within reference range despite symptomatic heart failure and ongoing treatment with diuretics in all but five patients. In a substudy of the SOLVD study, Francis et al. [29] found normal PRA levels in patients with left ventricular dysfunction who did not receive diuretic treatment and who had no symptoms of heart failure. In their study, heart failure was mainly caused by ischaemic heart disease, but there were also patients with dilated cardiomyopathy included. The daily dose of furosemide in the present study was only in the region of 60 mg, and Fitzpatrick et al. [5] reported that a daily maintenance dose of furosemide lower than 80 mg had only a modest stimulatory effect on renin and aldosterone levels in patients with stable heart failure. However, they found increasing levels of renin, A II and aldosterone when increasing the daily maintenance doses of furosemide to above 80 mg. On the other hand, Anand et al. [30] observed that a daily dose of 40 mg of furosemide was enough to increase PRA. Even if the patients in the present study did not have increased PRA, angiotensin II or urinary excretion of aldosterone, they had obvious findings of congestive heart failure, clinically and at cardiac catheterization [28]. In this study, the baseline values of ANP were twice ( Fig. 3a) the reference value, but the level of ANP did not correlate to NYHA function class or to the filling pressures in the right or left atria as previously reported [31]. Still, there are studies which do not find a correlation between the filling pressure and the level of ANP [32]. Nevertheless, in the present study, the ANP levels were reduced during exercise in the metoprolol group, and this group had a greater reduction in heart rate and left ventricular filling pressure in the haemodynamic study [28]. ANP is known to have an inhibitory effect on renin secretion in healthy subjects [33] and this may, to some extent, explain the normal PRA and A II concentration in our patients, more than half of whom belonged to NYHA function class I or II. However, Richards et al.[33] did not see this inhibitory effect in patients with congestive heart failure.
Therapy with metoprolol. This reduced PRA and A II concentration as expected, which is in accordance with Eichhorn et al.[34] who reported that bucindolol, a non-selective beta-antagonist, reduced PRA in a similar fashion. Moreover, the mean urinary excretion of aldosterone was reduced, which clearly demonstrates the beta-receptor antagonist’s ability to inhibit neurohormonal activation [34, 35]. The levels of ANP at rest were unchanged throughout the study.
Therapy with captopril. This increased PRA as expected. The level of A II was within the reference range at baseline, and remained unchanged at rest throughout the study. The reason for this may be that the formation of A II is dependent on factors other than ACE. There are several mechanisms affecting A II formation that may be involved during chronic ACE inhibition. These may involve enzymes that are not affected by ACE inhibitors, such as endothelial cell peptidyl dipeptidase [36], renal carboxypeptidase [37] or the heart chymase enzyme [38]. Furthermore, angiotensin II assays are difficult and cross-reactivity with the decapeptide angiotensin I may occur and could to some extent explain why the level of A II remained unchanged. The reduction in the urinary excretion of aldosterone, indicating that at least the A II-dependent secretion of aldosterone was decreased, supports the latter. The level of ANP was elevated at baseline but did not change during therapy. ANP is reported to be secreted mainly from the two atria in response to increased atrial stretch [39]. Webster et al. [40] found the level of ANP to be reduced by vasodilation when using nitroglycerin intravenously. Their patients appeared to have a more severe degree of heart failure judging by the daily doses of furosemide used and the ANP levels, which were both much higher than in our study. Another explanation could be different doses and timing. In our investigation, captopril was given twice daily (and metoprolol three times daily) and the plasma samples for ANP concentration were taken 4–5 h after the medication was given; therefore its maximal ACE inhibitory action may have waned.
Exercise
We found that the PRA and angiotensin II levels at baseline did not increase during exercise compared with the levels at rest in both the metoprolol and captopril groups. However, the plasma concentrations of ANP increased during exercise compared with the resting values at baseline in both groups. Physical exercise is known to stimulate the release of renin, to activate the RAAS and to increase plasma ANP in healthy normotensive subjects [41–44]. In patients with DCM, Kirlin et al. [7] observed that PRA remained largely unchanged (compared with the level at rest) during moderate (50 W) upright exercise, but increased when the patients performed strenuous exercise (100 W). The duration of exercise may be important. The half-life of the peptide is reported to be 10–13 min [45], which means that the duration of exercise may have been too short.
Therapy with metoprolol. Physical exercise has been reported to increase PRA, angiotensin II and ANP [41–43] in normal subjects, but there are also reports that do not confirm these results [7, 46]. These conflicting results could be the result of either patient selection or the degree of exhaustion during the exercise test, or both. We observed that the PRA and A II levels during exercise were not different from those at rest, and that metoprolol reduced the PRA at rest as well as during exercise during treatment. Since strenuous exercise is known to increase PRA [7], metoprolol therapy seemed to be effective in inhibiting the otherwise expected increase. The observed increase in the level of ANP during exercise at baseline, compared with the values at rest, indicates that the exercise test was strenuous enough to cause an increased release of ANP and that this increase was effectively suppressed by metoprolol therapy. Therapy with metoprolol is also reported to improve exercise tolerance and LV function in patients with congestive heart failure due to dilated cardiomyopathy [11–15]. However, there are also studies suggesting no, or even negative, effects of beta-blockade in these patients [17–18].
Therapy with captopril. Physical exercise did not cause any increase in PRA or A II, either at baseline or during treatment with captopril. This latter finding is in accordance with Aldigier et al.[47], who also found no suppressive effect on the level of A II during exercise. This may indicate that there are other pathways for the synthesis and formation of A II than via ACE, as we have previously discussed.
Conclusions
There is no general consensus on the degree of neurohormonal activation in patients with different kinds of heart failure. In the present study, there were only minor changes in the renin–angiotensin system and the circulating concentrations of ANP during therapy with either captopril or metoprolol. This could partly be due to patient selection, since the patients were studied during a rather stable phase of their disease and were receiving rather modest doses of diuretics. This implies that the RAAS was only partly activated. However, we found that metoprolol reduced the level of ANP during exercise. Unfortunately, when comparing the effects of metoprolol with the effects of captopril, this difference was not statistically significant.
Treatment with a beta-blocker in patients with heart failure due to dilated cardiomyopathy and only mild-to-moderate symptoms seems to affect the neurohormonal system in a favourable way, even if the renin–angiotensin system is only partly activated.
References
Received 1 September 1997; accepted 18 September 1998.