Diseases of tunas, Thunnus spp.
Abstract
Much is known about those aspects of tuna health which can be studied in wild populations, e.g. helminth parasites. However, because aquaculture of these species is in its infancy, knowledge of microbial, nutritional and environmental diseases is limited. This review is an attempt to bring together the available information on those diseases of Thunnus spp. which cause significant morbidity, mortality or economic loss. In doing so it has become clear that much more research needs to be undertaken on the physiology of the species (southern, northern and Pacific bluefin tuna) currently used in aquaculture in order for the pathogenesis of some conditions to be properly understood. Attempts at hatchery culture of Pacific bluefin tuna has indicated that Thunnus spp. will be problematic to hatch and propagate.
Introduction
Tunas of the genusThunnus are very important commercial species which have, until recently, been exclusively wild-caught (Kailola, Williams, Stewart, Reichelt, McNee & Grieve 1993). However, the drastic reduction in stocks resulting from uncontrolled harvesting has led to the imposition of stringent quotas for certain species and concurrent establishment of aquaculture of some of these species (Lee 1998). There is little information on diseases of these fish and this review is an attempt to assemble this information in one place for the use of interested parties. Both pathological and pathophysiological conditions will be addressed.
Tunas are superb athletes and a number of species maintain stable body temperatures by having relatively high metabolic rates complemented by the use of heat-exchange mechanisms. Some species have been extensively studied at the physiological level, despite the practical problems involved in working with such fish (reviewed by Brill 1996; Farrell 1996; Korsmeyer, Dewar, Lai & Graham 1996). These studies have provided a sound basis for understanding pathophysiological conditions encountered in these species but more work is required, especially in southern bluefin tuna, Thunnus maccoyii Castlenau, which is the basis for the Australian tuna aquaculture industry.
With regard to pathology, there is little published information available with the exception of parasitological studies which have mainly been undertaken for parasite taxonomical studies or identification of discrete stocks of Thunnus spp. (Yamaguti 1970; Jones 1991a; Williams & Bunkley-Williams 1996). It is notable that adult tunas appear to be relatively resistant to bacterial infections even when subjected to trauma and other factors likely to predispose to such infections.
This review is based on the published literature supplemented with unpublished data from the authors and colleagues. The diseases are divided into those of infectious and non-infectious origins with appropriate sub-divisions.
Infectious diseases
Virus diseases
Red sea bream iridoviral infection
Aetiology.
The disease is caused by red sea bream iridovirus (RSIV) which is a member of a recently recognized group of very pathogenic viruses affecting marine species in the Asian region (Miyata, Matsuno, Jung, Danayadol & Miyazaki 1997). Whereas many fish iridoviruses belong to the genus Ranavirus (Hyatt, Gould, Zupanovic, Cunningham, Hengstberger, Whittington, Kattenbelt & Coupar 2000), RSIV does not. Young Pacific bluefin tuna, Thunnus orientalis (Temminck & Schegel), are often infected with this virus, but the disease never appears in bluefin tuna more than 1 year of age. Occurrence of the disease is restricted to periods of higher water temperature (>24 °C). Sometimes the mortality reaches some tens of percent for young fish.
Clinical signs.
Infected fish have dark body colour and anorexia. If the fish do not die during the acute phase of the disease they become emaciated and die later.
Pathology.
As in other fish species, basophilic, hypertrophied cells (probably leucocytes) are observed in sections of spleen from diseased Pacific bluefin tuna.
Epidemiology.
Among tunas, this infection has only been reported in Pacific bluefin tuna (Kawakami & Nakajima 2002).
Net cages for young wild-caught tuna are often sited near cages containing other fish which are susceptible to this virus. Therefore, it is likely that wild-caught young tuna for aquaculture, which are not infected at the time of capture, become infected by being caged alongside other cultured fish.
Diagnosis.
The histopathological picture of enlarged cells in the spleen, liver, kidney and gills which stain strongly with Giemsa is very characteristic of RSIV infection. Further confirmation can be obtained by demonstrating iridovirus virions by electron microscopy, culture of the virus in RTG-2, CHSE-214, FHM, BF-2 or KRE-3 cells at 20–25 °C, detection of specific antibody by immunofluorescence using monoclonal antibodies and detection of specific genomic sequences by PCR (Inouye, Yamano, Maeno, Nakajima, Matsuoka, Wada & Sorimachi 1992;Nakajima, Maeno, Yokoyama, Kaji & Manabe 1998; Oshima, Hata, Hirasawa, Ohtaka, Hirono, Aoki & Yamashita 1998).
Treatment.
There is no available treatment.
Prevention.
Apart from normal hygienic precautions, especially siting tuna cages well away from other aquaculture species such as red sea bream, Chrysophrys major (Temminck & Schlegel), and yellowtail, Seriola quinqueradiata Temminck & Schlegel, there are no current specific control measures for the disease. However, promising vaccination trials have been reported (Nakajima, Maeno, Honda, Yokoyama, Tooriyama & Manabe 1999; Nakai & Nakajima 2002).
Bacterial diseases
Opportunistic bacterial infections
Aetiology.
Aeromonas sp. infections have been reported in association with Caligus elongatus damage to the eyes of southern bluefin tuna (Rough, Lester & Reuter 1999). Buchanan (2002) and R. Reuter (personal communication) have reported a variety of Aeromonas and Vibrio spp. in the kidney and other internal organs of southern bluefin tuna, especially those which have suffered trauma.
Clinical signs.
Rough et al. (1999) reported dissolution of the lens and consequent loss of the eye in Caligus elongatus infections. In instances of external trauma there may be large wounds which do not heal and the fish eventually die, presumably from a combination of bacteraemia and osmoregulatory failure.
Epidemiology.
The organisms concerned are normal environmental inhabitants which are able to colonize wounds resulting from mechanical or parasitic trauma.
Diagnosis.
Isolation of the organisms from the orbit or kidney is regarded as diagnostic. However, isolation of such bacteria from superficial wounds may only indicate contamination from the water column.
Treatment.
It is difficult to treat captive tuna and it is doubtful if severely affected animals would respond to therapy.
Prevention.
The prevalence and severity of superficial wounds can be reduced by careful handling of the fish. At present, C. elongatus infections are not a sufficient problem to warrant treatment but this may be necessary in the future to reduce morbidity.
Photobacterium spp. infections
Aetiology.
Peric (2002) reported lesions consistent with Photobacterium damsela subsp. piscicida in a single northern bluefin tuna, T. thynnus (Bonnaterre). Hamaguchi & Kusuda (1992) reported on experimental infections of Pacific bluefin tuna with P. phosphoreum.
Pathology.
Peric (2002) described the spleen of the tuna as being enlarged with a rough surface. The cut surface showed multiple granulomas which, histologically, contained small numbers of short plump rods. He indicated that the lesions were similar to those seen in sparids with chronic pasteurellosis (P. damsela subsp. piscicida infection).
Epidemiology.
Pasteurellosis is essentially a warm-water (20–25 °C) disease and as the causative organism does not survive for long outside the host. Transmission is presumed to be lateral between fish (AQIS 1999).
Diagnosis.
The macroscopic lesions are not diagnostic as such granulomas can be caused by a variety of agents such as Mycobacterium or Nocardia spp. The detection of short, plump Gram-negative bacteria should enable a presumptive diagnosis but a definitive diagnosis requires isolation and identification of P. damsela subsp. piscicida.
Hamaguchi & Kusuda (1992) confirmed experimental infections with P. phosphoreum by isolating the organism from the kidneys of diseased fish.
Treatment and prevention.
The reported very low prevalence of pasteurellosis in northern bluefin tuna would not warrant attempts at treatment or specific prophylaxis.
Mycobacteriosis or piscine tuberculosis
Aetiology.
The cause of putative tuberculosis reported in a single northern bluefin tuna (Biavati & Manera 1991) is not known. However, most isolates from marine species are Mycobacterium marinum (Austin & Austin 1987).
Clinical and pathological findings.
Biavati & Manera (1991) reported a granulomatous peritonitis. Histologically, there were granulomas composed predominantly of epithelioid cells and fibroblasts. Bacilli within the granulomas were Gram positive and stained with modified Ziehl–Neelsen stain. The authors believed that the diagnosis was more likely to be mycobacteriosis than nocardiosis.
Epidemiology.
It is possible that the tuna could have eaten a fish which itself had mycobacteriosis. Otherwise it most likely became infected from the environment as aquatic mycobacteria are capable of existing as environmental organisms.
Diagnosis.
There are a number of granulomatous diseases of marine fish which can be difficult to differentiate, e.g. mycobacteriosis and nocardiosis, pseudotuberculosis. The latter can be differentiated from the first two on the basis that the organisms are Gram negative but the other two require isolation and identification of the causal bacteria.
Treatment and prevention.
Treatment is not practicable. As most cultured tuna are currently fed raw baitfish, care must be taken not to include fish with tuberculosis in the diet.
Protozoan diseases
Coccidiosis
Aetiology.
Only Goussia auxidis has been reported from tunas.
Clinical signs.
No clinical signs have been reported.
Pathology.
Jones (1990) reported that oocysts occurred in both the liver and spleen where they produced minimal host response.
Epidemiology.
The method of transmission of infection is unknown. Jones (1990) found 98% (140 of 143) of albacore, T. alalunga (Bonnaterre), and an individual yellowfin tuna, T. albacares (Bonnaterre), from the South Pacific were infected. He found no infection in 11 southern bluefin tuna although R. Reuter (personal communication) has noted coccidial bodies in the liver of this species.
Treatment and prevention.
Not required.
Scuticociliate infection
Aetiology.
Munday, O'Donoghue, Watts, Rough & Hawkesford (1997) reported an encephalitis in young adult southern bluefin tuna caused by Uronema nigricans. Williams & Bunkley-Williams (1996) reported an apparently similar condition in northern bluefin tuna in the Pacific. As their record was not their own work, and such a report has not appeared in the mainstream scientific literature, it is possible that their citation actually applies to U. nigricans in southern bluefin tuna.
Unidentified scuticociliates have caused significant mortalities of larval Pacific bluefin tuna at 14–18 days post-hatch in hatcheries in Japan (Miyashita & Kumai 2002; Y. Sawada, unpublished data).
Clinical signs.
Adult southern bluefin tuna with Uronema encephalitis suffer from the so-called ‘swimmer syndrome’. Typically, such fish come to the surface, turn light blue and swim vigorously around the cage. Eventually, they cease compulsive swimming and tend to alternately sink and rise to the surface until they finally sink and die (Munday et al. 1997). Morbidity and mortality rates are comparable (B.L. Munday & K.M. Rough, unpublished data) and have decreased from about 5% in captive fish in 1993 to 1.34% in 1995 and <1% in 2001.
In the case of infection of 14-day post-hatch Pacific bluefin tuna larvae, approximately half of the larvae died within 3 days (Y. Sawada, unpublished data). The smaller larvae showed the heaviest mortality and signs of infection ceased at 18 days post-hatch.
Pathology.
In adult southern bluefin tuna pathology is restricted to the olfactory system and brain. Although parasites containing food vacuoles can be found in the brain (Fig. 1) there is no host response except where the meninges are involved. However, lymphocytic responses are frequent in the olfactory nerves and rosettes.
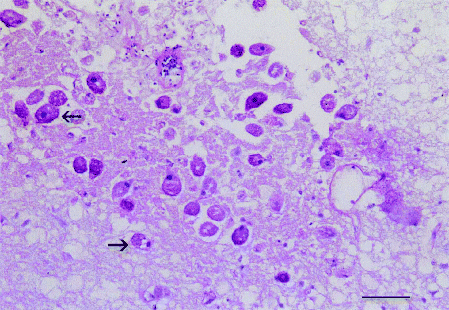
Brain of southern bluefin tuna showing presence of Uronema nigricans (arrows) containing food vacuoles. Note lack of host response (PAS, bar = 50 μm).
The parasites preferentially invade the epidermis and muscle of tuna larvae.
Epidemiology.
In the case of the ‘swimmer syndrome’ it is believed that the scuticociliates proliferate in the undercage sediment and invade the olfactory rosette of the fish when water passes through the nares during olfaction. The reduction in prevalence of the disease in recent years appears to be associated with improved undercage environmental conditions. It is not known what predisposes the olfactory rosette to colonization by Uronema, but water temperature appears to be an important variable because the disease does not occur when the water temperature is above 18 °C (Munday et al. 1997).
The build-up of organic matter which occurs in hatcheries is conducive to the development of high concentrations of scuticociliates and outbreaks of disease.
Diagnosis.
Cases of ‘swimmer syndrome’ can be diagnosed by demonstrating Uronema in wet preparations or sections of brain from affected fish. Watts, Burke & Munday (1996) developed a fluorescent antibody stain for specific identification of U. nigricans.
Scuticociliate infection of larval/juvenile tuna is diagnosed by demonstrating the parasites in wet preparations or tissue sections.
Treatment.
Treatment is not practicable for adult tuna reared in open sea cages. Copper treatment has been found to be effective for scuticociliate infections of many fish which are reared in tanks in Japan. Usually, copper ions in the range of 50–80 ppb concentration are effective. In Japan, this concentration of copper ions is achieved by the use of a simple electrolytic apparatus which consists of copper electrodes installed in the pipework carrying the influent water. However, larval and juvenile fish are less tolerant to copper ions than adult fish (S. Miyashita, personal communication) and the tolerance of larval and juvenile tuna to copper is unknown.
It is helpful to increase the water exchange rate in larval rearing tanks and, of course, the removal of dead fish is necessary. In the case of larval Pacific bluefin tuna, a higher water temperature of 28 °C has been found to be effective in reducing losses. It is not clear whether the higher temperature prevents proliferation of the parasite or if higher temperatures accelerate the fish's development to the juvenile stage at which they develop higher tolerance toUronema spp. However, it may be pertinent that Crosbie (1996) found that the maximum U. nigricans densities, which were achieved during the exponential phase of cultured organisms, were lower at 30 °C than at 10–25 °C. This suggests that the effect of high water temperatures on the organisms may be the more important mechanism.
Prevention.
Prevention is by improved hygiene/husbandry.
Metazoan infections
As given in Table 1, many metazoans infect Thunnus spp. but only a few are of health and/or economic importance. These will be discussed here. Additionally, the reader needs to be aware that, while an effort has been made to identify errors in records of parasites of tunas, Table 1 may still contain contentious citations.
| Parasite | Species infected | Reference |
|---|---|---|
| Myxosporea | ||
| Hexacapsula neothunni | Alb, BET, YFT | Arai & Matsumoto (1953), Williams & Bunkley-Williams (1996) |
| Kudoa clupeidae | NBT | Williams & Bunkley-Williams (1996) |
| Kudoa crumena | Alb | Harshbarger pers. comm. |
| Kudoa nova | BET | Williams & Bunkley-Williams (1996) |
| Kudoa sp. | SBT | Langdon (1990) |
| Monogenea | ||
| Allopseudaxine sp. | YFT | Pozdnyakov (1990) |
| Areotestis sibi | Alb, BET,YFT | Pozdnyakov (1990) |
| Benedenia seriolae | PBT, SBT | Kohn & Cohen (1998) |
| Caballerocotyla abidjani | YFT | Pozdnyakov (1990) |
| Caballerocotyla albsmithi | NBT | Pozdnyakov (1990) |
| Caballerocotyla biparasitica | BET, SBT,YFT | Pozdnyakov (1990) |
| Caballerocotyla goueri | LTT, NBT | Murugesh (1995) |
| Caballerocotyla magronum | NBT | Pozdnyakov (1990) |
| Caballerocotyla paucispinosa | PBT | Pozdnyakov (1990) |
| Caballerocotyla pseudomagronum | BET | Pozdnyakov (1990) |
| Caballerocotyla verrucosa | BET,YFT | Pozdnyakov (1990) |
| Caballerocotyla sp. | NBT, SBT | Pozdnyakov (1990), Rough (2000) |
| Capsala foliacea | LTT | Young (1970) |
| Capsala gotoi | BET,YFT | Pozdnyakov (1990) |
| Capsala neothunni | YFT | Pozdnyakov (1990) |
| Capsala thynni | Alb, YFT | Pozdnyakov (1990) |
| Hexostoma acutum | BET, NBT | Williams & Bunkley-Williams (1996) |
| Hexostoma albsmithi | NBT | Pozdnyakov (1990) |
| Hexostoma dissimile | NBT | Pozdnyakov (1990) |
| Hexostoma grossum | BET, PBT | Pozdnyakov (1990) |
| Hexostoma sibi | Alb, BET,YFT | Pozdnyakov (1990) |
| Hexostoma thynni | BET, NBT, YFT | Pozdnyakov (1990) |
| Hexostoma sp. | BET, YFT | Pozdnyakov (1990) |
| Kuhnia thunni | NBT | Pozdnyakov (1990) |
| Metapseudaxine ventrosicula | LTT, NBT | Murugesh (1995) |
| Nasicola hogansi | NBT | Wheeler & Beverley-Burton (1987) |
| Nasicola klawei | BET,BFT, NBT,YFT | Walters (1980), Pozdnyakov (1990) |
| Neohexostoma euthynni | YFT | Pozdnyakov (1990) |
| Neohexostoma extensicaudatum | NBT | Pozdnyakov (1990) |
| Neohexostoma robustum | BET | Pozdnyakov (1990) |
| Neohexostoma thunninae | NBT | Williams & Bunkley-Williams (1996) |
| Neohexostoma sp. | SBT, YFT | Pozdnyakov (1990), Rough (2000) |
| Sibitrema poonui | BET, NBT, YFT | Pozdnyakov (1990) |
| Tristomella interrupta | NBT | Pozdnyakov (1990) |
| Tristomella nozawae | Alb, BET, NBT,YFT | Pozdnyakov (1990) |
| Tristomella onchidiocotyle | BET, NBT | Pozdnyakov (1990) |
| Udonella caligorum | YFT | Williams & Bunkley-Williams (1996) |
| Zeuxapta taylori | YFT | Payne (1990) |
| Digenea | ||
| Anaplerurus thynnusi | PBT | Srivastava & Sahai (1978) |
| Angionematoborium cephalodomus | YFT | Nikolaeva & Parukhin (1968) |
| Aponurus lagunculus | PBT | Williams & Bunkley-Williams (1996) |
| Atalostroppion sardae | SBT,YFT | Williams & Bunkley-Williams (1996), Rough (2000) |
| Botulus microporus | BET | Williams & Bunkley-Williams (1996) |
| Brachyphallus parvus | YFT | Nikolaeva & Parukhin (1968) |
| Bucephalopsis sibi | PBT | Williams & Bunkley-Williams (1996) |
| Cardicola ahi | BET, YFT | Yamaguti (1970) |
| Cardicola forsteri | SBT | Cribb et al. (2000) |
| Cetiotrema crassum | SBT | Manter (1970) |
| Coeliotrema thynni | Alb, PBT, YFT | Yamaguti (1938), Pozdnyakov (1990) |
| Colocyntotrema sp. | SBT | Rough (2000) |
| Decemtestis dollfusi | YFT | Ahmad (1983) |
| Dermatodidymocystis indicus | BET | Nikolaeva & Dubina (1978) |
| Dermatodidymocystis vivipira | BET, YFT | Yamaguti (1970) |
| Dermatodidymocystis viviparoides | BET, YFT | Yamaguti (1970) |
| Didymocylindrus filiformis | PBT | Ishii (1935) |
| Didymocystis acanthocybii | YFT | Williams & Bunkley-Williams (1996) |
| Didymocystis alalongae | Alb,BET | Yamaguti (1970) |
| Didymocystis bifurcata | Alb, BET, LTT, YFT | Yamaguti (1970), Nikolaeva & Dubina (1985), Murugesh & Madhavi (1995) |
| Didymocystis crassa | PBT | Ishii (1935) |
| Didymocystis dissimilis | NBT | Yamaguti (1971) |
| Didymocystis guernei | Alb | Dollfus (1952) |
| Didymocystis irregularis | YFT | Yamaguti (1970) |
| Didymocystis katsuwonicola | PBT | Ishii (1935) |
| Didymocystis lanceolata | Alb | Dollfus (1952) |
| Didymocystis macrorchis | Alb | Dollfus (1952) |
| Didymocystis nasalis | BET | Yamaguti (1970) |
| Didymocystis oesophagicola | LTT, YFT | Yamaguti (1970), Murugesh & Madhavi (1995) |
| Didymocystis opercularis | Alb | Yamaguti (1938) |
| Didymocystis orbitalis | YFT, BET | Yamaguti (1970) |
| Didymocystis ovata | LTT, PBT | Ishii (1935), Ku & Shen (1965) |
| Didymocystis palati | YFT | Yamaguti (1970) |
| Didymocystis philobranchia | Alb, BET, YFT | Yamaguti (1970), Williams & Bunkley-Williams (1996) |
| Didymocystis philobranchiarca | Alb, BET, YFT | Yamaguti (1970), Nikolaeva & Dubina (1985) |
| Didymocystis poonui | BET | Yamaguti (1970) |
| Didymocystis reniformis | NBT | Ariola (1902) |
| Didymocystis rotunditestis | Alb, BET, YFT | Nikolaeva & Dubina (1985), Williams & Bunkley-Williams (1996) |
| Didymocystis semiglobularis | PBT | Ishii (1935) |
| Didymocystis soleiformis | PBT | Williams & Bunkley-Williams (1996) |
| Didymocystis spirocauda | YFT | Yamaguti (1970) |
| Didymocystis superpalati | Alb, BET,LTT, YFT | Yamaguti (1970), Nikolaeva & Dubina (1985), Murugesh & Madhavi (1995) |
| Didymocystis thynni | Alb, NBT, PBT, SBT | Dollfus (1952), Yamaguti (1971), Williams & Bunkley-Williams (1996), Rough (2000) |
| Didymocystis wedli | Alb, LTT, NBT, PBT, SBT, YFT | Ariola (1902), Yamaguti (1934), Ishii (1935), Dollfus (1952), Murugesh & Madhavi (1995), Rough (2000) |
| Didymocystoides alalongae | Alb, LTT | Yamaguti (1938), Murugesh & Madhavi (1995) |
| Didymocystoides bifasciatus | BET, YFT | Williams & Bunkley-Williams (1996) |
| Didymocystoides buccalis | Alb | Yamaguti (1970) |
| Didymocystoides oesophagicola | YFT | Williams & Bunkley-Williams (1996) |
| Didymocystoides opercularis | Alb | Yamaguti (1971) |
| Didymocystoides pectoralis | BET | Yamaguti (1970) |
| Didymocystoides semiglobularis | PBT | Yamaguti (1971) |
| Didymocystoides superpalati | Alb, BET, YFT | Williams & Bunkley-Williams (1996) |
| Didymonaja branchialis | Alb | Pozdnyakov (1987a) |
| Didymoproblema fusiforme | PBT | Ishii (1935) |
| Didymostoma bipartitum | NBT | Ariola (1902) |
| Didymosulcus aahi | Alb, YFT | Pozdnyakov (1990) |
| Didymosulcus dimidiatus | Alb | Pozdnyakov (1990) |
| Didymosulcus katsuwonicola | BET, YFT | Pozdnyakov (1990) |
| Didymozoon filicolle | PBT | Ishii (1935) |
| Didymozoon longicolle | BET, NBT, PBT, YFT | Ishii (1935), Yamaguti (1970), Williams & Bunkley-Williams (1996) |
| Didymozoon pretiosus | PBT, NBT | Ariola (1902), Williams & Bunkley-Williams (1996) |
| Didymozoon thynni | NBT | Taschenberg (1879) |
| Dinurus breviductus | BET | Korotaeva & Korjakovtzeva (1983) |
| Distomum clavatum | NBT | Linton (1901) |
| Ectenurus lepidocybii | YFT | Korotaeva & Korjakovtzeva (1983) |
| Hirudinella ahi | YFT | Yamaguti (1970) |
| Hirudinella clavata | NBT | Yamaguti (1971) |
| Hirudinella fusca | Alb, NBT | Linton (1940), Dollfus (1952) |
| Hirudinella marina | YFT | Yamaguti (1971) |
| Hirudinella oxysoma | Alb | Dollfus (1952) |
| Hirudinella poirieri | Alb | Dollfus (1952) |
| Hirudinella spinulosa | Alb | Yamaguti (1938) |
| Hirudinella ventricosa | Alb, BFT, NBT, SBT, YFT | Gibson & Bray (1977), Williams & Bunkley-Williams (1996), Rough (2000) |
| Koellikerioides apicalis | BET | Yamaguti (1970) |
| Koellikerioides externogastricus | BET, YFT | Yamaguti (1970) |
| Koellikerioides internogastricus | BET, YFT | Yamaguti (1970) |
| Koellikerioides intestinalis | BET | Yamaguti (1970) |
| Koellikerioides orientalis | Alb, NBT, YFT | Pozdnyakov (1992) |
| Koellikerioides splenalis | YFT | Nikolaeva (1988) |
| Köllikeria abdominalis | YFT | Yamaguti (1970) |
| Köllikeria bipartita | Alb, NBT, YFT | Yamaguti (1971), Williams & Bunkley-Williams (1996) |
| Köllikeria globosa | NBT, PBT, YFT | Ishii (1935), Williams & Bunkley-Williams (1996) |
| Köllikeria orientalis | Alb, LTT, NBT, YFT | Yamaguti (1971), Shen (1990) |
| Köllikeria pylorica | BET | Yamaguti (1970) |
| Köllikeria reniformis | PBT | Ishii (1935) |
| Köllikeria retrorbitalis | BET, YFT | Yamaguti (1970) |
| Köllikeria submaxillaris | BET, YFT | Yamaguti (1970) |
| Lecithaster gibbosus | NBT | Williams & Bunkley-Williams (1996) |
| Lecithocladium excisum | NBT | Williams & Bunkley-Williams (1996) |
| Lobatozoum multisacculatum | PBT | Ishii (1935) |
| Metanematobothrium guernei | Alb | Yamaguti (1938) |
| Nematobothrium latum | Alb | Dollfus (1952) |
| Nematobothrium sp. | BET, PBT | Nikolaeva & Dubina (1985), Williams & Bunkley-Williams (1996) |
| Neonematobothrioides poonui | BET | Yamaguti (1970) |
| Neophrodidymotrema ahi | YFT | Yamaguti (1970) |
| Oesophagocystis sp. | PBT | Williams & Bunkley-Williams (1996) |
| Opisthorchinematobothrium nephrodomus | Alb | Nikolaeva & Dubina (1978) |
| Opisthorchinematobothrium parathunni | BET | Yamaguti (1970) |
| Orbitonematobothrium perioculare | BET, YFT | Yamaguti (1970) |
| Phyllodistomum thunni | YFT | Baudin-Laurencin & Richard (1973) |
| Platocystis alalongae | Alb | Yamaguti (1938) |
| Platocystis meridionalis | Alb | Pozdnyakov (1987b) |
| Platocystis sp. | YFT | Nikolaeva & Parukhin (1968) |
| Prosorhynchoides sibi | PBT | Yamaguti (1940) |
| Rhipidocotyle pentagonum | PBT | Eckmann (1932) |
| Rhipidocotyle septpapillata | NBT | Williams & Bunkley-Williams (1996) |
| Sterrhurus imocavus | NBT, PBT | Williams & Bunkley-Williams (1996) |
| Syncoelium filiferum | Alb, SBT | Williams & Bunkley-Williams (1996), Rough (2000) |
| Umatrema indica | YFT | Srivastava & Sahai (1978) |
| Univietellodidymocystis lingualis | Alb, BET, YFT | Nikolaeva & Dubina (1985) |
| Univietellodidymocystis neothunni | BET, YFT | Yamaguti (1970) |
| Uroproctinella attenuata | YFT | Hafeezullah (1971) |
| Uroproctinella spinulosa | Alb, YFT | Nikolaeva & Parukhin (1968), Bussieras & Baudin-Laurencin (1973) |
| Wedlia bipartita | Alb, NBT | Linton (1940), Dollfus (1952) |
| Wedlia lingualis | YFT | Nikolaeva & Dubina (1985) |
| Wedlia musseliusae | Alb | Nikolaeva & Dubina (1985) |
| Wedlia orientalis | Alb, NBT, YFT | Yamaguti (1934), Dollfus (1952) |
| Yamaguticystis ariellii | YFT | Nikolaeva & Dubina (1985) |
| Cestoda | ||
| Callitetrarhynchus gracilis | YFT, NBT, SBT | Dollfus (1942), Bussieras & Baudin-Laurencin (1973), Rough (2000) |
| Dasyrhynchus talismani | YFT, BET | Bussieras & Aldrin (1965), Baudin-Laurencin (1971) |
| Echeneibothrium sp. | YFT | Williams & Bunkley-Williams (1996) |
| Grillotia sp. | YFT, PBT | Williams & Bunkley-Williams (1996) |
| Gymnorhynchus gigas | YFT | Williams & Bunkley-Williams (1996) |
| Hepatoxylon trichiuri | Alb, YFT | Bussieras & Baudin-Laurencin (1973), Jones (1991a) |
| Lacistorhynchus tenuis | NBT | Dollfus (1942) |
| Monorygma grimaldi | Alb | Williams & Bunkley-Williams (1996) |
| Nybelinia lingualis | SBT | Rough (2000) |
| Nybelinia sp. | YFT | Williams & Bunkley-Williams (1996) |
| Pelichnibothrium speciosum | YFT, NBT, PBT | Schmidt (1986), Williams & Bunkley-Williams (1996) |
| Pseudobothrium grimaldi | Alb | Williams & Bunkley-Williams (1996) |
| Pterobothrium heteracanthum | SBT | Rough (2000) |
| Scolex pleuronectis | LTT | Murugesh (1995) |
| Sphyriocephalus dollfusi | BET | Bussieras & Aldrin (1965) |
| Sphyriocephalus tergestinus | Alb | Williams & Bunkley-Williams (1996) |
| Sphyriocephalus sp. | YFT, BET | Williams & Bunkley-Williams (1996) |
| Tentacularia coryphaenae | Alb, BFT, NBT, YFT | Williams & Bunkley-Williams (1996) |
| Tentacularia sp. | YFT | Williams & Bunkley-Williams (1996) |
| Tetraphyllidean larvae | Alb, BFT, NBT, YFT, | Williams & Bunkley-Williams (1996) |
| Nematoda | ||
| Anisakis simplex | YFT | Williams & Bunkley-Williams (1996) |
| Anisakis sp. | Alb, YFT, PBT | Williams & Bunkley-Williams (1996) |
| Capsularia marina | SBT | Humphrey (1995) |
| Contracaecum sp. | Alb, PBT | Williams & Bunkley-Williams (1996) |
| Heptachona caudata | PBT | Williams & Bunkley-Williams (1996) |
| Hysterothylacium aduncum | Alb, NBT | Williams & Bunkley-Williams (1996) |
| Hysterothylacium cornutum | Alb, NBT, SBT, YFT | Humphrey (1995), Williams & Bunkley-Williams (1996) |
| Metanisakis sp. | YFT | Williams & Bunkley-Williams (1996) |
| Monhysterides sp. | YFT | Williams & Bunkley-Williams (1996) |
| Oncophora albacarensis | YFT | Williams & Bunkley-Williams (1996) |
| Oncophora melanocephala | Alb, BFT, NBT, YFT | Williams & Bunkley-Williams (1996), Moravec, Kohn & Santos (1999) |
| Philometroides sp. | BET, YFT | Williams & Bunkley-Williams (1996) |
| Spirurida | SBT | Rough (2000) |
| Terranova sp. | LTT | Cannon (1977) |
| Acanthocephala | ||
| Bolbosoma vasculosum | Alb, PBT, YFT | Williams & Bunkley-Williams (1996) |
| Bolbosoma sp. | YFT | Williams & Bunkley-Williams (1996) |
| Gorgorhynchus sp. | Alb | Williams & Bunkley-Williams (1996) |
| Neorhadinorhynchus nudus | PBT | Williams & Bunkley-Williams (1996) |
| Neorhadinorhynchus sp. | YFT | Williams & Bunkley-Williams (1996) |
| Rhadinorhynchus cadenati | YFT | Williams & Bunkley-Williams (1996) |
| Rhadinorhynchus pristis | Alb, SBT, YFT | Williams & Bunkley-Williams (1996), Rough (2000) |
| Rhadinorhynchus trachuri | YFT | Williams & Bunkley-Williams (1996) |
| Rhadinorhynchus sp. | YFT | Williams & Bunkley-Williams (1996) |
| Copepoda | ||
| Brachiella thynni | Alb, BET, NBT, SBT, YFT | Jones (1991b), Williams & Bunkley-Williams (1996) |
| Caligus alalongae | Alb, BET | Williams & Bunkley-Williams (1996) |
| Caligus asymmetricus | YFT | Williams & Bunkley-Williams (1996) |
| Caligus balistae | NBT | Williams & Bunkley-Williams (1996) |
| Caligus bonito | NBT | Williams & Bunkley-Williams (1996) |
| Caligus coryphaenae | Alb, BET, BFT, NBT, YFT | Hogans (1985), Williams & Bunkley-Williams (1996) |
| Caligus elongatus | SBT | Rough et al. (1999) |
| Caligus productus | Alb, BET, BFT, NBT, YFT | Williams & Bunkley-Williams (1996) |
| Caligus quadratus | YFT | Williams & Bunkley-Williams (1996) |
| Caligus sp. | YFT | Williams & Bunkley-Williams (1996) |
| Caligus robustus | YFT | Williams & Bunkley-Williams (1996) |
| Euryphorus brachypterus | Alb, BET, BFT, NBT, YFT | Walters (1980), Williams & Bunkley-Williams (1996) |
| Euryphorus nordmanni | Alb, BET, YFT | Williams & Bunkley-Williams (1996) |
| Pennella filosa | Alb, NBT, YFT | Williams & Bunkley-Williams (1996) |
| Pennella sp. | YFT | Williams & Bunkley-Williams (1996) |
| Pseudocynus appendiculatus | Alb, BET, BFT, LTT, NBT, YFT | Williams & Bunkley-Williams (1996) |
| Isopoda | ||
| Rocinella signata | Alb | Williams & Bunkley-Williams (1996) |
- Alb, albacore Thunnus alalunga (Bonnaterre); BET, bigeye tuna Thunnus obesus (Lowe); BFT, blackfin tuna Thunnus atlanticus (Lesson); LTT, longtail tuna Thunnus tonngol (Bleeker); NBT, northern bluefin tuna Thunnus thynnus (L.); PBT, Pacific bluefin tuna Thunnus orientalis (Temminck & Schlegel); SBT, southern bluefin tuna Thunnus maccoyii (Castelnau); YFT, yellowfin tuna Thunnus albacares (Bonnaterre).
Postmortem liquefaction of muscle due to myxosporean infections
Aetiology.
Hexacapsula neothunni in albacore, yellowfin tuna and bigeye tuna, T. obesus (Lowe), Kudoa nova in bigeye tuna and, possibly, K. clupeidae in poorly identified Thunnus spp. (Williams & Bunkley-Williams 1996).
Clinical signs and pathology.
The parasites produce no clinical signs and, while with heavy infections cysts may be visible in the musculature, it is the postmortem liquefaction of the muscle caused by the release of proteases from the parasites that is the most dramatic result of the infection (Ogawa 1996). Histologically, the myxosporean spores are found aggregated in the cystic structures and usually produce minimal host response.
Epidemiology.
Most myxosporeans, for which life cycles are known, have a two-host cycle with the myxosporean in a fish and an actinosporean in an invertebrate (Kent, Andree, Bartholomew, El-Matbouli, Desser, Devlin, Feist, Hedrick, Hoffman, Khattra, Hallett, Lester, Longshaw, Palenzeula, Siddall & Xiao 2001). As most juvenile tuna consume invertebrates such as squid and crustaceans (Kailola et al. 1993), it is conceivable that these prey species could be alternative hosts.
Diagnosis.
Typical myxosporean spores can be easily found in wet preparations or histological sections of affected muscles. Hexacapsula neothunni spores measure 6.2 × 11.0 μm and have six shell valves each containing one polar capsule (Lom & Dykova 1992). Kudoa nova spores measure 5.3–6.5 × 8.5–9.8 μm and have four shell valves each containing one polar capsule.
Treatment and prevention.
Neither treatment nor prevention is practicable.
Kudoa sp. infection of northern and southern bluefin tuna producing lesions in the musculature
Aetiology.
Kent et al. (2001) reported K. crumena in a yellowfin tuna. Similar lesions because of an unidentified Kudoa sp. have been reported in southern bluefin tuna and Langdon (1990) suggested that the parasite could be K. nova, but as it does not produce myoliquefaction, this seems unlikely.
Clinical signs.
None reported.
Pathology.
The infection in southern bluefin tuna produces white cysts 1–10 mm in diameter which are apparently in the muscle (J.C. Harshbarger, personal communication; Rough 2000) although Langdon (1990) produced evidence to suggest that most, if not all, cysts in southern bluefin tuna were in peripheral nerves, especially the intercostal nerves. Histologically the cysts are found to consist of numerous Kudoa spores surrounded by a fibrous capsule (Fig. 2).
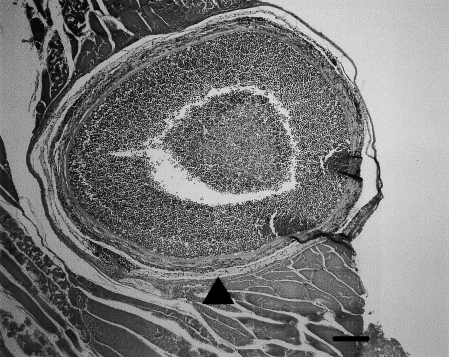
Kudoa sp. nodule in muscle of southern bluefin tuna. Note the small nerve (arrowhead) embedded in/apposed to, the capsule surrounding the mass of spores (H & E, bar = 100 μm).
Epidemiology.
The infections described by Langdon (1990) were in southern bluefin tuna caught off south-western Western Australia when the fish would have been 1–3 years of age and feeding on cephalopods, crustaceans and salps (Kailola et al. 1993). Infections have also been reported in southern bluefin tuna in South Australia (Rough 2000) and wild fish caught off the New South Wales coast where the prevalence of about 1% affected fish was a cause of commercial loss (B.L. Munday, unpublished data). The prevalence of K. crumena in albacore was reported as 5% (J.C. Harshbarger, personal communication).
Diagnosis.
Typical Kudoa spores measuring 7.5 × 9.9 (K. crumena) and 4.4–5.6 × 7.8–10.0 μm (Kudoa sp.) can be seen in wet preparations or histological sections.
Treatment and prevention.
Neither treatment nor prevention are practicable.
Monogenean infection of gills
Aetiology.
An unidentified, capsalid monogenean (Rough 2000, type 3) infecting southern bluefin tuna.
Clinical signs.
The parasite does not cause mortality but Rough (2000) stated that heavy infections lead to respiratory stress.
Pathology.
Rough (2000) described white patches on the gills which appear histologically as areas of focal lamellar fusion.
Epidemiology.
Rough (2000) stated ‘It is more common in farmed fish but its distribution is often confined to only a few cages, and heavy infections to individual fish’.
Diagnosis.
The presence of white patches on the gills together with the presence of monogeneans permits a presumptive diagnosis. A definitive diagnosis is currently not possible in the absence of a description of the parasite.
Treatment.
Therapeutic treatment would not be practicable nor warranted.
Prevention.
Control would most likely be achieved by reducing stocking densities for both cages and fish in cages, i.e. fewer cages per site and fewer fish per cage.
Blood fluke infections of tuna
Aetiology.
Cardicola ahi has been reported from yellowfin and bigeye tunas (Smith 1997). Cardicola forsteri (Cribb, Daintith & Munday 2000) occurs in southern bluefin tuna.
Clinical signs.
Cardicola forsteri infections of cultured southern bluefin tuna lead to increased mucus on the gills and have been associated with signs of respiratory distress, lethargy and slightly increased mortality (Rough 2000; B.L. Munday, unpublished data).
Pathology.
Colquitt, Munday & Daintith (2001) described multifocal, white to yellow lesions involving the gills of infected southern bluefin tuna. The lesions ranged in size from 2 to 12 mm and often extended in an arc across the gills (Fig. 3).
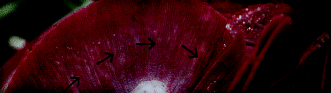
Gill of southern bluefin tuna with Cardicola forsteri infection. Note the focal, pale lesions arranged in an arc formation (arrows).
Histopathological lesions in the gills of southern bluefin tuna have been described in detail by Colquitt et al. (2001). The lesions appeared to be the result of the fluke ova impacting in the afferent filamentary arteries where they stimulated a host response (Fig. 4). Bussieras & Baudin-Laurencin (1973) also reported similar lesions in yellowfin tuna infected with a Cardicola sp. (probably C. ahi).
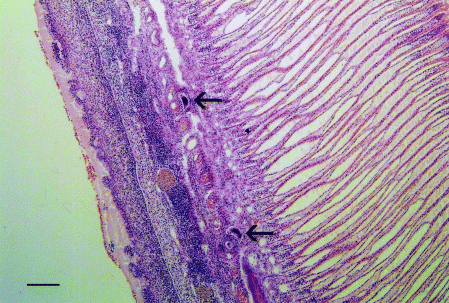
Gill of southern bluefin tuna with Cardicola forsteri infection. Note the presence of sanguinicolid eggs (arrows) and associated cellular response (H & E, bar = 100 μm).
Cardiac lesions were also reported by Colquitt et al. (2001) who noted many ova surrounded by granulomas. There was marked hypertrophy of the cardiac spongiosa (Fig. 5), presumably because of increased resistance to blood passing through the partly occluded branchial vasculature. It may not be a coincidence that bigeye tuna, which are known to be infected with Cardicola ahi, have a much more compact ventricular myocardium (74%) compared with northern bluefin tuna (30–50%) which have not been reported to be to be infected with blood flukes (Santer & Greer-Walker 1980; Smith 1997).
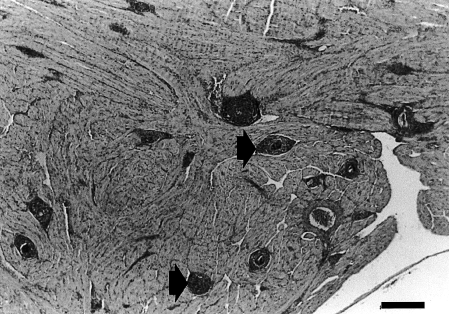
Spongiosa of southern bluefin tuna heart with Cardicola forsteri infection showing myocardial hypertrophy and presence of granulomas surrounding parasite eggs (arrows) (H & E, bar = 250 μm). Illustration from Colquitt et al. (2001).
Epidemiology.
The intermediate hosts of Cardicola ahi and C. forsteri are not known. It is also not known if teleosts, other than Thunnus spp., act as final hosts. Colquitt et al. (2001) reported that the prevalence and severity of the infection increased with the time that southern bluefin tuna were held in captivity suggesting that the life cycle was maintained in the vicinity of the cages. Additionally, tuna farmers have reported that infections tend to be more severe at new cage sites suggesting that the parasite may also have a deleterious effect on the intermediate host (B.L. Munday, unpublished data).
Diagnosis.
In southern bluefin tuna the gross lesions are characteristic enough to enable a presumptive diagnosis. Histopathology is even more diagnostic, but definitive diagnosis depends upon flushing the adults from the heart and identifying them.
Treatment.
There is no practicable treatment at present.
Prevention.
Prevention of blood fluke infections depends upon an understanding of the parasites’ life cycle and, therefore, is not possible at present.
Larval cestode infection
Aetiology.
Larval cestodes (plerocercoids) do not usually cause disease in tunas and, therefore, it is only those which involve the musculature which are of commercial importance. Tentacularia coryphaenae does produce muscle lesions in a range of tuna species and will be considered here.
Clinical signs.
The infection is covert.
Pathology.
Plerocercoids up to 9.5 mm in length have been reported in albacore, T. alalunga (Bonnaterre), blackfin tuna T. atlanticus (Lesson), northern bluefin tuna, bigeye tuna and yellowfin tuna (Williams & Bunkley-Williams 1996).
Epidemiology.
Sharks are definitive hosts for this cestode which also occurs in a range of pelagic fish apart from tuna.
Diagnosis.
Presumptive diagnosis can be made on the basis of the plerocercoids having a long scolex with four shallow, elongate bothridia and four short, retractile, hook-bearing tentacles.
Treatment and prevention.
Not practicable at present.
Anisakid nematode infection
Aetiology.
These parasites are of importance because they can potentially infect humans. Parasites of interest are Anisakis simplex and Hysterothylacium cornutum (Williams & Bunkley-Williams 1996).
Clinical signs.
The infections are covert.
Pathology.
The small third-stage larvae are found encapsulated in the peritoneal mesenteries and, sometimes, the liver. If the fish are not quickly eviscerated it is possible for the larvae to migrate to the abdominal muscles.
Epidemiology.
The definitive hosts of these parasites are marine mammals. Many other species of fish can act as intermediate hosts.
Diagnosis.
The presence of tightly coiled, encapsulated larval nematodes in the mesenteries of tunas is suggestive of anisakid nematode infection, but definitive identification of the larvae can be difficult.
Treatment.
There is no practicable treatment.
Prevention.
Human infection can be prevented by rapid evisceration of the fish and/or cooking of the flesh. In most instances tuna destined for sashimi are eviscerated soon after capture.
Copepod infections
Aetiology.
A number of copepods parasitise Thunnus spp. (Table 1) but only C. elongatus, Euryphros brachypterus and Penella filosa are potentially pathogenic.
Clinical signs.
Rough et al. (1999) reported that C. elongatus grazes on the integument of southern bluefin tuna and may produce grazing trails including over ocular tissues. Damage to the eye results in keratitis, panopthalmitis and cataract formation.
Very heavy infections of Euryphorus brachypterus have been reported in northern bluefin tuna in which the pseudobranch has been carpeted with the parasite leading to ulceration and bleeding. Similar, but less severe lesions may be present on the gills and skin (Williams & Bunkley-Williams 1996.
The very large copepod Penella filosa (≥50 mm long) penetrates into the muscles of a number of tuna species. It has been reported to cause the fish considerable discomfort (Williams & Bunkley-Williams 1996).
Pathology.
Lesions because of the above copepods are related to their grazing behaviour (C. elongatus) or the damage caused by their attachment to the host (Euryphorus brachypterus and Penella filosa).
Epidemiology.
As C. elongatus and Penella filosa have multiple hosts, tuna may become infected from a variety of sources. However, Euryphorus brachypterus is almost genus specific for Thunnus spp. (Williams & Bunkley-Williams 1996).
In the case of C. elongatus infecting captive southern bluefin tuna, capture trauma and high stocking densities are believed to predispose to heavy infections (Rough et al. 1999).
Diagnosis.
Experienced diagnosticians can make a presumptive diagnosis of these copepod infections based on the morphology of the parasites and the types of lesions induced by their activities. However, definitive diagnosis is only possible by a scientist skilled in identifying the parasites.
Treatment.
Although a number of therapeutants are capable of killing copepod parasites (Lester & Roubal 1999) it is impracticable to use these agents under current tuna aquaculture conditions. In addition, at the present level of loss of production, such treatments would be uneconomical.
Prevention.
As Rough et al. (1999) have suggested that trauma may predispose to Caligus elongatus infections then reduction of damage because of capture, towing and harvesting should simultaneously reduce the level of infestation/damage caused by this copepod. Additionally, as this parasite and Penella filosa are carried by other species of fish, it would be appropriate to keep other forms of aquaculture separate from tuna farms.
Non-infectious diseases
Nutritional diseases
In the early stage of the Pacific bluefin tuna aquaculture in Japan, morbidity and mortality was reported caused by a shortage of thiamin (Yamaguchi 1986). At that stage only Pacific saury, Cololabis saira (Brevoort), and/or Japanese anchovy, Engraulis japonicus Temminck & Schlegel, was fed to the fish resulting in a large reduction of thiamin stores in the cultured fish. These baitfish contain thiaminase which is capable of producing an induced thiamin deficiency in tuna. At present, several kinds of baitfish are fed to tuna in Japan and this disease no longer occurs.
There have been no reports of nutritional diseases in cultured southern bluefin tuna. However, relatively low levels of vitamin E have been found in the livers (mean 33 μg g−1) of southern bluefin tuna fed on baitfish (B.L. Munday, unpublished data). These are comparable with those (40 μg g−1) reported by McLoughlin, Kennedy & Kennedy (1992) in rainbow trout with vitamin E-responsive myopathy.
Toxicoses
Microalgal toxicosis
Aetiology.
There are no unequivocal reports of mortalities in tuna because of toxic microalgae but the evidence for Chattonella marina causing the mass mortality episode in southern bluefin tuna at Port Lincoln, South Australia in 1996 is compelling (Munday & Hallegraeff 1998).
Clinical signs.
Ishimatsu, Sameshima, Tamura & Oda (1996) reported that fish affected by Chattonella toxicosis had excessive production of gill mucus leading to respiratory distress. These signs were reported in southern bluefin tuna dying in the 1996 incident. In that incident mortalities varied from 22 to 92%.
Pathology.
Munday & Hallegraeff (1998) reported histopathological lesions in the gills with epithelial swelling, variable lifting of the epithelium (Fig. 6a,b) and apparent blockage of gill fenestrations by mucus. These lesions are consistent with those produced experimentally in yellowtail exposed to Chattonella marina (Ishimatsu et al. 1996).
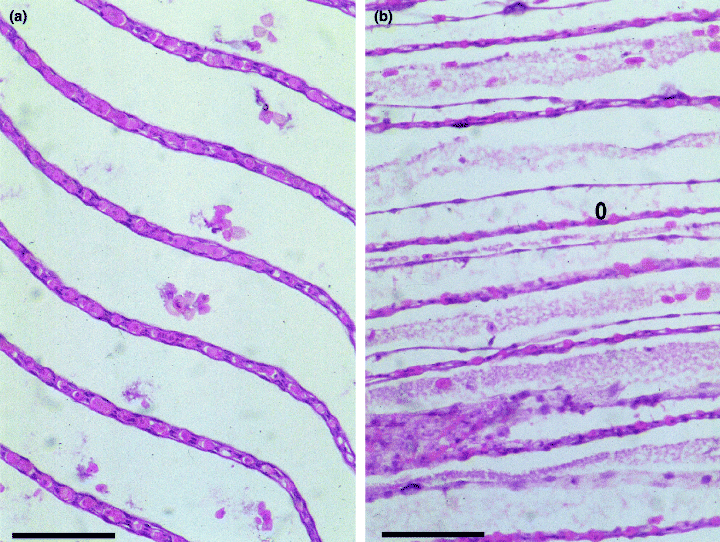
Comparison of normal and abnormal southern bluefin tuna gills. (a) Normal southern bluefin tuna gill secondary lamellae (H & E, bar = 50 μm). (b) Abnormal southern bluefin tuna gill secondary lamellae in a fish exposed to Chattonella marina. Note subepithelial oedema (o) (H & E, bar = 50 μm).
Epidemiology.
The toxicity of Chattonella marina and other toxic algae is governed by a range of variables including the stage of growth, temperature, availability of iron and level of irradiation (Kawano, Oda, Ishimatsu & Muramatsu 1996; Khan, Haruyama, Iwashita, Ono & Onoue 2000; Pickell & Trick 2000). In relation to the latter it is important to note that the Australian Chattonella marina isolates are adapted to much higher irradiances than Japanese isolates (Marshall & Hallegraeff 1999).
Diagnosis.
Unequivocal diagnosis of microalgal toxicosis can often be problematic unless facilities for microalgal collection, identification and quantification are already in place when an episode occurs. Even when such facilities are available, diagnosis depends upon observing typical clinical signs and pathology in association with appropriate levels of microalgal cells in the water column at the time of the mortality.
Okaichi, Ochi, Nishio, Takano, Matsuno, Morimoto, Murakami & Shimada (1989) reported that 500 Chatonella marina cells mL−1 were lethal to yellowtail. Additionally, Hishida, Katoh, Oda & Ishimatsu (1998) found that yellowtail were much more suceptible to this toxicosis than red sea bream or Japanese flounder and the relative susceptibilities were directly related to the ventilation volumes of these fish. As yellowtail have a ventilation volume of 1099.6 mL/kg/min and yellowfin tuna (no data are available for southern bluefin tuna) have a ventilation volume of 3900 mL kg−1 min−1 (Bushnell, Brill & Bourke 1990) then it is to be expected that a much lower concentration of Chattonella marina (≈170 mL−1) would be toxic for Thunnus spp.
Other host-associated factors to be considered are the additive effects of low dissolved oxygen in the water and cardiopathy because of blood fluke infection.
Treatment.
There are no practicable treatments.
Prevention.
The most efficacious means of prevention is by towing the tuna cages away from the bloom (Rensel 2000). Other strategies are the use of perimeter skirts in association with airlifts and spreading certain types of clays to remove toxic microalgae from net-pens by flocculation (Rensel 2000).
Trauma
Trauma to wild-caught fish
Aetiology.
Seedlings (20–40 cm total length) for Pacific bluefin tuna aquaculture in Japan are caught by trolling and these fish are sometimes injured in their jaws or other parts of the body. In the case of southern bluefin tuna caught by seine net when about 3 years of age, trauma can occur during capture, towing, transfer and eventual harvest. Often this is exacerbated by unfavourable weather conditions. Attempts by predators to attack fish in cages can lead to substantial trauma.
Clinical signs.
Affected fish have skin wounds of variable size and commonly there is damage to the eyes which may lead to blindness. Severely damaged fish usually die.
Pathology.
As would be expected damaged fish frequently have local and systemic infections with opportunistic bacteria such as Aeromonas and Vibrio spp.
Epidemiology.
Although most tuna harvesters and/or farmers use basically the same techniques there are differences based on the species and age of the tuna and the distance between the capture site and the farms. Pacific bluefin tuna caught by Japanese operators are usually between 0.2 and 1 kg and are rarely more than 48 h in transit (Miyashita, Sawada, Hattori, Nakatsukasa, Okada, Murata & Kumai 2000). In contrast, southern bluefin tuna sourced by Australian operators are usually 12–20 kg and may be towed for up to 2 weeks before reaching the farming sites. However, it is suggested by operators that southern bluefin tuna are more robust and, therefore, do not suffer as much injury, especially once in farm cages, as might be expected.
The mesh size of nets is important otherwise fish may become ‘gilled’ and die or are damaged. In addition, operators suggest that a degree of net-fouling makes the net more visible and, therefore, less likely to be impacted by the fish.
Siting net cages near seal colonies can lead to increased attacks and discharge of blood during harvesting can attract sharks.
Diagnosis.
The injuries are usually self-evident but determining the cause(s) can be problematic.
Treatment.
Treatment is usually not practicable.
Prevention.
Prevention is mainly by applying good husbandry principles such as not towing cages at an excessive speed. Areas which require special attention are selection of mesh sizes for net pens and methods of harvesting (hook and line rather than gaffing).
Predators should be excluded by use of predator nets or tightly tensioned, heavy nets.
Morbidity and mortality of Pacific bluefin tuna during hatchery and early growout culture
Aetiology.
The end cause of the syndrome is collision of juvenile tuna with the walls of tanks or mesh of nets (Miyashita et al. 2000).
Clinical signs.
The fish have been observed to respond to external stimuli by panicking and colliding with the sides of tanks or net pens (Fig. 7).

Juvenile Pacific bluefin tuna embedded in PVC sheeting used to prevent the fish impacting directly on the walls of the tank. Illustration from Miyashita et al. (2000).
Miyashita, Sawada, Hattori, Nakatsukasa, Okada, Murata & Kumai (2000) reported that mortality increased from 4.9% on day 33 after hatch to 8.9% on day 52 and then stabilized at about 4% day−1 leading to very few survivors at day 60 after hatch.
Pathology.
Affected fish have had considerable damage to the vertebral column and the parasphenoid bones.
Epidemiology.
The syndrome is apparently the result of confining free-ranging young fish in a restricted area. Such fish usually escape danger by rapid flight, but this is not possible within the confines of tanks or cages. In addition, Miyashita et al. (2000) make the point that in Pacific tuna juveniles development of muscle and caudal fin shape occurs earlier than development of pectoral fins and caudal keels, i.e. swimming ability develops in advance of steering ability. Even weak stimuli such as flashes of light or vibrations were reported to lead to panic responses in the juvenile tuna resulting in collisions with the sides of the culture vessels/nets.
Diagnosis.
Diagnosis can be made by observing the fish and/or by finding the typical skeletal lesions.
Treatment.
Treatment is impracticable.
Prevention.
Miyashita et al. (2000) reported that apart from minimizing stimuli, the most useful preventive measure was to institute a 24-h light regime so that the sides of the tanks/nets were visible to the fish at all times.
Management problems
Cannibalism in Pacific bluefin larvae
Aetiology.
Cannibalism is related to the predatory nature of tuna and becomes a problem when there are variable sizes in a cohort of these fish.
Clinical signs.
There is a continual reduction in numbers of larvae from 14 to 20 days post-hatch (Sawada, Kato, Okada, Kurata, Mukai, Miyashita, Murata & Kumai 1999). Also, some fish may be observed swallowing smaller fish.
Pathology.
Cannibalism is often accompanied by fin and eye nipping with resultant damage to these areas.
Epidemiology.
As indicated above, the predatory nature of tuna is a major factor. Also, stocking densities, feed availability and other managerial factors may be involved.
Treatment.
Treatment is not possible.
Prevention.
Stocking densities may be reduced and care taken that fish do not become excessively hungry.
Adhesion of Pacific bluefin larvae to the water surface
Aetiology.
The thick skin mucus layer which is present from hatching and develops with age causes the larvae to adhere to the surface of the water when carried there by aeration currents. As the fish become older and more competent swimmers they are able to extricate themselves (Sawada 1999).
Clinical signs.
The condition is quite characteristic with the larvae being trapped at the surface of the water and suffering from desiccation.
Pathology.
The pathology has not been described.
Epidemiology.
As larval Pacific bluefin tuna have a very high oxygen requirement (Miyashita, Hattori, Sawada, Ishibashi, Nakatsukasa, Okada, Murata & Kumai 1999) aeration must be vigorous, thus inducing the condition.
Diagnosis.
The presence of larvae trapped at the surface of the water is quite obvious.
Treatment.
Affected larvae can be removed to static containers but are not likely to survive.
Prevention.
Use of oxygen rather than air as a source of water oxygenation over the first 7 days after hatch may allow less vigorous ‘aeration’ and thus reduce losses. Additionally, providing an oil film on the water surface is very effective. The oil film prevents the larvae being exposed to the air when they are transported to the water surface. This preventive measure is used for other fish larvae, such as grouper larvae, in which the same problem occurs during seedling production (Sawada et al. 1999).
Neoplasia
Clinical signs.
Superficial lipomas constitute the majority of reported tumours in Thunnus spp. (Easa, Harshbarger & Hetrick 1989; Harshbarger, personal communication; Lester & Kelly 1983) and may constitute up to 10% of body mass.
Pathology.
Of eight reported tumours, three were lipomas (op.cit.), two were osteomas (J.C. Harshbarger, personal communication) and one each a fibroma, an invasive schwannoma (J.C. Harshbarger, personal communication) and a melanoma (Anders 1988).
Diagnosis.
Diagnosis relies upon histological examination of the tumours.
Other conditions
‘Burnt tuna’
Aetiology.
The most plausible explanation for this condition is that it is a form of calpain proteolosis (Watson 1995). The suggestion is that low intracellular ATP concentrations lead to the breakdown of calcium homeostasis with an increase in cytosolic calcium, which activates calpain. Calpain is an enzyme which attacks non-contractile proteins, such as the Z-discs of muscle. Low intracellular ATP concentrations are likely to occur in fish which have low glycogen reserves and are subjected to intense physical exertion, e.g. yellowfin tuna caught by handline.
Clinical signs.
There are no clinical signs as this is a post-mortem condition.
Pathology.
Instead of being red, translucent and firm affected muscles are pale, watery and soft.
Epidemiology.
‘Burnt tuna’ are mainly associated with the yellowfin tuna handline fishery (Watson 1995) and the condition has been reported occasionally in southern bluefin tuna (Williams 1986).
As indicated above, Watson (1995) suggested that the stress involved in handline fishing leads to catecholamine release which promotes glycogenolysis. If the fish have limited glycogen reserves, ATP is reduced and muscle pH elevated leading to calpain proteolysis of the Z-discs of the musculature.
Diagnosis.
The condition can be diagnosed by observing the typical muscle lesions.
Prevention.
Cultured tuna are unlikely to be affected by this condition because they usually have adequate stores of muscle glycogen (B.L. Munday, unpublished data). More efficient catching methods such as polling for wild fish should obviate the problem.
Eye lesions
Aetiology.
Some are traumatic but the aetiologies of others are unknown.
Clinical signs.
Abnormalities vary from cataracts, to corneal ulcers, to complete loss of the orbit.
Pathology.
The pathology has not been described in tunas.
Epidemiology.
Most of the corneal lesions are probably caused by contact with nets. The aetiology of cataracts is not known, although UV irradiation has been suggested as one possible cause.
Diagnosis.
The lesions are self-evident although small cataracts can be difficult to detect.
Treatment.
There are no practicable means of treating captive southern bluefin tuna for these conditions.
Prevention.
Improved management should reduce the prevalence of corneal lesions. Until the cause of cataracts is established it is not possible to suggest means of preventing them.
General discussion including future directions
The present system of using wild-caught seedstock for aquaculture purposes has been characterized by the occurrence of relatively few bacterial diseases. The reasons for this situation are probably manifold but the observation that tunas’ immune responses may be independent of ambient temperatures would seem to be a key factor when tuna are held at relatively low temperatures (Watts, Munday & Burke 2002).
It is paradoxical that a great number of first class physiological studies have been undertaken on skipjack, Katsuwonas pelamis (L.), and yellowfin tuna, whereas most tunas used for aquaculture are southern, northern or Pacific bluefin tuna. While it is possible to extrapolate from one species to another, there is ample evidence that significant differences in physiology do occur (Bushnell, Brill & Bourke 1990) and such extrapolation could lead to erroneous decisions being made. Obviously, this is an area requiring urgent attention.
The use of oily baitfish as a source of food for captive tuna poses a number of problems. First, the presence of thiaminases and oxidized lipids in these fish has been, or is likely to be, responsible for nutritional problems in the tuna (B.L. Munday, unpublished data; Yamaguchi 1986). Also, baitfish are known to carry important viral diseases such as viral haemorrhagic septicaemia (Kocan, Hershberger, Elder & Winton 2001) and pilchard herpesvirus (Hyatt, Hine, Jones, Whittington, Kearns, Wise, Crane & Williams 1997). Consequently, the development of a palatable artificial feed which produces desirable carcass composition is an urgent need.
Experiences of Japanese workers with preliminary attempts to hatch and raise tuna have indicated that hatchery propagation is fraught with many problems and it may be some years before such fish will replace seedstock caught from the wild. However, it is important that this research continues because, as in the case of the threatened southern bluefin tuna, completing the life cycle has conservation in addition to commercial implications.
Acknowledgements
The authors wish to acknowledge the valuable assistance provided by Drs I. Beveridge of the University of Melbourne, J. B. Jones of the Western Australian Department of Fisheries and J. Harshbarger of the George Washington University. The authors also thank Dr Miyashiata of Kinki University and three anonymous reviewers for their helpful comments.
References
Received: 2 September 2002 Accepted: 29 January 2003




