Analysis of exotoxins produced by atypical isolates of Aeromonas salmonicida, by enzymatic and serological methods
Abstract
In this study, exotoxins produced by 62 Aeromonas salmonicida strains and the bacterium Haemophilus piscium were analysed. Enzymatic assays, zymograms and serological detection were used to monitor secretion by bacterial strains of the previously described exotoxins P1, GCAT and AsaP1 and also the extracellular P2 metallo-gelatinase and a serine caseinase, which is different from the P1 protease and has not yet been characterized. Based on the results, the strains were divided into five groups. One comprised the type strains for A. salmonicida ssp. masoucida, H. piscium and 36% of the atypical isolates, and another, a type strain for A. salmonicida ssp. smithia together with 14% of the atypical isolates. A second type strain of A. salmonicida ssp. smithia was grouped with 8% of the atypical isolates. The largest group contained the type strains for A. salmonicida ssp. achromogenes and 38% of the atypical isolates. The type strains for A. salmonicida ssp. salmonicida were in the last group with all the four typical strains and 4% of the atypical isolates. The combination of zymogram and serological detection used is recommended as the most reliable method for characterizing A. salmonicida strains according to their exotoxin secretion.
Introduction
The fish pathogenic bacterium Aeromonas salmonicida has a world-wide geographical distribution and is one of the most important pathogens, infecting salmonids and numerous other fish species. Epizootics occur both in cultivated and wild fish stocks (Eggset & Gudmundsdóttir 1999). According to Holt, Krieg, Sneath, Stanley & Williams (1994), A. salmonicida is classified into four subspecies: ssp. salmonicida, ssp. achromogenes, ssp. masoucida and ssp. smithia. Recently, a fifth subspecies, pectinolytica, has been described (Pavan, Abbott, Zorzópulos & Janda 2000), although it has not yet been associated with disease in fish. Aeromonas salmonicida ssp. salmonicida, the causative agent of classical furunculosis of salmonids, is designated typical, and other strains atypical. Haemophilus piscium was originally described by Snieszko, Griffin & Friddle (1950) as the causal agent of ulcer disease, but its reclassification as atypical A. salmonicida has been suggested (Paterson, Douley & Desauteles 1980; Belland & Trust 1988; Boyd, Hiney, Peden & Smith 1994; Austin, Austin, Dalsgaard, Gudmundsdóttir, Høie, Thornton, Larsen, O'hici & Powell 1998).
Typical A. salmonicida strains are described as a homogeneous taxon, with respect to biochemical and genotypic characteristics (Eggset & Gudmundsdóttir 1999). Conversely, atypical A. salmonicida strains show a wide variety of host specificity (Wiklund & Dalsgaard 1998) and biochemical, molecular and virulence characteristics (Hirväla-Koski, Koski & Niiranen 1994; Gudmundsdóttir 1996; Gunnlaugsdóttir & Gudmundsdóttir 1997; Austin et al. 1998; Høie, Dalsgaard, Aase, Heum, Thornton & Powell 1999).
Atypical and typical strains of A. salmonicida have been reported to share cell-associated antigens like lipopolysaccharide (LPS) and outer membrane proteins, but extracellular virulence factors produced by typical and atypical strains differ (Chart, Shaw, Ishiguro & Trust 1984; Evenberg, Versluis & Lugtenberg 1985; Gudmundsdóttir 1996, 1998; Gunnlaugsdóttir & Gudmundsdóttir 1997; Austin et al. 1998; Eggset & Gudmundsdóttir 1999). It has been shown by genetic methods that there are atypical strains possessing genes encoding the P1 serine protease (AspA) and/or the glycerophospholipid:cholesterol acyltransferase (GCAT) cytotoxin, two major exotoxins of typical strains (Ellis 1997; Austin et al. 1998; Høie et al. 1999). Furthermore, atypical strains producing either of these enzymes have been reported (Gudmundsdóttir 1996; Gunnlaugsdóttir & Gudmundsdóttir 1997; Austin et al. 1998). A metallo-caseinase, AsaP1, has been isolated from the extracellular products (ECP) of some atypical strains and identified as a major exotoxin of a group of atypical strains, including the type strains for A. salmonicida ssp. achromogenes (Gudmundsdóttir, Hastings & Ellis 1990; Gudmundsdóttir & Magnadóttir 1997; Gunnlaugsdóttir & Gudmundsdóttir 1997). Some strains have also been found to produce a gelatinolytic metallo-protease, P2 (Sheeran & Smith 1981; Rockey, Fryer & Rohovec 1988; Gudmundsdóttir 1996; Wagner, Lachmann, Hädge & Drössler 1997) and several other proteolytic factors (Gudmundsdóttir 1996) which have not so far been related to A. salmonicida virulence.
Concurrent with the cultivation of more fish species, infections caused by atypical A. salmonicida strains have increased and bacteria differing from the already described subspecies are detected more frequently (Gudmundsdóttir 1998). The classification of atypical A. salmonicida isolates is unclear and proper identification of strains is problematic (Austin et al. 1998; Dalsgaard, Gudmundsdóttir, Helgason, Høie, Thoresen, Wichardt & Wiklund 1998). There is evidence that grouping of strains according to their exotoxin production can be reproducible (Gudmundsdóttir 1996; Gunnlaugsdóttir & Gudmundsdóttir 1997; Austin et al. 1998) and useful for selection of strains for vaccine development (Gudmundsdóttir 1998; Lillehaug, Nygaard, Eggset & Poppe 1999).
The aim of this study was to analyse by enzymatic and serological identification, exotoxins and proteolytic factors produced by reference strains of four subspecies of A. salmonicida ssp. salmonicida, ssp. masoucida, ssp. achromogenes and ssp. smithia, 50 atypical and four typical A. salmonicida isolates and a closely related bacterium, H. piscium. In addition, the sensitivity and reproducibility of enzymatic and serological methods for the detection of A. salmonicida antigens were compared.
Materials and methods
Bacterial strains
All the reference strains used in this study are listed in Table 1. In order to analyse the influences of laboratory maintenance on strain characters, two reference strains originating from a different strain collection were included for each of the four A. salmonicida subspecies. The 54 A. salmonicida isolates, listed in Table 2, were collected and examined in a concerted action coordinated by Dr Richard Powell (EU Contract no.: AIR 3-CT94-1884). Antigens of strains NCIMB13076, 251 and 265 were used in the generation of monoclonal antibodies (Mabs). The bacteria were routinely cultured on blood agar at 15 °C. Authenticity and purity of the isolates were established by examination of Gram-stained smears and by whole cell granular pattern agglutination tests, using the MONO-As kit (BIONOR AS, Skien, Norway), which consists of antibody-coated latex beads and is designed for specific identification of A. salmonicida. The absence of motility and growth at 37 °C were examined by the hanging drop method and incubation of seeded agar plates at 37 °C, respectively. In order to group the strains as typical or atypical, the following tests were applied using standard bacteriological methods: ampicillin (33 μg) and cephalothin (66 μg) sensitivity, aesculin hydrolysis, haemolysis on horse blood agar, production of gas from glucose, ability to produce indole and the capability to use sucrose as a carbon source. Stock cultures were stored in brain heart infusion (BHI; Oxoid, Lyfjaverslun Íslands, Reykjavík, Iceland) broth containing 15% (v/v) glycerol at −80 °C.
| Strain | Subspecies | Host species | Origin of isolation | Reference |
|---|---|---|---|---|
| Aeromonas salmonicida | ||||
| CCM4103 | smithia | Roach | UK | Czechoslovakian Collection of Microorganisms, Brno, Czechoslovakia |
| ATCC19261 | achromogenes | Sea trout | UK | American Type Culture Collection, Rockville, Maryland |
| ATCC27013 | masoucida | Masou salmon | Japan | |
| ATCC14174 | salmonicida | Brook trout | USA | |
| NCIMB1102 | salmonicida | Atlantic salmon | UK | National Collection of Industrial and Marine Bacteria, Aberdeen, Scotland |
| NCIMB1110 | achromogenes | Brown trout | UK | |
| NCIMB2020 | masoucida | Masou salmon | Japan | |
| NCIMB13210 | smithia | Roach | UK | |
| NCIMB13076 | salmonicida | Atlantic salmon | UK | |
| Haemophilus piscium | ||||
| NCIMB1952 | Rainbow trout | USA | National Collection of Industrial and Marine Bacteria, Aberdeen, Scotland |
| Collection strain no. | Original no. of strain | Host species | Origin of isolation |
|---|---|---|---|
| Atypical | |||
| 1 | SAB-1 | Sable fish | Canada |
| 4 | 81377 | Atlantic cod | Canada |
| 6 | F1542-2 | Atlantic salmon | Norway |
| 10 | MT373 | Atlantic salmon | UK |
| 16 | 87480 | Atlantic salmon | Canada |
| 19 | 91549 | Eel | Canada |
| 24 | Fin-7 | Brown trout | Finland |
| 28 | 3,111 | Goldfish | USA |
| 30 | V234/81 | Carp | Netherlands |
| 42 | 890902403 | Atlantic salmon | Norway |
| 46 | 880902221 | Arctic char | Norway |
| 47 | 880902778 | Turbot | Norway |
| 48 | 900900115 | Wrasse | Norway |
| 51 | 760900856 | Rainbow trout | Norway |
| 54 | 860902120 | Rainbow trout | Norway |
| 59 | 900903441 | Arctic char | Norway |
| 62 | 880903377 | Atlantic salmon | Norway |
| 69 | 870902303 | Atlantic salmon | Norway |
| 74 | 920901777 | Spotted wolf-fish | Norway |
| 78 | 930900914 | Atlantic cod | Norway |
| 81 | 930901392 | Wrasse | Norway |
| 89 | 940901074 | Flounder | Tasmania |
| 92 | 921113-1/3a | Plaice | Denmark |
| 93 | 921113-1/10a | Plaice | Denmark |
| 94 | 870626-1/1a | Blenny | Denmark |
| 102 | 841018-1/2 | Sand-eel | Denmark |
| 103 | 841116-3/1 | Sand-eel | Denmark |
| 108 | 114/1 (T1) | Flounder | Finland |
| 109 | 2F7-190 (T6) | Flounder | Finland |
| 112 | 8F12-38/1 (T19) | Flounder | Finland |
| 121 | 920501-3/1 | Dab | Denmark |
| 122 | 920501-3/3 | Dab | Denmark |
| 128 | 930719-1/30 | Dab | Denmark |
| 130 | 920720-2/4 | Turbot | Norway |
| 135 | 860625-4/3 | Atlantic salmon | Faroe Islands |
| 145 | M283/89 | Atlantic salmon | Iceland |
| 163 | TP-9 | Grayling | Finland |
| 167 | TP-121 | Whitefish | Finland |
| 176 | 5127/88 | Arctic char | Finland |
| 182 | 184/76 | Goldfish | Italy |
| 183 | M45/89 | Arctic char | Iceland |
| 200 | T5/92 | Whiting | Iceland |
| 201 | BA174 | Wolf-fish | Canada |
| 203 | – | Wolf-fish | Canada |
| 204 | V179 | Wrasse | UK |
| 209 | – | Wolf-fish | UK |
| 221 | AFHRL 1 | Goldfish | Australia |
| 222 | RN 85:1923-A | Goldfish | Australia |
| 224 | WN 87:442-E | Goldfish | Australia |
| 265 | 265/87 | Atlantic salmon | Iceland |
| Typical | |||
| 60 | 900902717 | Turbot | Norway |
| 231 | MT004 | Atlantic salmon | Scotland |
| 234 | 339/95 | Atlantic salmon | Iceland |
| 251 | F216.1/83 | Rainbow trout | Germany |
- Sources of isolates: Dr B. Austin, Department of Biological Sciences, Heriot-Watt University, Edinburgh, Scotland;
- Dr E.-M. Bernoth, CSIRO Australian Animal Health Laboratory, Victoria, Australia;
- Dr I. Dalsgaard, Fish Disease Laboratory, Danish Institute of Fisheries Research, Denmark;
- Dr B. K. Gudmundsdóttir, Institute for Experimental Pathology, University of Iceland, Reykjavík, Iceland;
- Dr S. Høie, Department of Fish Health, Central Veterinary Laboratory, Oslo, Norway;
- Dr G. Olivier, Department of Fisheries and Oceans, Halifax Fisheries Research Laboratory, Halifax, Canada;
- Dr R. Powell, Department of Microbiology, University College Galway, Ireland; Dr T. Wiklund, Institute of Parasitology, Åbo, Finland. The isolates were collected and examined in a concerted action, EU Contract no. AIR 3-CT94-1884, coordinated by R. Powell.
Preparation of ECP
The bacteria were cultivated on cellophane (BioRad, Lyfjaverslun Íslands, Reykjavík, Iceland) covered agar plates, using BHI agar supplemented with 5% (v/v) new-born calf serum (Gibco, Lyfjaverslun Íslands, Reykjavík, Iceland) at 22 °C. The bacteria and ECPs were washed off the cellophane with minimal amounts of phosphate-buffered saline (PBS; pH 7.2) when cells showed adequate growth (48–144 h) and centrifuged (2000 g for 30 min). The supernatant (ECP) was filtered (0.22-μm porosity filters; Millipore, Lyfjaverslun Íslands, Reykjavík, Iceland), protein concentration measured with the BioRad protein assay using bovine serum albumin as a standard, and the ECPs subsequently stored at −80 °C until required.
Analysis of enzyme activities
GCAT assay
The GCAT activity was detected by the method described by Lee & Ellis (1990). Phospholipase activity was determined by applying 20 μL samples into wells cut in 1% (w/v) agarose in PBS containing 1%l-α-lecithin (Sigma, Lyfjaverslun Íslands, Reykjavík, Iceland) and incubated at 22 °C for 48 h. The appearance of transparent zones around a well in the agar indicated a positive phospholipase reaction. Haemolytic activity of ECP against salmon red blood cells (RBC) was estimated by adding 100 μL of two fold serial dilutions of ECP in PBS to 100 μL of 1% (v/v) RBC suspension in PBS in a 96-microwell plate (Nunc, Lyfjaverslun Íslands, Reykjavík, Iceland). As a negative control, PBS was used instead of the enzyme solution. Incubation was performed for 3 h at 22 °C. One unit of haemolytic activity (HU) was defined as the dilution causing 50% haemolysis. The GCAT activity of ECP was determined as a positive phospholipase reaction together with pronounced haemolytic activity (≥128 HU).
Caseinase assay
Caseinase activity of ECP was determined as previously described (Gudmundsdóttir 1996) using azocasein (Sigma) as a substrate. Released azodye was measured spectrophotometrically at 450 nm (A450) against a reagent blank. The assay was performed in triplicate. One caseinase unit was defined as an increase A450 of 0.001 under the assay conditions.
Gelatinase assay
Gelatinase detection was performed by a radial diffusion method, as described by Gudmundsdóttir (1996), using 3% gelatine (Difco, Lyfjaverslun Íslands, Reykjavík, Iceland) in 1% agarose gels. Proteolytic activity was determined from a standard curve using trypsin (bovine pancreas type III, Sigma). One unit of gelatinase activity was defined as equivalent to that of 1 μg trypsin.
Protease inhibition
Solutions of 0.025 m phenylmethylsulphonyl fluoride (PMSF) and 0.050 m 1,10-phenanthroline in ethanol were freshly prepared. Equal volumes of enzyme and inhibitor solutions were mixed and incubated at 22 °C for 10 min prior to proteolytic assay. Controls contained only the solvent. The inhibition was designated positive if ≥15% of the activity was reduced by treatment.
Zymograms
Analysis of proteases by substrate gel electrophoresis was performed as described by Gudmundsdóttir (1996), using 12% gels supplemented with 0.1% substrate. Analysis of GCAT by sodium dodecylsulphate–polyacrylamide gel electrophoresis (SDS–PAGE) was performed using the α-naphthyl acetate-azo dye method for detection of esterolytically active GCAT as described by Lachmann, Wagner, Hädge & Drössler (1997), using 14% gels.
Mab-based ELISA test
The enzyme-linked immunosorbent assay (ELISA) test employed has been described in detail previously (Wagner, Gudmundsdóttir & Drössler 1999). All antibodies used in the test are listed in Table 3. The Mab-containing hybridoma supernatants (diluted 1:10) were used as the primary antibody. The Mab-captured antigens were detected by incubation with different rabbit antisera (diluted 1:4000), which, in turn, was detected with peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (Jackson ImmunoResearch, Lyfjaverslun Íslands, Reykjavík, Iceland) (diluted 1:10 000). Reactions were monitored colorimetrically using 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) diammonium salt as a chromogen and read at A450 after 1 h at 22 °C. In order to record non-specific ECP bindings, Mab 13C7 binding to l(−)-carnitine dehydratase of Escherichia coli was used as an irrelevant control antibody. Two anti-P2 Mabs were employed recognizing, at least in part, different molecular forms of this metallo-gelatinase. Every sample was applied in eight replicates in each test. An A450 reading higher or equal to 0.4 was deemed as positive (+5× SD of a zero-dose sample).
| Origin of bacterial antigen strain no. | Antigen | Reference of hybridoma generation | Hybridoma (capture antibody) | Rabbit antisera (secondary antibody) | Reference of rabbit antisera production |
|---|---|---|---|---|---|
| A. salmonicida ssp. salmonicida, NCIMB13076 | Glycerophospholipid:cholestrol acyltransferase, GCAT | Lachmann et al. (1998) | 1D11 (anti-GCAT) | Ranti-GCAT | Arnesen, Bjørnsdóttir, Jørgensen & Eggset (1993) |
| A. salmonicida ssp. salmonicida, isolate 251 | Serine protease, P1 | Wagner et al. (1997) | 9D5 (anti-P1) | Ranti-ECP251 | Wagner et al. (1997) |
| A. salmonicida ssp. salmonicida, isolate 251 | Metallo-gelatinase, P2 | Wagner et al. (1997) | 3B11 (anti-P2) | ||
| 15C2 (anti-P2) | |||||
| A. salmonicida ssp. achromogenes,isolate 265 | Metallo-caseinase, AsaP1 | Wagner et al. (1999) | 9F9 (anti-AsaP1) | Ranti-ECP265 | Gudmundsdóttir & Magnadóttir (1997) |
| Escherichia coli O44 K74 | L(−)-carnitine dehydratase (irrelevant control antigen) | Preusser, Wagner, Elssner & Kleber (1999) | 13C7 (negative control) | Either of the three above antisera |
Production of polyclonal murine antibodies
Polyclonal antibodies to AsaP1 were prepared in mouse ascitic fluid according to the method of Overkamp, Mohammed-Ali, Cartledge & Landon (1988). Antigen for immunization was prepared as follows. The ECP of isolate 265 (a known AsaP1 producer, Gudmundsdóttir et al. 1990) was subjected to SDS–PAGE, using 12% gels. Ten micrograms of protein in sample buffer (8% SDS and 10% 2-mercaptoethanol in Tris–HCl, pH 6.8) were placed in each gel pocket. One part of each gel was blotted onto a nitrocellulose (NC) membrane (Hybond ECL; Amersham Biosciences i Danmark Hørsholm, Denmark) and immunostained with Mab 9F9 (anti-AsaP1). Bands containing the AsaP1 protease were cut out of the gels and fragmented. Gel pieces from eight lanes (8 μg protein) were fragmented in 50 μL of PBS and emulsified with 1 mL of adjuvant. The ascitic fluid was clarified by centrifigation at 750 g, filtered through 0.22 μm filters (Millipore) and stored at −80 °C, until required. The specificity of the polyclonal antibodies was tested against purified protease and various A. salmonicida ECPs, using Western blotting.
Western blotting
The SDS–PAGE was performed using 12% gels. Various A. salmonicida ECPs were applied as antigens and transferred to an NC membrane in an electroblotter. The buffer used was 25 mm Tris–glycine, pH 8.8, containing 20% methanol. The membranes were blocked overnight at 4 °C with 0.1% skimmed milk powder in Tris-buffered saline (TBS), 0.1 m, pH 7.8, containing 0.1% Tween-20 (TBS/T). Immunostaining was performed by polyclonal murine anti-AsaP1 antibodies diluted 1:2000 or rabbit anti-P1 serum (donated by Trevor Hastings, FRS Marine Laboratory, Aberdeen, UK) diluted 1:500, followed by goat antimouse (BioRad; diluted 1:3000) or goat anti-rabbit (Dako, Lyfjaverslun Íslands, Reykjavík, Iceland; diluted 1:1000) immunoglobulin antibodies conjugated with alkaline phosphatase. The antibodies were diluted in TBS/T and incubations performed at room temperature for 2 h. The blots were developed with 0.1 m ethanolamine/HCl buffer (pH 9.6) containing 1 mg mL−1p-nitroblue tetrazolium (NBT), 0.1 m MgCl2 and 4 mg mL−1 5-bromo-4-chloro-3-indolyl phosphate (BCIP) in methanol:acetone (2:1).
Results
Bacteriological examinations
All the reference strains, including the type strain for H. piscium, and the 54 A. salmonicida isolates were found to be Gram-negative non-motile short rods that did not grow at 37 °C and were positive in the MONO-As test. The four strains designated typical (Table 2) fulfilled all the following characteristics: sensitive to both ampicillin and cephalothin, degraded aesculin and horse blood, produced gas from glucose, were unable to produce indole and did not produce acid from sucrose. The 50 isolates termed atypical varied from the typical isolates in one or more of the listed characteristics.
Detection of exoenzymes and comparison of enzymatic and serological methods
The results of exoenzyme analyses are listed in Table 4 and 1-5. The isolates fell into 11 groups according to the exoenzyme profile obtained (Table 4) and five groups according to the exotoxins detected by enzymatic and serological methods (Table 5).
| Profile observed | Number of isolates | GCAT activity | GCAT- ELISA | Serine caseinase | Serine gelatinase | P1-ELISA | Metallo- caseinase | AsaP1- ELISA | Metallo- gelatinase | P2-ELISA | Factors detected in zymograms | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| activity* | activity* | activity** | activity** | Mab3B11 | MAb15C2 | |||||||
| 1 | 6 | − | − | − | − | − | − | − | − | − | − | None |
| 2 | 15 | − | − | − | − | − | − | − | + | + | + | P2 |
| 3 | 3 | − | − | + | + | + | − | − | − | − | − | P1 |
| 4 | 5 | − | − | + | + | + | − | − | + | + | + | P1 and P2 |
| 5 | 2 | − | − | + | + | + | + | + | + | + | + | P1, AsaP1 and P2 |
| 6 | 1 | − | − | + | + | + | + | + | + | − | − | P1 and AsaP1 |
| 7 | 10 | − | − | + | − | − | + | + | + | − | − | AsaP1 and a serine caseinase |
| 8 | 7 | − | − | + | − | − | + | + | + | + | + | AsaP1, a serine caseinase and P2 |
| 9 | 6 | − | − | − | − | − | + | + | + | + | + | AsaP1 and P2 |
| 10 | 1 | + | + | + | + | + | − | − | − | − | − | GCAT and P1 |
| 11 | 7 | + | + | + | + | + | − | − | + | + | + | GCAT, P1 and P2 |
- Caseinase activity: +, ≥10 units; Gelatinase activity: +, ≥2 units; *inhibition of activity by PMSF ≥15%; **inhibition of activity by 1,10-phenanthroline ≥ 15%; GCAT activity: +, a positive phospholipase reaction and haemolytic activity against salmon-RBC ≥128 HU; ELISA reactivities were expressed as difference in A450 values to negative controls containing the irrelevant Mab 13C7; +, >0.4 ; −, <0.4; P1, a 70 kDa serine protease (Tajima Takahashi, Ezura & Kimura 1983; Wagner et al. 1997); P2, high and low molecular phospholipid:cholestrol acyltransferase with molecular weight of 26-27 kDa (Eggset et al. 1994; Lachmann et al. 1998). Isolates in profile 1: NCIMB1952 (H. piscium), 19, 54, 89, 128 and 182; Isolates in profile 2: ATCC27013 (masoucida), NCIMB2020 (masoucida), 4, 24, 28, 30, 46, 51, 92, 163, 167, 176, 221, 222 and 224; Isolates in profile 3: 93, 108 and 109; Isolates in profile 4: NCIMB13210 (smithia), 59, 121, 122 and 130; Isolates in profile 5: CCM4103 (smithia) and 204; Isolate in profile 6: 112; Isolates in profile 7: ATCC19261 (achromogenes), NCIMB1110 (achromogenes), 10, 47, 78, 135, 145, 183, 200, and 265; Isolates in profile 8: 42, 74, 94, 102, 103, 201 and 203; Isolates in profile 9: 6, 16, 48, 62, 69 and 81; Isolate in profile 10: ATCC14174 (salmonicida); Isolates in profile 11: NCIMB1102 (salmonicida), 1, 60 (typical), 209, 231(typical), 234 (typical) and 251 (typical).
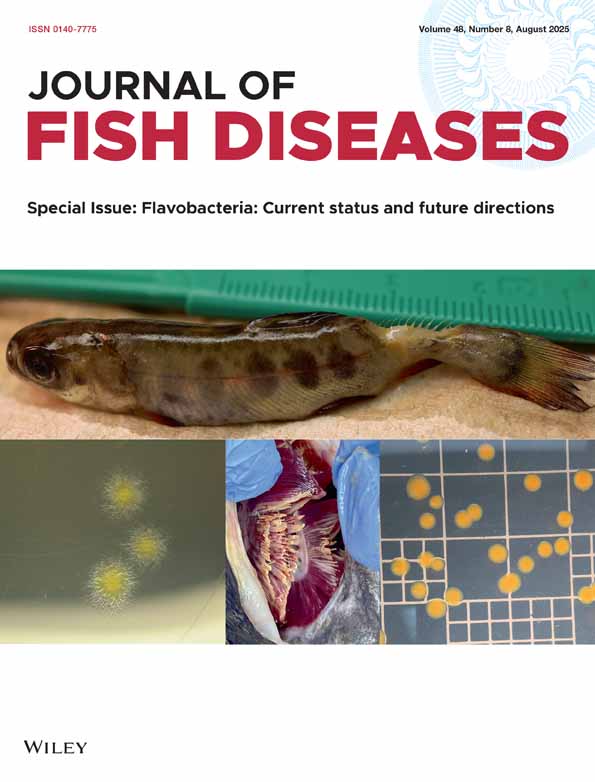
Caseinolytic zymograms of ECPs. (a) Lane 1, A. salmonicida ssp. masoucida (strain ATCC27013); lane 2, A. salmonicida ssp. masoucida (strain NCIMB2020); lane 3, A. salmonicida ssp. smithia (strain CCM4103); lane 4, A. salmonicida ssp. achromogenes (strain ATCC19261); lane 5, A. salmonicida ssp. achromogenes (strain NCIMB1110); lane 6, A. salmonicida ssp. smithia (strain NCIMB13210); lane 7, A. salmonicida ssp. salmonicida (strain NCIMB1102); lane 8, A. salmonicida ssp. salmonicida (strain ATCC14174) and lane 9, H. piscium (strain NCIMB1952). (b) lane 1, isolate 74 and lane 2, isolate 108.

Western blots of ECPs from the reference strains used in the study. Proteins were detected by immunostaining with rabbit anti-P1 serum, diluted 1:500. Lane 1, A. salmonicida ssp. masoucida (strain NCIMB2020); lane 2, H. piscium (strain NCIMB1952); lane 3, A. salmonicida ssp. salmonicida (strain ATCC14174); lane 4, A. salmonicida ssp. smithia (strain CCM4103); lane 5, A. salmonicida ssp. achromogenes (strain ATCC19261).
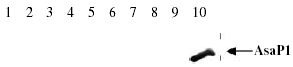
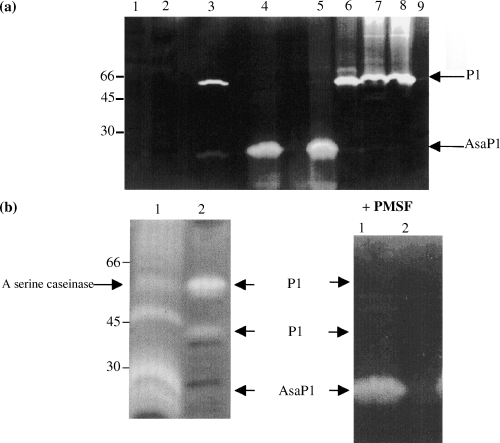
Western blots of extracellular products (ECP) from A. salmonicida isolates from the strain collection. Proteins were detected by immunostaining with murine anti-AsaP1 ascitic fluid diluted 1:2000. Lane 1, isolate 251; lane 2, isolate 1; lane 3, isolate 93; lane 4, isolate 163; lane 5, isolate 209; lane 6, isolate 221; lane 7, isolate 222; lane 8, isolate 224; lane 9, isolate 60 and lane 10, isolate 265.
Esterolytic zymograms of extracellular products (ECP). (a) Lane 1, pre-stained MW standards (80; 52; 35; 30 and 22 kDa); lane 2,H. piscium (strain NCIMB1952); lane 3, A. salmonicida ssp. smithia (strain CCM4103); lane 4, A. salmonicida ssp. masoucida (strain NCIMB2020); lane 5, A. salmonicida ssp. masoucida (strain ATCC27013); lane 6, A. salmonicida ssp. achromogenes (strain ATCC19261); lane 7, A. salmonicida ssp. salmonicida (strain ATCC14174) and lane 8, A. salmonicida ssp. salmonicida (strain NCIMB1102). (b) Lane 1, isolate 265; lane 2, isolate 60; lane 3, prestained MW standards (80; 52; 35; 30 and 22 kDa) and lane 4, A. salmonicida ssp. salmonicida (strain NCIMB1102).
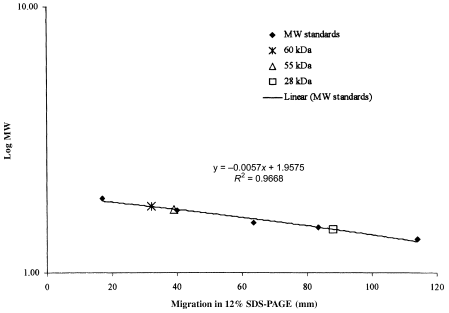
Diagram showing the migration of pre-stained molecular weight (MW) standards and esterolytic components of A. salmonicida ECPs in a 12% SDS–PAGE, plotted against the logarithmic value of the MW (kDa) of the respective proteins.
| Collection strain no. | Host species | Host order | Origin of isolation | Virulence related factor(s) detected in zymograms |
|---|---|---|---|---|
| NCIMB1952 (H. piscium) | Rainbow trout | Salmoniformes | USA | None |
| ATCC27013 (masoucida) | Masou salmon | Salmoniformes | Japan | |
| NCIMB2020 (masoucida) | Masou salmon | Salmoniformes | Japan | |
| 4 | Atlantic cod | Gadiformes | Canada | |
| 19 | Eel | Anguilliformes | Canada | |
| 24 | Brown trout | Salmoniformes | Finland | |
| 28 | Goldfish | Cypriniformes | USA | |
| 30 | Carp | Cypriniformes | Netherlands | |
| 46 | Arctic char | Salmoniformes | Norway | |
| 51 | Rainbow trout | Salmoniformes | Norway | |
| 54 | Rainbow trout | Salmoniformes | Norway | |
| 89 | Flounder | Pleuronectiformes | Tasmania | |
| 92 | Plaice | Pleuronectiformes | Denmark | |
| 128 | Dab | Pleuronectiformes | Denmark | |
| 163 | Grayling | Salmoniformes | Finland | |
| 167 | White fish | Salmoniformes | Finland | |
| 176 | Arctic char | Salmoniformes | Finland | |
| 182 | Goldfish | Cypriniformes | Italy | |
| 221 | Goldfish | Cypriniformes | Australia | |
| 222 | Goldfish | Cypriniformes | Australia | |
| 224 | Goldfish | Cypriniformes | Australia | |
| NCIMB13210 (smithia) | Roach | Cypriniformes | UK | P1 |
| 59 | Arctic char | Salmoniformes | Norway | |
| 93 | Plaice | Pleuronectiformes | Denmark | |
| 108 | Flounder | Pleuronectiformes | Finland | |
| 109 | Flounder | Pleuronectiformes | Finland | |
| 121 | Dab | Pleuronectiformes | Denmark | |
| 122 | Dab | Pleuronectiformes | Denmark | |
| 130 | Turbot | Pleuronectiformes | Norway | |
| ATCC19261 (achromogenes) | Sea trout | Salmoniformes | UK | AsaP1 |
| NCIMB1110 (achromogenes) | Brown trout | Salmoniformes | UK | |
| 6 | Atlantic salmon | Salmoniformes | Norway | |
| 10 | Atlantic salmon | Salmoniformes | UK | |
| 16 | Atlantic salmon | Salmoniformes | Canada | |
| 42 | Atlantic salmon | Salmoniformes | Norway | |
| 47 | Turbot | Pleuronectiformes | Norway | |
| 48 | Wrasse | Perciformes | Norway | |
| 62 | Atlantic salmon | Salmoniformes | Norway | |
| 69 | Atlantic salmon | Salmoniformes | Norway | |
| 74 | Spotted wolf-fish | Perciformes | Norway | |
| 78 | Atlantic cod | Gadiformes | Norway | |
| 81 | Wrasse | Perciformes | Norway | |
| 94 | Blenny | Perciformes | Denmark | |
| 102 | Sand-eel | Perciformes | Denmark | |
| 103 | Sand-eel | Perciformes | Denmark | |
| 135 | Atlantic salmon | Salmoniformes | Faroe Islands | |
| 145 | Atlantic salmon | Salmoniformes | Iceland | |
| 183 | Arctic char | Salmoniformes | Iceland | |
| 200 | Whiting | Gadiformes | Iceland | |
| 203 | Wolf-fish | Perciformes | Canada | |
| 201 | Wolf-fish | Perciformes | Canada | |
| 265 | Atlantic salmon | Salmoniformes | Iceland | |
| CCM4103 (smithia) | Roach | Cypriniformes | UK | P1 and AsaP1 |
| 112 | Flounder | Pleuronectiformes | Finland | |
| 204 | Wrasse | Perciformes | UK | |
| 1 | Sable fish | Scorpaeniformes | Canada | GCAT and P1 |
| 209 | Wolf-fish | Perciformes | UK | |
| ATCC14174 (salmonicida) | Brook trout | Salmoniformes | USA | |
| NCIMB1102 (salmonicida) | Atlantic salmon | Salmoniformes | UK | |
| 60 (a typical strain) | Turbot | Pleuronectiformes | Norway | |
| 231 (a typical strain) | Atlantic salmon | Salmoniformes | UK | |
| 234 (a typical strain) | Atlantic salmon | Salmoniformes | Iceland | GCAT and P1 |
| 251 (a typical strain) | Rainbow trout | Salmoniformes | Germany |
- P1, a 70 kDa serine protease (Tajima et al. 1983; Wagner et al. 1997).
- AsaP1, a toxic metallo-protease (Gudmundsdóttir et al. 1990; Wagner et al. 1999).
- GCAT, a glycerophospholipid:cholestrol acyltransferase with MW of 26–27 kDa (Eggset et al. 1994; Lachmann et al. 1998).
No exoenzyme production detected
According to the defined criteria for a positive reaction of the analysed enzymes (listed in Materials and Methods and Table 4), the type strain for H. piscium and 10% (five of 50) of the atypical strains were not found to produce any of the respective exoenzymes.
GCAT
A positive GCAT assay (phospholipase assay and haemolytic titration) was detected in the two type strains for ssp. salmonicida, all the four typical and 4% (two of 50) of the atypical isolates (Table 4). The same ECPs were the only products that were defined as positive in the GCAT-ELISA. In all these eight ECPs, a strong band of 28 kDa and bands of 55 and 60 kDa were stained in esterolytic zymograms (Fig. 4a, lanes 7 and 8; Fig. 4b, lanes 2 and 4; Fig. 5). The 60 kDa band was stained in the zymogram of ECPs of all the tested strains and a 55 kDa band was revealed in the majority of the ECPs. On the other hand, the 28 kDa band was detected only in the ECPs that were found to be GCAT positive by GCAT assay and GCAT-ELISA. The ECPs of the two type strains for ssp. masoucida and one atypical isolate (16) were designated negative in the GCAT-ELISA (A450 = 0.34 and 0.37 and 0.36, respectively) and also in the phospholipase assay. A strong band of 60 kDa was stained in zymograms (Fig. 4a, lanes 4 and 5) of these ECPs and they all induced pronounced haemolytic activity against salmon RBC (128–256 HU after 3 h).
P1 serine protease
The P1 protease was identified by all methods (gelatinase and caseinase assays, protease inhibition assay, zymogram detection and P1-ELISA) applied to the reference strains for ssp. smithia and salmonicida, 20% (10 of 50) of the atypical isolates and all the typical strains (four of four) involved in the study. One isolate (204) was P1 positive in the enzymatic assays and zymogram, but negative in the Mab based P1-ELISA (A450 = 0.16). A Western blot of isolate 204 ECP was, however, immunostained with rabbit anti-P1 (data not shown). The ssp. masoucida type strains were designated P1 negative in the enzymatic tests and the Mab-based ELISA (A450 = 0.05 and 0.06, respectively), but a factor of the same size as that of P1 was stained with rabbit anti-P1 serum in Western blotting (data not shown).
AsaP1 metallo-protease
The AsaP1 metallo-protease was detected by all methods (caseinase assay, protease inhibition assay, zymogram detection and AsaP1-ELISA) in the ECPs of both reference strains for ssp. achromogenes, one of the two for ssp. smithia and 46% of the atypical isolates. In the casein zymogram the apparent molecular weight (AMW) of AsaP1 was close to 30 kDa. There was good correspondence between results from the enzymatic assay and casein zymogram, but some discrepancies were observed between enzymatic and serological detection. Thus, 14% of the atypical isolates (isolates no. 1, 93, 163, 209, 221, 222 and 224) and one typical isolate (60) were designated positive (A450 = 0.87, 0.83, 0.47, 0.48, 0.72, 0.45, 0.56 and 0.87) in the AsaP1-ELISA, although enzymatic methods did not detect the enzyme. Western blotting analysis using reduced samples of the eight listed ECPs, the ECPs of isolate 265 as a positive control and 251 as a negative control, respectively, was performed and the blots immunostained with polyclonal mouse anti-AsaP1 antibodies. Bands were not stained in any of the ECPs except that of isolate 265 (Fig. 3).
Serine caseinase
A serine caseinase activity was detected in the ECPs of both ssp. achromogenes reference strains and also in 30% (15 of 50) of the atypical isolates, which all produced the AsaP1 protease. A PMSF sensitive gelatinolytic activity was not detected in any of these ECPs and they were all negative in the P1-ELISA. The PMSF sensitive factors with AMW close to that of P1 and below were detected in the same ECPs (Fig. 1B, lane 1). There was 100% agreement between the recognition of the factor in casein SDS–PAGE and detection of caseinase activity inhibited by PMSF. A faint band of the same size as P1 was revealed in these ECPs in Western blots stained by rabbit anti-P1 (Fig. 2, lane 5).
P2 metallo-gelatinase
The P2 protease was detected in the ECPs of the two reference strains for ssp. smithia and masoucida, one of the two for ssp. salmonicida, 74% (37 of 50) of the atypical isolates and the four typical isolates. There was a good correlation between detection of P2 by enzymatic and serological methods with the exception that the ECPs of three goldfish isolates from Australia (isolates no. 221, 222 and 224) were all negative in the P2-ELISA, although a metallo-gelatinase activity was detected and the gelatine SDS–PAGE revealed gelatinolytic bands with AMW comparable with that of the P2 protease.
Comparison of virulence-associated antigens detected by enzymatic and serological analyses with the host and geographical origin of isolation
Grouping of the strains according to detection of three factors, P1, GCAT and AsaP1, which have been associated with A. salmonicida virulence, was performed and compared with the host and geographical origin of isolation (Table 5). A criterion for a strain to be considered positive was that both zymogram and serological detection were positive. The strains were allocated into five different groups based on the detection of the named virulence-related factors.
The only patterns observed between the exotoxins produced and the host were as follows. All goldfish isolates were in the group with strains not producing any of the known exotoxins. All isolates from salmonid fish in Finland were also in this group. All the flatfish isolates from Finland, three from Denmark and one from Norway were in the group producing P1. The only obvious relationship between geographical origin of isolation and exotoxin production of atypical isolates was that all five isolates from Iceland and the Faroe Islands were grouped together and also all the three isolates from Australia. Twenty-eight per cent of the atypical isolates originated from Norway and were represented in four groups with three, two, eight and one isolates in each, respectively.
Discussion
In the present study, the production of the previously described exotoxins, P1, GCAT and AsaP1 of the bacterium A. salmonicida and two exoproteases, the P2 metallo-gelatinase and a serine caseinase, were analysed by enzymatic and serological methods. The bacterial strains studied included two reference strains of each of the four described A. salmonicida subspecies that have been associated with infections in fish, a reference strain of the bacterium H. piscium, four typical and 50 atypical A. salmonicida isolates. The isolates fell into 11 groups according to the exoenzyme profile obtained, but were divided into five groups based on their secretion of known exotoxins.
The type strain for H. piscium was found to agglutinate with species-specific anti-A. salmonicida antibodies and was grouped with atypical A. salmonicida, which is in agreement with results from previous studies suggesting that the bacterium should be classified as atypical A. salmonicida (Paterson et al. 1980; Belland & Trust 1988; Boyd et al. 1994; Austin et al. 1998).
According to the results of the analyses, the type strains for A. salmonicida ssp. masoucida and ssp. achromogenes did not group together. There are, however, previous proposals of McCarthy & Roberts (1980) and Belland & Trust (1988), which suggest that they could be combined.
Both type strains for A. salmonicida ssp. masoucida, the type strain for H. piscium, and 36% of the atypical isolates were not found to produce any of the known virulence-related factors in detectable amounts. The two type strains for ssp. achromogenes and 42% of the atypical isolates were in another group, producing only the AsaP1 exotoxin. One of the two type strains for ssp. smithia was placed together with 14% of the atypical isolates in a group that was found to produce only the P1 protease. The second reference strain for ssp. smithia and 4% of the atypical isolates were found to produce P1 and AsaP1. The type strains for ssp. salmonicida, all the four typical isolates and 4% of the atypical isolates were in the last group producing P1 and GCAT.
A pair of reference strains for each of the four A. salmonicida subspecies, which originated from different strain collections, was included in order to analyse for different influences on strain characters as a result of handling. Each pair of type strains used, originated from the same initial isolate. The only discrepancy obtained was that one of the two type strains for ssp. smithia (CCM4103) was found to produce the AsaP1 exotoxin, indicating that it may have lost the ability as a result of environmental influences.
The variety of secreted toxins produced by strains of A. salmonicida may well contribute to their wide geographical distribution and ability to infect various fish species. However, the only definite pattern regarding origin of atypical isolates and their exotoxin production observed were the following. All goldfish isolates were in the group with strains not producing any of the known exotoxins. This group included the isolates from Australia and all the isolates from salmonid fish from Finland. All the flatfish isolates from Finland, three from Denmark and one from Norway were in the group producing P1, together with one of the type strains for ssp. smithia. All atypical isolates from Iceland and the Faroe Islands were among the 21 atypical isolates producing only the AsaP1 exotoxin. Ten of these isolates originated from salmonid fish and eight from perciform fish. This is in accordance with previously reported observations (Gudmundsdóttir 1996; Gunnlaugsdóttir & Gudmundsdóttir 1997; Wiklund & Dalsgaard 1998).
The correlation of results from protease and GCAT activity assays and detection of the respective factors in zymograms was very good, but some discrepancies were observed between enzymatic and serological detection of the different proteolytic factors and also between the serological tests, ELISA and Western blotting. Thus, the two ssp. masoucida type strains were designated P1 negative in the enzymatic tests and the Mab-based ELISA (A450 = 0.05 and 0.06, respectively), but a factor of the same size as that of P1 was weakly stained with polyclonal rabbit anti-P1 serum on Western blots. The Mab used in the ELISA has been shown to bind the native 70 kDa serine protease of A. salmonicida with high affinity and a related caseinase of A. hydrophila (Wagner et al. 1997). The results, therefore, indicate that the polyclonal anti-P1 antibodies detected inactive molecules of the protease.
The ECPs of 14% of the atypical isolates and one typical isolate were designated weakly positive for AsaP1 in the ELISA, although neither enzymatic assays nor zymograms detected the enzyme. Polyclonal murine anti-AsaP1 antibodies also failed to identify the exotoxin on blots of these ECP samples. A previous study suggested that the Mab 9F9-based ELISA used is highly specific and far more sensitive than the caseinase assay (Wagner et al. 1999). However, the possibility of detection of inactive protease molecules or degradation products has to be considered.
In all the ECPs that were designated GCAT positive in ELISA, the enzyme was also detected by enzymatic methods. Additionally, type strains for ssp. masoucida, achromogenes and smithia produced an esterolytic enzyme revealing two factors with MW of 55 and 60 kDa in zymograms. The Mab 1D11 used for ELISA is reported to bind GCAT of A. salmonicida with high specific activity (Lachmann, Wagner, Hädge & Drössler 1998). However, this ELISA did not detect the exotoxin in the ECPs of the strains used here and none of them were found to be GCAT-positive by enzymatic methods. The ssp. masoucida type strains and one atypical isolate (16), which were found to possess pronounced haemolytic activity, but not detectable phospholipase activity, were just under the limit (A450 = 0.4) to be defined as weakly positive (A450 = 0.34–0.37) in the GCAT-ELISA. Furthermore, the same three ECPs revealed stronger 60 kDa bands in zymograms than those of other GCAT negative strains. The GCAT monomer (28 kDa) was never seen in ECPs of GCAT negative strains. It is known that the proform of the GCAT enzyme consists of two polypeptides connected by a disulphide bridge, which is proteolytically digested during activation (Brumlik & Buckley 1996). Furthermore, the tendency of activated GCAT to form dimers has been documented (Ausio, van der Goot & Buckley 1993). In a paper describing the screening of 205 isolates of atypical Aeromonas strains by polymerase chain reaction (PCR)-based amplification of genes encoding for GCAT, using appropriate primer sets, it was demonstrated that all strains tested had the GCAT-gene (Høie et al. 1999). The results of this study indicate that the 55–60 kDa factors may represent a proform of the GCAT enzyme, or an enzyme which is somewhat different from the described GCAT enzyme of A. salmonicida, although genetically related.
Analyses of the P2 metallo-gelatinase were included in order to increase the accuracy of AsaP1 detection, as AsaP1 also comprises metallo-protease activity (Gudmundsdóttir et al. 1990). Furthermore, the study confirmed previous data (Gudmundsdottir 1996; Wagner et al. 1999), that typical and atypical strains share this ECP antigen. There was a good correlation between detection of the P2 gelatinase by enzymatic and serological methods, with the exception that three goldfish isolates from Australia were negative in the ELISA, but positive in the enzymatic assays. This indicates that enzymatic detection was either the most sensitive method or that the gelatinase detected was immunologically different from the P2 protease.
A serine caseinase activity was detected by enzymatic methods in the ECPs of both ssp. achromogenes reference strains and 15 of 23 AsaP1-positive isolates. No inhibitory effect of PMSF on gelatinolytic activity of these strains, a characteristic feature of P1, was detected. A c. 50 kDa band was exposed in casein SDS–PAGE of all these ECPs. Although these ECPs were P1-negative in the ELISA, immunostaining with rabbit anti-P1 serum stained bands, although very weakly, in ECPs of some of these isolates, possibly indicating some common epitopes with P1. Staining of bands with AMW close to 66 and 43 kDa in A. salmonicida ECP with rabbit anti-P1 serum has been reported (Ellis, do Vale, Bowden, Thompson & Hastings 1997). In a genetic analysis by Høie et al. (1999)A. salmonicida ssp. achromogenes (ATCC19261) and 11 of the 15 named isolates (no. 10, 47, 78, 94, 102, 103, 135, 145, 183, 200 and 201) were found to be AspA-negative in two assays using two different PCR primer sets, and three isolates (isolates no. 42, 74 and 203) were positive in PCR with both primer sets (one isolate, 265, was not involved in the PCR analysis). It is therefore most probable that some A. salmonicida strains produce a serine caseinase, which is different from the P1 protease and has not yet been characterized.
Although the Mab-based ELISA may be the most sensitive method in detection of the AsaP1 exotoxin, this was not observed for the P1- and GCAT-ELISA. Zymogram detection has the advantage of revealing enzymatic activity and the size of the respective factor simultaneously and in this study was sensitive in giving reproducible results. Based on the results from this study, the combination of zymogram and serological detection is recommended as the most reliable method for grouping A. salmonicida strains according to their exotoxin secretion.
As the growth rate of the isolates involved in this study were quite different, all strains were grown to stationary phase before the ECPs were harvested. This was performed to minimize growth phase affects. Measures were taken to avoid the effect of storage. Thus, all procedures were performed using ECPs produced and stored under identical conditions. Each test was carried out twice, using different ECP preparations and was repeated in the case of discrepancies. However, the results may indicate that the strains used had not been passaged in fish in order to recover virulence lost during repeated subcultivation on agar plates, as was indicated by the difference obtained between the exotoxin production of the pair of type strains for ssp. smithia.
The clinical presentation of atypical A. salmonicida infections in fish may be manifested differently (Gudmundsdóttir 1998; Wiklund & Dalsgaard 1998; Eggset & Gudmundsdóttir 1999). In some cases, they cause a disease with clinical signs comparable with those of typical furunculosis, but may also cause an ulcerative disease with a more superficial variety of pathological changes than seen in typical furunculosis. Furthermore, the same strain has been shown to cause different pathology in different hosts (Gudmundsdóttir 1998). The exact roles played by the various virulence factors of A. salmonicida are still unclear. When injected into fish, purified factors or combinations of factors can result in disease signs similar to those observed during infection with A. salmonicida (Ellis 1997; Gudmundsdóttir 1997). However, more research is needed before functions can be assigned to each of the virulence factors.
Currently atypical furunculosis causes epidemics in farming of fish such as cod, spotted wolffish, halibut, turbot and in salmonids. Commercial furunculosis vaccines have been used with different results (Lillehaug et al. 1999). Grouping of A. salmonicida strains after exotoxin secretion may be a useful approach for selecting furunculosis vaccines and strains for vaccine production.
Acknowledgements
This study was supported by EU Contract No: AIR 3-CT94-1884. We wish to thank the other partners in the project R. Powell, B. Austin, I. Dalsgaard, S. Høie, J.L. Larsen and others for the supply of strains used in the study. The Federation of European Microbiological Societies, FEMS, provided a fellowship to Iris Hvandal to visit Leipzig. We also wish to thank T. Hastings for supplying P1 antiserum, and S. Vilhjálmsdóttir and Eggert Gunnarsson for their help with immunization of mice.
References
Received: 17 June 2002 Accepted: 3 September 2002