Effects of ethanol ingestion on epididymal glycosidases and fertility in the rat
Abstract
Epididymal glycosidases play a role in sperm maturation by modifying sperm surface glycoproteins. To study the effects of ethanol on epididymal sperm maturation, ethanol (3 g/kg body weight as 25%, v/v) was administered to a group of rats by gastric-intubation twice daily for 30 days. In another group, rats were also treated with alcohol for 30 days but were then withdrawn from treatment for 30 days to assess the reversibility of ethanol-induced effects. Ethanol-induced changes in epididymal tissue and sperm glycosidases, cauda epididymal sperm motility and the fertility of rats were assessed. Ethanol treatment caused a marked decrease in the specific activities of glycosidases in both tissues and spermatozoa from epididymal segments. Cauda epididymal sperm motility and the fertility of ethanol-treated rats were significantly impaired compared to control rats fed an isocaloric diet. These changes are likely to be the consequence of direct and indirect effects of ethanol mediated through subnormal testosterone and dihydrotestosterone. Most of these changes were found to be reversible. The present study suggests that impaired activity of sperm glycosidases may be one of the factors responsible for defective sperm motility and fertilizing potential in ethanol-treated rats.
Introduction
Studies on semen samples from alcoholics have shown decreased numbers and motility of spermatozoa with increased teratozoospermia ( Kucheria et al., 1985 ; Brzek, 1987; Gomathi et al., 1993 ), suggesting impaired epididymal sperm maturation. The latter process is not an intrinsic property of spermatozoa but is brought about by interaction of the spermatozoa with secretory products of the epididymal epithelium ( Amann et al., 1993 ). A number of components including glycoproteins become associated with the sperm surface, and both membrane-integrated and surface-adsorbed glycoproteins are altered ( Orgebin-Crist, 1987). The synthesis and secretion of various glycoproteins by the different regions of the epididymis as well as the incorporation of these glycoproteins onto/into different regions of the sperm plasma membrane have been well documented ( Robaire & Hermo, 1988). The addition and deletion of sugars to and from glycoproteins of the sperm plasma membrane are mediated by glycosyltransferases ( Tadolini et al., 1976 ; Bernal et al., 1980 ; Hamilton, 1980) and glycosidases ( Chapman & Killian, 1984; Skudlarek & Orgebin-Crist, 1986; Skudlarek et al., 1992 ), respectively. Changes in the lectin-binding ability of the sperm plasma membrane during epididymal maturation ( Nicolson et al., 1977 ; Hammerstedt et al., 1982 ) indicate the action of glycosidases to alter terminal saccharide residues. In addition to their hydrolytic activity, the active site of glycosidases have been implicated as non-catalytic carbohydrate-binding proteins that mediate sperm–oocyte recognition as an early step in fertilization ( Tulsiani et al., 1989 , 1990; Cornwall et al., 1991 ; Miller et al., 1993 ; Grimalt et al., 1995 ). In view of the pivotal role played by epididymal glycosidases in sperm maturation and fertilization, the impact of ethanol toxicity on these processes was studied.
Materials and methods
Chemicals
Ethanol (99.9%) was purchased from Riedel-de-Haen, Germany. Fine chemicals such as substrates, enzymes, coenzymes, standards and the ethanol measurement kit were purchased from Sigma Chemical Company, USA. Other chemicals and reagents were from Merck and Sisco Research Laboratories, India.
Animals
Healthy adult male albino rats of the Wistar strain, weighing 175–225 g, were used. Rats were kept in clean cages in a temperature-controlled room with a 12-h light: dark regime. Animals were divided into the following treatment groups and provided with drinking water ad libitum.
Group I: control. Rats received an isocalorific quantity of sucrose in the same volume in which experimental rats received ethanol.
Group II: ethanol-treated. Ethanol was administered twice daily (at 0930 and 1530 h) at a dose of 3 g/kg body weight, as a 25% (v/v) aqueous solution for 30 days by gastric intubation.
Group III: ethanol treatment withdrawn. Rats treated with ethanol as in group II for 30 days were withdrawn from treatment for a further period of 30 days, to assess the reversibility of the ethanol effect.
Each group consisted of 30 rats. The control group had two equal subgroups, namely the control for ethanol treatment and the control for ethanol withdrawal. As there was no significant difference between these two control subgroups in terms of the parameters studied (except body weight), they were pooled to provide a single control group. Bodyweight was recorded at regular intervals (twice weekly) and the volume of ethanol administered was adjusted accordingly. Animals were pair-fed by providing the same weight of pelleted rat feed to control animals (Lipton India Ltd, India) as the corresponding experimental animal had eaten the previous day. In addition, the calorie value derived from ethanol (in experimental animals) was replaced isocalorifically by feeding sucrose to control animals. After the respective experimental period, rats were killed by decapitation. Epididymides were dissected free from adhering fat and connective tissues and weighed.
Collection of spermatozoa
Spermatozoa were expressed from the ends of cauda epididymal tubules by cutting with a sharp blade. Spermatozoa with epididymal fluid were diluted with physiological saline and placed on a thin glass slide. Fifty spermatozoa were observed from each epididymis. The rate of forward motility (100 spermatozoa per rat) and percentage of forward motile spermatozoa were assessed at a magnification of ×150 using a light microscope fitted with a pre-calibrated ocular micrometer. The ocular micrometer was rotated accordingly to follow the direction of movement of spermatozoa, and the time taken (in seconds) to travel 10 divisions was recorded ( Gomathi et al., 1993 ).
Epididymal spermatozoa were separated into KRP buffer (pH 7.4) according to the method of Brooks (1976). The epididymal segments were cut into ≈1.0 mm3 pieces using a sharp razor blade, after division of the epididymis into caput, corpus and cauda regions as per the guidelines of Hamilton (1975). Spermatozoa from the epididymal pieces were completely removed by vortexing gently in KRP buffer. Spermatozoa released into the buffer was aspirated, centrifuged at 800 g and used for estimations. Spermatozoa were counted using a Neubauer heamocytometer as per the method of Zaneveld & Polakoski (1977).
Ethanol, hormone and glycosidase measurement
Blood and epididymal (caput, corpus and cauda) ethanol was estimated using the ethanol estimation kit. Tissue levels of testosterone and dihydrotestosterone (DHT) were measured by radioimmunoassay using a kit purchased from Amersham International Plc, UK. α-Glucosidase ( Carlsen & Pierce, 1972), α- L-fucosidase and α-mannosidase ( Grandmont et al., 1983 ) were estimated spectrophotometrically using p-nitrophenyl substrates as per the respective methods. β-Galactosidase, β-glucosidase, β-N-acetyl glucosaminidase and β-N-acetyl galactosaminidase were estimated fluorimetrically using respective 4-methylumbelliferone substrates, according to the method of Chapman & Killian (1984).
Fertility
The fertility of control, ethanol-treated and ethanol treatment-withdrawn rats was assessed by co-habiting each male with two proven fertile proestrus female rats. The presence of spermatozoa in the vaginal smear or vaginal plug confirmed mating. The number of rats that became pregnant was noted and females were then isolated until parturition. The number of female rats that become pregnant and the number of pups delivered were considered as indices of fertility and fecundity, respectively. Results are expressed in terms of the percentage change in experimental (ethanol-treated or ethanol treatment-withdrawn) rats when compared to control.
Statistical analysis
Data were analysed using one-way analysis of variance ( Zar, 1974). When this revealed a significant (p < 0.05) difference, the data were further subjected to the Student-Newman-Keul's test.
Results
Ethanol and hormone concentrations
Ethanol concentrations in blood and epididymis (caput, corpus and cauda) reached a peak at 20 min after ingestion and returned to baseline levels at 360 min ( Table 1). The percentage of progressively motile spermatozoa and the rate of forward motility of morphologically normal spermatozoa recovered from the cauda epididymis decreased significantly in ethanol-treated rats. Following withdrawal of ethanol treatment, these changes reverted to normal. Ethanol administration caused a marked fall in both testosterone and DHT concentrations in epididymal (caput, corpus and cauda) tissues. These changes also reverted to normal following withdrawal of ethanol treatment ( Table 2).
Activity of epididymal glycosidases
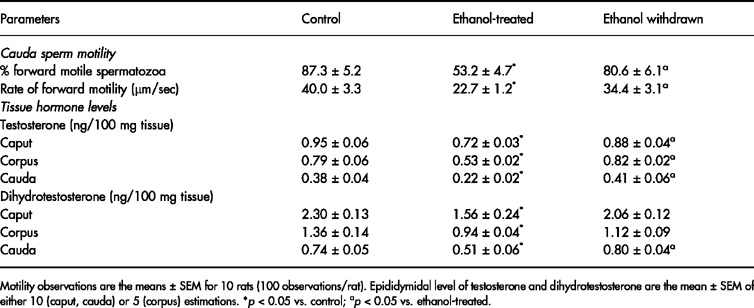
Activity of β- D-galactosidase declined markedly in caput, corpus and cauda epididymis as well as in spermatozoa obtained from these regions. Withdrawal of ethanol treatment restored the enzyme activity in epididymal tissues to normal. However, in spermatozoa, restoration of enzyme activity was found only for cells obtained from the corpus and cauda regions ( Fig. 1).

0 (caput and cauda) or five (corpus) estimations. ap < 0.05 vs. control; bp < 0.05 vs. ethanol-treated.
Activity of fucosidase showed a marked fall in tissue from all three epididymal regions, while sperm fucosidase declined specifically in the corpus and caudal segments only. Withdrawal of ethanol treatment restored the enzyme activity in both tissue and sperm to normal levels ( Fig. 2).

. Effects of ethanol treatment on activity of α-fucosidase in (a) epididymal tissue and (b) spermatozoa. Values are mean ± SEM of 10 (caput and cauda) or five (corpus) estimations. ap < 0.05 vs. control; bp < 0.05 vs. ethanol-treated.
Following ethanol treatment, activity of α-glucosidase declined perceptibly in caput, corpus and cauda epididymis, while activity of β-glucosidase remained unaltered. However, in spermatozoa obtained from all three segments, activities of both α- and β-glucosidase exhibited a decrease in response to ethanol treatment. With the exception of β-glucosidase activity in spermatozoa from the caput epididymis, all of the changes in epididymal tissues and spermatozoa were restored to normal following discontinuation of ethanol treatment (Figs 3 & 4 3).
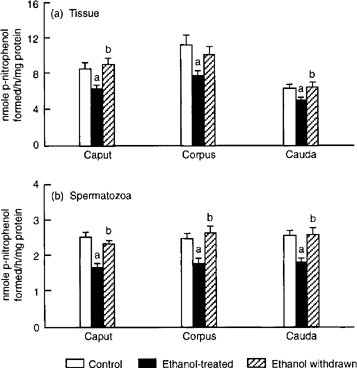
. Effects of ethanol treatment on activity of α-glucosidase in (a) epididymal tissue and (b) spermatozoa. Values are mean ± SEM of 10 (caput and cauda) or five (corpus) estimations. ap < 0.05 vs. control; bp < 0.05 vs. ethanol-treated.
In ethanol-treated rats, the activities of β-N-acetyl glucosaminidase and β-N-acetyl galactosaminidase were reduced in both tissue and spermatozoa from all three epididymal segments. After withdrawal of ethanol treatment, only the decrease in activity of β-N-acetyl galactosaminidase of caput spermatozoa persisted (Figs 5 & 6 5. Effects of ethanol treatment on activity of β-N-acetyl- D-glucosaminidase in (a) epididymal tissue and (b) spermatozoa. Values are mean ± SEM of 10 (caput and cauda) or five (corpus) estimations. ap < 0.05 vs. control; bp < 0.05).
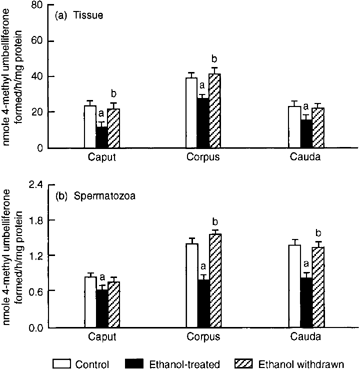
vs. ethanol-treated.
A marked fall in α-mannosidase activity was evident in caput, corpus and cauda epididymal tissues as well as in spermatozoa collected from these segments. Changes in enzyme activity were returned to normal in epididymal tissues following withdrawal of treatment. However, the ethanol-induced decline in α-mannosidase activity persisted in caput and corpus spermatozoa ( Fig. 7).
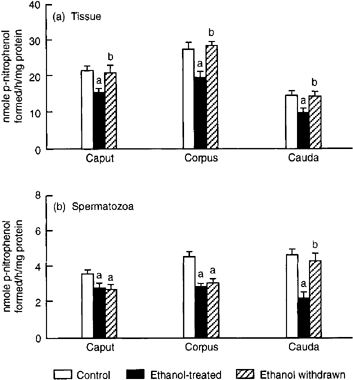
. Effects of ethanol treatment on the (a) epididymal tissue and (b) sperm α-mannosidase activity. Values are mean ± SEM of 10 (caput and cauda) or five (corpus) estimations. ap < 0.05 vs. control; bp < 0.05 vs. ethanol-treated.
Ethanol treatment caused a marked fall in the number of rats that became pregnant (60%) and in litter size (50%) after mating. Withdrawal of ethanol treatment restored fertility to normal ( Fig. 8).

. Effects of ethanol treatment on the fertility and fecundity of male rats. Values are mean ± SEM of 25 estimations. ap < 0.05 vs. control; bp < 0.05 vs. ethanol-treated.
Discussion
In accordance with the present study, Roine et al. (1991 ) have also shown a peak in blood alcohol concentration at around 20 min after administration by oral gavage at a dose of 1 g/kg body weight. Penetration of ethanol into tissues is reported to be dependent on its water content and permeability ( Kalant, 1971). The higher ethanol concentration in the caput than in the corpus and cauda epididymis may be due to its high fluid content ( Hamilton, 1975).
The epididymis receives androgens from the testis and the peripheral circulation ( Robaire & Hermo, 1988). The ethanol-induced decrease in testicular ( Salonen & Huhtaniemi, 1990; Adams et al., 1991 ) and serum testosterone could be responsible for the decreased epididymal tissue levels of testosterone and DHT. Oestrogens impair the production of 5α-reduced androgens by inhibiting 5α-reductase in the prostate ( Lee et al., 1973 ; Jenkins & Mc Caffrey, 1974) and by causing their retention within the epididymis ( Vazquez et al., 1986 ). Therefore, the decrease in epididymal DHT may also be due to an ethanol-induced increase in estradiol. Ethanol treatment increased the conversion of DHT to 5α-androstane-3ß,17β-diol and 5α-androstane-3α,17β-diol in Leydig cells ( Murono & Fisher-Simpson, 1984, 1985a) and liver ( Murono & Fisher-Simpson, 1985b). A similar mechanism is probably operative in the epididymis to produce low levels of DHT.
The impairment of sperm motility in ethanol-treated rats indicates a defect in the acquisition and/or maintenance of sperm motility. A similar decrease in average sperm velocity was observed in ethanol-treated C57B1/6 J mice ( Anderson et al., 1983 ). Exposure of human spermatozoa to ethanol in vitro (21.7 mmol/L) for up to 9 h also decreased the percentage of motile cells and their average speed ( von Doepfmer & Hinckers, 1966). Therefore, it is suggested that ethanol in the epididymis might have disturbed sperm motility directly and/or indirectly through altering the biochemical function of the epididymis.
In control rats, a regional difference was observed in the specific activity of all glycosidases in the caput, corpus and cauda epididymis as well as in spermatozoa obtained from these segments. Tissue glycosidases, in general, showed maximum activity in the corpus, lower activity in the caput and much lower activity in the cauda. On the other hand, the specific activity of sperm glycosidases showed an increase from the caput to the cauda. Others have recorded a similar trend ( Chapman & Killian, 1984; Hall & Killian, 1987; Gupta & Setty, 1995; Hall et al., 1996 ). The regional difference in the activities of these enzymes may be ascribed to differences in the rate of synthesis, secretion or degradation of these enzymes.
Ethanol treatment reduced both epididymal tissue and sperm β- D-galactosidase activity in all regions, suggesting the impairment of enzyme synthesis, its reduced availability in luminal fluid and subsequent attachment to spermatozoa. Sperm-associated β- D-galactosidase has been suggested to have a role either as a lectin or as a hydrolase during fertilization ( Majumder & Turkington, 1974; Nikolajczyk & O'Rand, 1992). The lowered sperm β- D-galactosidase activity due to ethanol treatment may have resulted in an adverse effect in the recognition of the active site of sugars on the surface of the oocyte during fertilization.
β-N-acetyl glucosaminidase plays a vital role in the formation of the acrosomal matrix ( NagDas et al., 1996 ), in the acrosome reaction ( Miller et al., 1993 ; Takada et al., 1994 ) and in fertilization ( Miller et al., 1993 ; NagDas et al., 1996 ). It is therefore possible that the low activity of sperm N-acetyl glucosaminidase may be one of the factors that contributed to the impaired fertility of ethanol-treated rats.
The diminished activity of α-fucosidase in caput, corpus and cauda epididymal tissues suggests the defective synthesis of this enzyme. A specific decline in the activity of this enzyme in spermatozoa from the corpus and cauda epididymis suggests impaired availability of fucosidase in epididymal luminal fluid and uptake of the same by spermatozoa.
The potential role of α-fucosidase in fertilization is evident from the study of Taylor et al. (1989 ) who have demonstrated that male English Springer Spaniels which are deficient in α-fucosidase because of a genetic mutation are sterile. Low activity of α-fucosidase observed in spermatozoa may be one of the reasons for the decreased fertility of ethanol-treated rats. The recovery of α-fucosidase in both epididymal tissues and spermatozoa following withdrawal of ethanol treatment shows clearly that the ethanol-induced changes are reversible.
α-Glucosidase activity in human seminal plasma has been acknowledged to be a marker of epididymal function ( Cooper et al., 1988 ; Guérin et al., 1990 ) and could provide information concerning the maturation of spermatozoa during their transit through the epididymis ( Guérin et al., 1990 ). Data on both tissue and sperm α-glucosidase activity suggest an inhibitory effect of ethanol. The inhibitory response of α-glucosidase may be attributed to low epididymal testosterone and DHT levels, since earlier studies have shown androgen dependence of epididymal α-glucosidase activity in the rat and mouse ( Conchie & Findlay, 1958; Grandmont et al., 1983 ). In the present study, activity of α-glucosidase recovered along with restoration of epididymal androgens in ethanol-withdrawn rats. α-glucosidase has also been suggested to play a role in destruction of the cytoplasmic droplets of spermatozoa or in the digestion of adsorbed polysaccharides and glycoproteins, as well as in the processing of glycoproteins synthesized by the epididymis ( Jauhiainen & Vanha-Pertula, 1985). From these observations, it is suggested that the low activity of sperm α-glucosidase may be one of the factors responsible for the high incidence of spermatozoa containing cytoplasmic droplets recorded in the present study. Supporting evidence comes from ethanol-withdrawn rats, in which these values returned to normal.
Unlike other glycosidases, activity of β-glucosidase did not show any regional difference in either tissue or spermatozoa. Tissue and sperm β-glucosidase activity was considerably lower than that of other glycosidases. The low activity of this enzyme may be due to the fact that β-glucosidase will not function in the modification of membrane carbohydrate moieties, since glucose is not present as a structural component of sperm membranes ( Chapman & Killian, 1984). Ethanol treatment did not affect β-glucosidase activity in epididymal tissues but specifically decreased activity in spermatozoa obtained from all three epididymal regions, suggesting the differential effect of ethanol on β-glucosidase activity in epididymal tissues and spermatozoa.
α-Mannosidase has been implicated as a non-catalytic binding protein during sperm–oocyte interaction ( Tulsiani et al., 1989 , 1990; Cornwall et al., 1991 ). Mannosyl residues on the zona pellucida and α- D-mannosidase on the spermatozoa are involved in a ligand–receptor interaction to facilitate sperm–oocyte binding in the rat and mouse ( Tulsiani et al., 1989 , 1990). Shalgi et al. (1986 ) demonstrated that rat oocytes pre-treated with mannosidase showed complete inhibition of sperm–oocyte binding. Sperm–oocyte binding is also reduced by inhibitors of this enzyme ( Cornwall et al., 1991 ). Therefore, in the present study, low activity of α-mannosidase in spermatozoa may have an adverse effect on the fertilization process.
In general, all glycosidases in both epididymal tissues and spermatozoa showed inhibition after ethanol treatment. The reduced activity of sperm glycosidases could be due to reduced uptake of these enzymes from epididymal luminal fluid which, in turn, may be due to impaired synthesis and secretion of these enzymes, as it has been shown that glycosidases secreted by the epididymal epithelium become associated with, tightly bound to, or incorporated into the sperm plasma membrane ( Hall & Killian, 1987; Barbieri et al., 1994 ). Since epididymal glycosidases have been reported to be regulated by androgens ( Conchie & Findlay, 1958; Grandmont et al., 1983 ), the observed changes in the present study may be attributed to low levels of tissue testosterone and DHT. These changes in epididymal glycosidases culminating in defective glycoprotein metabolism may be responsible for impaired fertility.
Partial restoration of the enzyme activities in caput and/or corpus spermatozoa following withdrawal of ethanol treatment indicates that 30 days of withdrawal is insufficient to restore the activity to normal or that restoration to normal levels requires the involvement of factors other than androgens.
A number of studies have shown that, in non-reproductive tissues, both alcohol and its metabolite, acetaldehyde, exert their effects directly on the endoplasmic reticulum and Golgi apparatus (the site of glycosylation) not only by altering their morphology but also by interfering with several steps in glycoconjugate synthesis and release ( Matsuda et al., 1979 ; Baraona & Lieber, 1982; Fernandez-Briera et al., 1990 ). In the epididymis also, it is probable that ethanol and its metabolite, acetaldehyde, exert their effects directly by impairing the synthesis and release of glycosidases which are also glycoproteins ( Khar & Anand, 1977; Kornfeld & Mellman, 1989; Hancock et al., 1993 ). The presence of ethanol as well as ADH in the epididymis ( von Wartburg & Buhler, 1984) lends support to the above proposal.
In the present study, copulation of male rats was confirmed by the formation of vaginal plugs or the presence of spermatozoa in the vaginal smear. A look at the data revealed that only 60% of the female rats were mated successfully. The failure of mating in the remaining percentage of females may be due to the adverse impact of chronic ethanol ingestion on sexual behaviour of male rats.
The substantial increase in abnormal spermatozoa and decreased motility of morphologically normal spermatozoa in ethanol-treated rats may be two of the reasons for the observed decrease in fertility and litter size. A decrease in glycosidases culminating in defective glycoprotein metabolism is a clear manifestation of impaired sperm maturation. The decreased fertility and fecundity may also be due to the inability of spermatozoa to undergo physiologically important events (i.e. capacitation, acrosome reaction) which are prerequisites for fertilization of oocytes ( Zaneveld, 1978). Abnormal spermatogenesis and sperm maturation could produce metabolically deficient spermatozoa or spermatozoa deficient in enzymes necessary for oocyte recognition and sperm penetration of the outer investments of the oocyte ( Zaneveld, 1975). Therefore, in the present study, the diminished fertility of ethanol-treated male rats could be due to an increase in morphologically abnormal spermatozoa, a decrease in the percentage of progressively motile spermatozoa and impaired forward motility of morphologically normal spermatozoa or incomplete sperm maturation within the epididymis.
Acknowledgements
Financial assistance from the Council of Scientific and Industrial Research, New Delhi is gratefully acknowledged.