NIM-R7, a novel marker for resting B1 and marginal-zone B lymphocytes, is also expressed on activated T and B cells
Summary
In mice, follicular B cells have been studied in detail, while two other B-cell subpopulations – marginal-zone B and B1 cells – are less well understood. In this work we report the expression pattern of p58, a lymphocyte-activation marker, recognized by rat monoclonal antibody, NIM-R7, and present on the latter two cell subpopulations. Staining with NIM-R7 showed that undisturbed marginal-zone B cells, as well as peritoneal cavity and splenic B1a cells, constitutively expressed p58, whereas follicular B cells and resting T lymphocytes did not. Ontogenic analysis of different compartments showed that p58 did not appear at any stage of development, prior to the development of mature T or B2 lymphocytes. Upon polyclonal stimulation, however, p58 appeared on both T and B2 lymphocytes. Finally, ricin A-conjugated NIM-R7 was able to kill the BCL1 lymphoma without effect on mature resting B2 cells. Therefore, p58 may be a potential target for diagnosis or therapy of B1 and marginal-zone B-cell malignancies.
Abbreviations
-
- Con A
-
- concanavalin A
-
- FITC
-
- fluorescein isothiocynate
-
- FO
-
- follicular
-
- IL
-
- interleukin
-
- LPS
-
- lipopolysaccharide
-
- mAb
-
- monoclonal antibody
-
- MZ
-
- marginal zone
-
- PE
-
- phycoerythrin.
Introduction
The process of lymphocyte maturation has been greatly clarified through defining the sequential and differential expression of cell-surface antigens.1 At a functional level, the relationship between cell-surface marker expression and the resulting cell physiology has enlightened our understanding of the biology of lymphocytes. Nonetheless, there remain many unanswered questions and relatively unexplored areas.2 For example, within the long-lived ‘naïve’ B-cell compartment, follicular (FO) B cells (also named B2) are more numerous and have been studied in detail,3 whereas the B-cell population within the marginal zone (MZ B cells) and the minor B1 subpopulation, typically enriched in peritoneal cavity but also found in spleen and lymph nodes, have been less well studied. Indeed, it is only recently that the important role of MZ B cells to respond to particulate blood-borne antigens has been defined.4
Similarly, B1 cells derived mainly from fetal lymphopoiesis5 behave differently in some experimental situations to peritoneal B1 cells,6 and differ in some surface markers.7 Therefore, FO, MZ and B1 cells may have different roles in the immune system, not only in health8 but also in disease. For example, the relationship of B1 and MZ B cells to autoimmune processes9,10 and chronic lymphocytic leukemias11,12 makes the study of these subpopulations of great importance and also suggests practical applications of their markers as possible targets for diagnosis and, perhaps, directed therapy.12
In this work we characterized cellular expression of the selectively expressed lymphocytic glycoprotein, p58, recognized by the rat mAb, NIM-R7.13 Within the resting lymphocyte populations, p58 was uniquely expressed on undisturbed B-cell subpopulations of the MZ in the spleen and on B1 cells of both the peritoneal cavity and the spleen. Both activated T and B cells, also expressed p58. Importantly, NIM-R7 coupled to ricin killed BCL1 lymphoma cells, without any significant death of the follicular (B2) B-cell compartment.
Materials and methods
Mice
BALB/c mice were bred and maintained in the animal facility of Centro de Investigación y de Estudios Avanzados (CINVESTAV). Animals were age- (newborn to 8 weeks old) and gender-matched.
Reagents
Monoclonal antibodies (mAbs) NIM-R7 (anti-p58) and 1C10 have been described previously.13,14 Lipopolysaccharide (LPS), from Escherichia coli serotype 055:B5, and concanavalin A (Con A) were purchased from Sigma (St Louis, MO). Interleukin (IL)-4 and IL-5 were purchased from Genzyme (Cambridge, MA). RPMI-1640 supplemented with 2 mm glutamine and 10% (vol/vol) fetal calf serum (FCS) (all from Gibco, Grand Island, NY) were used in all cell cultures.
Cell-surface staining
mAb NIM-R7 was conjugated to biotin in our laboratory. Fluorescein isothiocyanate (FITC)-labelled anti-immunoglobulin (Ig)M and anti-Mac1; phycoerythrin-labelled (PE) anti-IgD and anti-IgM; as well as Spectral Red-labelled (SPRD) anti-B220 and anti-CD5 were purchased from Southern Biotechnology Associates, Inc. (Birmingham, AL); anti-B220-FITC, anti-CD3-FITC, anti-CD21-FITC, anti-B220-PE, anti-CD23-PE, anti-B220-biotin, anti-CD138-biotin and IgG1-biotin were purchased from PharMingen (San Diego, CA).
Spleens were removed from mice at different ages. Briefly, 106 cells, depleted of red blood cells (RBC) by lysis in an ammonium chloride-containing buffer and recovered from an anti-B220 panning, as well as peritoneal cavity cells, were incubated with a mixture of fluorescein-, PE-, SPRD- and biotin-conjugated Abs followed by incubation with streptavidin-allophyco cyanin (SA-APC) (PharMingen). Cells were incubated for 15 min at each step and washed with phosphate-buffered saline (PBS) containing 0·5% bovine serum albumin (BSA) between steps. Data were acquired using a fluorescence-activated cell sorter (FACSCalibur; Becton-Dickinson, San Jose, CA) and analysed using CellQuest software (Becton-Dickinson).
p58 expression after stimulation
One million splenocytes were obtained by Ficoll (Sigma) gradient separation and incubated in complete media with one of the following as stimulus: LPS (20 µg/ml) plus IL-4 (100 U/ml); 1C10 (10 µg/ml) plus IL-4 (100 U/ml); or Con A (2·5 µg/ml). All cells were incubated for 24, 48, 72 or 96 hr at 37°. After incubation, the cells were washed and labelled with anti-CD3-FITC, B220-PE and NIM-R7-biotin or an isotype control, and SA-APC to develop biotinylated reagents, as described above. B220+ or CD3+ cells were selected, and the staining of p58 in activated cells (according to size and granularity) was analysed by flow cytometry, as described above.
Killing normal spleen lymphocytes or the BCL1 lymphoma cells with ricin A-conjugated NIM-R7
The antibody, NIM-R7, was conjugated to ricin A, as described previously.13,15 Briefly, NIM-R7, or an irrelevant isotype control, was conjugated to ricin A chain and gel filtrated through Sephacryl S200. BCL1 cells or splenic lymphocytes were incubated for 1 hr at 4° with different concentrations of the ricin A-conjugated antibodies, after which the cells were washed three times with cold medium and then assayed for proliferation according to the following protocol: BCL1 cells were stimulated with recombinant IL-5 (10 U/ml) and spleen lymphocytes were stimulated with LPS (20 µg/ml) plus IL-4 (100 U/ml). After 48 or 72 hr, respectively, of incubation, cells were pulsed for 4 hr with 0·5 µCi of [3H]thymidine before being harvested and counted.13
Results
The activation molecule, p58, is expressed on activated follicular cells, and MZ and B1a cell subpopulations from the spleen
B cells in the spleen are not homogeneous. Three major subpopulations have been identified by their phenotype and biological role: the major follicular (FO) B-cell population and the minor subpopulations B1 and MZ B cells.16 The distribution of p58 on these subpopulations was determined by four-parameter FACS analysis, using NIM-R7 to stain B-cell populations defined by expression of IgM, CD21 and CD5. Because B1 and MZ B cells are minor subpopulations in the spleen, we collected not less than one hundred thousand events. Figure 1 shows that undisturbed B1a (CD5+) and MZ B cells were positive for the expression of p58, while immature B cells did not express p58. Importantly, when FO B cells were separated according to size and granularity to distinguish between resting and activated cells, the activated cells expressed p58 (Fig. 1b). These results, in sum, point out that p58 is a marker of undisturbed MZ and B1a and activated FO B cells.
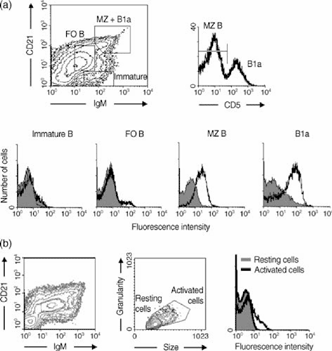
Expression of p58 on splenic B-cell subpopulations. p58 is expressed on marginal-zone (MZ) B cells and on B1a cells, but not on immature B cells (a), while it is expressed on follicular B cells (FO) with activated characteristics (b). A total of 106 B cells, enriched from panning with anti-B220 antibody, were stained with immunoglobulin M antibody (anti-IgM), CD21 antibody (anti-CD21), CD5 antibody (anti-CD5) and NIM-R7 monoclonal antibody (mAb) or an isotype-matched control mAb. The shadow line shows the staining with the isotype-control mAb.
The activation molecule, p58, is expressed on peritoneal cavity B cells
To continue with the analysis of the B-cell subpopulations, we stained peritoneal cavity B cells, where we found a population positive for staining with NIM-R7. Because peritoneal cavity contains T and B lymphocytes, we used B220 and CD3 to define the lineage. As seen in Fig. 2(a), T cells were negative for the expression of p58. In contrast, more than half of the B lymphocytes stained positively with NIM-R7. B cells in the peritoneum can be separated into two main populations, which are mainly defined by the amount of IgM and IgD on their surface. B1 cells are IgM bright, IgD dim and express Mac-1, while B2 cells are IgM medium, IgD bright and do not express Mac-1. As seen in Fig. 2(b), 2(c), B1 lymphocytes were the only cells expressing p58. The results from this figure, along with p58 expression on splenic B1a cells, clearly show that p58, as defined by staining with the mAb, NIM-R7, is a marker for undisturbed B1 cells.
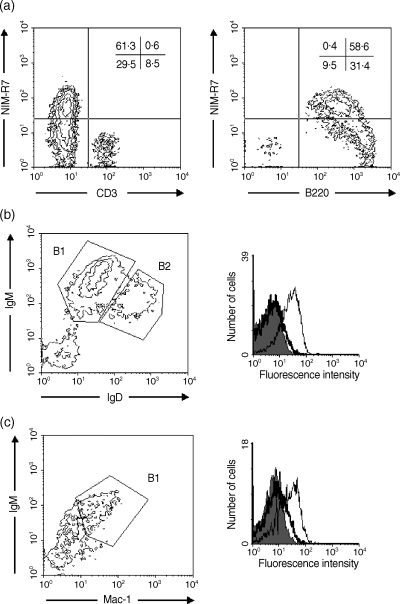
The p58 molecule is expressed on peritoneal cavity B1 cells. p58 is expressed on B cells, but not on T cells from the peritoneal cavity (a). p58 is expressed on B1 cells, defined by immunoglobulin (Ig)M and IgD staining (b), or on B1 cells, as defined by Mac-1 and IgM staining (c). A total of 106 cells were stained with anti-B220 and anti-CD3 (a), anti-IgM and anti-IgD (b), or anti-Mac-1 and IgM (c), and NIM-R7 monoclonal antibody (mAb) or an isotype-matched control mAb. In all panels, a gate in the lymphocyte region (according to size and granularity) is shown. In (b) and (c) the shadow lines show the staining with the isotype control; the thick lines are B2 cells and the thin lines are B1 cells.
p58 is induced after lymphocyte stimulation
Because p58 was originally reported to be induced upon activation in B cells,13 we analysed the induction of this antigen on B cells as well as its expression on activated T cells. We activated splenic cells with LPS, anti-CD40 or Con A. The results shown in Fig. 3 demonstrate that p58 is expressed on B cells after 24 hr of stimulation, increasing in intensity at later time-points. The maximum expression of p58 was reached at 72 hr, with anti-CD40 being a more potent stimulus than LPS (Fig. 3). B cells, activated as a consequence of the Con A-induced T-cell activation, also showed this pattern of expression. Interestingly, splenic lymphocytes activated with Con A showed a strong expression of p58 on the surface of T cells. The induction of p58 on T cells occurred more rapidly than on B cells, with maximum expression at 24 hr of stimulation and a decrease at 72 hr (Fig. 3d). These results confirm and extend the observation that p58 is a B- and T-lymphocyte activation antigen.
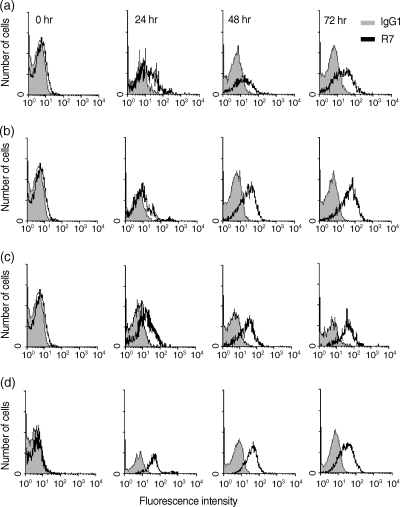
The p58 molecule is expressed upon lymphocyte activation. p58 is expressed on anti-CD40 activated (a), lipopolysaccharide (LPS)-activated (b), or concanavalin A (Con A)-activated (c) B cells, and on Con A-activated T cells (d). A total of 106 cells were activated for 0, 24, 48, 72 or 96 hr, and then stained with anti-B220 or anti-CD3 and NIM-R7 monoclonal antibody (mAb) or an isotype-matched control mAb. The analysis was performed on activated cells (according to size and granularity). Shadow histograms show the staining with the isotype control.
Ontogeny of the expression of p58
Next, the ontogeny of the expression of p58, in spleens of BALB/c mice at different ages and in liver from newborns, was analysed. When looking for the expression of p58 on the surface of B cells (defined by the marker B220), it was seen that p58 was not expressed on B220+ cells until week 8 of age, when a small percentage of NIM-R7-positive cells appeared (Table 1). To extend these results, bone marrow samples from adult mice were also analysed. As shown in Table 1, the expression of p58 was not seen on B cells or on the remaining populations that reside in the bone marrow. The same type of analysis was performed in thymus, and once again p58 was not found (Table 1). Taken together, we can conclude that p58 its not expressed at the early stages of lymphocyte development.
| Age | Mean fluorescence intensity | |||
|---|---|---|---|---|
| B220 | CD3 | |||
| IgG1 | NIM-R7 | IgG1 | NIM-R7 | |
| Liver (newborn) | 3·0 | 3·0 | – | – |
| Spleen (newborn) | 2·7 | 2·9 | – | – |
| Spleen (2 weeks) | 3·3 | 3·5 | 5·0 | 5·5 |
| Spleen (4 weeks) | 3·4 | 4·0 | 4·0 | 5·0 |
| Spleen (8 weeks) | 3·4 | 4·6 | 3·0 | 3·7 |
| Adult bone marrow | 6·0 | 6·1 | – | – |
| Thymus | – | – | 2·3 | 2·6 |
- A total of 106 cells were stained with anti-CD3 or anti-B220 and the antibody NIM-R7 or an isotype-matched control monoclonal antibody.
- IgG1, immunoglobulin G1.
Expression of p58 on lymphomas
Analysis of the expression of p58 was extended with the staining of different T- and B-cell lymphomas, representing different stages of development. As shown in Table 2, several B-cell lymphomas, representing immature (CH31), activated (BCL1) and differentiated (SP2/0 and C1.18.4) cells, were positive for the expression of p58. T-cell lymphomas, such as BW5147 and EL-4 (immature), were negative, and CTLL-2, representing an activated T-cell lymphoma, showed a dim staining with the mAb NIM-R7. As expression of p58 was higher in the plasmacytomas, we looked for the expression of this molecule on normal plasma cells from spleen and bone marrow. Spleen and bone marrow cells were stained with B220, CD138 and NIM-R7, in order to clearly define the plasma cells (B220+, CD138+). However, p58 was not detected on cells of this phenotype, either in the spleen (mainly derived from MZ B lymphocytes) or in the bone marrow plasma cells (germinal centre-derived) (data not shown). These results suggest that p58 is critically expressed during activation and may be lost on the surface of fully differentiated plasma cells from normal healthy mice.
| Name | Description | Mean fluorescence intensity | |
|---|---|---|---|
| IgG1 | NIM-R7 | ||
| BaF/3 | Pro-B cells | 4 | 5 |
| 70Z | Pre-B cells | 3 | 3 |
| WEHI-231 | Immature B cell | 4 | 11 |
| CH31 | Immature B cell | 4 | 12 |
| CH33 | Immature B cell | 4 | 22 |
| BCL1 | Activated B cell | 5 | 119 |
| A20 | Differentiated B cell | 4 | 9 |
| Ag8 | Plasmacytoma | 3 | 3 |
| SP2/0 | Plasmacytoma | 17 | 224 |
| C1.18.4 | Plasmacytoma | 7 | 157 |
| LBRM33 1A5 | T-cell precursor | 4 | 4 |
| BW5147 | Immature T cell | 3 | 3 |
| EL-4 | Immature T cell | 4 | 4 |
| CTLL2 | Mature T cell | 5 | 7 |
| YAC-1 | Lymphoma | 4 | 5 |
| RAW309.F1 | Lymphoma | 8 | 8 |
| L-929 | Fibroblast | 3 | 3 |
| WEHI-164 | Fibrosarcoma | 9 | 9 |
Ricin A-conjugated NIM-R7 antibody kills BCL1 lymphoma in vitro
BCL1 is a transplantable murine B-cell lymphoma resembling a chronic lymphocytic leukaemia. This lymphoma is an interesting model for using to analyse possible targets for diagnosis and therapy (immunotoxins).13 The search for magic bullets has long been a dream, and the differential expression of some antigens in malignancies has made this an option for directed therapy.12,17 Because BCL1 membranes were used to generate the mAb NIM-R7, we assessed the potential of the antibody as an immunotoxin. As seen in Table 3, the mAb NIM-R7, conjugated to ricin A, was able to kill the BCL1 lymphoma in vitro, but it was unable to kill resting T and FO B lymphocytes. Therefore, p58, besides being a marker for B-cell subpopulations, may be a possible target for selective therapy of certain lymphomas.
| Concentration of the immunotoxin | [3H]Thymidine incorporation (%) ± SD | |||
|---|---|---|---|---|
| Splenocytes activated with: | ||||
| BCL1 cells | LPS | Con A | ||
| IgG1 | NIM-R7 | NIM-R7 | NIM-R7 | |
| Medium | 100 | 100 | 100 | 100 |
| 1 × 10−10m | 100 | 91 ± 4 | 100 | 97 ± 3 |
| 5 × 10−10m | 97 ± 1 | 70 ± 1 | 96 ± 4 | 96 ± 5 |
| 1 × 10−9m | 95 ± 9 | 45 ± 8 | 91 ± 6 | 94 ± 7 |
| 5 × 10−9m | 93 ± 5 | 30 ± 6 | 92 ± 8 | 96 ± 6 |
| 1 × 10−8m | 94 ± 6 | 11 ± 3 | 94 ± 6 | 93 ± 9 |
| 5 × 10−8m | 93 ± 7 | 7 ± 5 | 94 ± 1 | 94 ± 4 |
- The immunotoxins were titrated using the assays described in the Materials and Methods. After 2–3 days, proliferation was assessed by uptake of [3H]thymidine. Results show the percentage of [3H]thymidine incorporation ± standard deviation (SD), from three independent experiments.
- Con A, concanavalin A; IgG1, immunoglobulin G1; LPS lipopolysaccharide.
Discussion
Our results highlight that p58 is not expressed either during the early stages of lymphocyte maturation or by fully mature resting ‘naïve’ populations. However, p58 is present on activated B and T cells, as it can be detected both in vivo and in vitro after polyclonal stimulation. The different patterns of expression of p58 seen in vitro, on activated T and B cells, is intriguing and, at this moment, we can only guess about the role that this molecule plays during the activation of these cells.
The selective expression of p58 by the B1 cell subpopulation, both in the peritoneal cavity and the spleen, is interesting as markers of B1 cells from the peritoneal cavity are not always shared by splenic B1 cells, and so p58 may have a correspondingly specialized role in the biology of B1 cells. The expression of p58 on ‘undisturbed’ B1 cells may reflect the often-quoted opinion that these cells are ‘already activated’,8 and raises the possibility that p58 may play a role during the activation process.
MZ B cells have a low threshold for activation. Therefore, the expression of p58 on these cells may once again be part of this ‘ready-to-react’ state,4 and is consistent with the similar phenotype of MZ B cells and B1 lymphocytes.16 Moreover, the shared expression of p58 on the ‘already activated’ B1, the ‘ready-to-react’ MZ and the ‘truly’ activated B2 cells, strongly suggest a role of p58 in the activation process. In sum, the data presented are consistent with the hypothesis that p58 is an activation marker, induced upon polyclonal stimulation in vitro and possibly by specific stimuli in vivo. The fact that p58 is undetectable in plasma cells may indicate that it is essentially required during activation, rather than differentiation; the lack of p58 expression on plasma cells makes this molecule a more restricted marker for B1 and MZ B cells than CD9, which is also expressed on plasma cells.18 Therefore, the identity of p58 and the characterization of agonistic or antagonistic antibodies against p58 will provide tools and directions to probe the role of this molecule in the activation process of B and T lymphocytes.
There exist lymphocyte surface proteins similar in molecular weight (MW) to p58, e.g. PD-1, a 50 000–55 000-MW protein expressed by activated T and B cells, but whose expression, in contrast with p58, is not induced in vitro. In contrast to p58, PD-1 is expressed on thymic cells.19 Another candidate is the 55 000-MW activation marker, CD25, which is induced on T and B cells upon stimulation but, in contrast to p58, CD25 is expressed during the early stages of T- and B-cell development.20,21
The ability of NIM-R7 antibody to selectively kill BCL1 cells in vitro supports the idea that p58 (and its possible human homologue) could be a therapeutic target and diagnostic tool for B1 malignancies. However, its capacity to kill BCL1 cells in vivo, and its use in the therapy of autoimmune diseases with B1 cells, remain to be investigated.
The exquisite stage-specific expression of p58 and its implications makes the further characterization of this molecule very interesting. Unfortunately, NIM-R7 seems to recognize a very labile conformational epitope, because our efforts to purify this molecule have, to date, been unsuccessful, and so it will be necessary to prepare more suitable mAbs to p58. These new set of antibodies may also allow us to analyse the biological role of p58, because NIM-R7, on its own, does not have agonistic or antagonistic functions in a variety of biological assays. The expression of p58 may be also studied on cells participating in phenomena such as autoimmune diseases or inflammatory processes, where lymphocyte activation is part of the pathological process. In summary, the findings of this work corroborated and extended the characterization of the mAb NIM-R7, showing that p58 is also expressed on activated T cells and may be an important marker for undisturbed B1 and MZ B lymphocytes.
Acknowledgments
This work was supported by a grant from Consejo Nacional de Ciencia y Tecnología (Conacyt), México (33497-N). The authors thank Q. F. B. Héctor Romero-Ramírez and Q. F. B. Víctor Rosales-García for their technical assistance.




