Apoptotic cell death in conjunction with CD80 costimulation confers uveal melanoma cells with the ability to induce immune responses
Summary
Uveal melanoma is a rare malignancy with a poor prognosis despite current therapeutic intervention. The current investigation focuses on the immunogenicity of uveal melanoma cells genetically modified with recombinant adenovirus encoding CD80 (AdCD80) in contrast to their parental counterpart. We demonstrate that costimulation provided by uveal melanoma cells improved immune responses in vitro as determined by mixed lymphocyte tumour cell cultures and cytotoxic T-cell assays using lymphocytes from healthy donors and uveal melanoma patients. Flow cytometry revealed T-cell stimulation by activated CD4+ and CD8+ T cells. Additionally, autologous lymphocytes proliferated in response to CD80-expressing primary uveal melanomas, indicating that this patient group is suitable for immunotherapy. Moreover, this study utilized AdCD80 modified and parental apoptotic tumour cells, loaded onto immature dendritic cells, as a source of tumour antigen. The ability of live or apoptotic tumour cells to stimulate lymphocyte proliferation and activation was determined. Apoptotic uveal melanoma cells expressing CD80 were efficient at inducing an immune response and served as a potent immunogen. The use of apoptotic uveal melanoma cells in combination with expression of costimulatory molecules could prove a novel adjuvant therapy for the treatment of this disease.
Abbreviations
-
- DC
-
- dendritic cell
-
- Ad
-
- adenoviral vector
-
- GFP
-
- green fluorescent protein
-
- WT
-
- wild type
-
- MOI
-
- multiplicity of infection
-
- MLTC
-
- mixed lymphocyte tumour cell culture
-
- MMC
-
- mitomycin-C
-
- NR
-
- non-replicating.
Introduction
Uveal melanoma is the most common intraocular malignancy in adults.1 In spite of this it is a rare, but aggressive malignancy with 50% of patients developing metastases primarily to the liver2,3 remaining the leading cause of death.4 The prognosis of patients with metastatic disease is poor, with a median survival time of only 4–6 months. Although many current treatments are available for the primary tumour, no effective therapy is available for metastases, hence the need for novel therapies is urgent. Uveal melanoma is an antigenic tumour, expressing higher than normal levels of tyrosinase, glycoprotein (GP)100 and melanoma antigen recognized by T lymplocytes (MART)-1.5 In addition, uveal melanoma patients have precursor lymphocytes, which can recognize and kill target cells presenting these peptides.6,7 Despite arising in an immune privileged site, tumour infiltrating lymphocytes (TILs),8 CD68 positive macrophages9 and CD1a positive immature dendritic cells (ImDC) (manuscript submitted for publication) have been found within these tumours, suggesting immunogenicity. Current immunotherapies to melanoma have been complicated by pre-existing self-tolerance, as well as the possibility of inducing autoimmune vitiligo;10 however, the disease prognosis of uveal melanoma far exceeds these complications.
An efficient lymphocyte response requires optimal lymphocyte activation, which occurs via two signals. Tumour-associated antigen delivered in the context of the major histocompatibility complex (MHC) to the T-cell receptor is the first of these two signals.11 Interaction between the T-cell receptor and the antigen–MHC complex is necessary but not sufficient for the activation of T cells. Full activation of T cells after antigen recognition is mediated through signal two, provided by costimulatory molecules such as CD80 and CD86,12,13 whereby strong T-cell activation through CD28 interactions is triggered. The absence of costimulatory molecules delivered to T cells by malignant cells is among the factors that contribute to low proliferation in vitro and tumour escape in vivo and has been extensively investigated.14 One study has demonstrated15 that optimal induction of effector T cells in a plasmacytoma model required direct antigen presentation by the tumour itself in concert with professional antigen-presenting cells (APC), highlighting the importance of costimulation by tumour cells. Several recent publications have reported that dendritic cells (DCs) acquire antigen from apoptotic cells, but not necrotic cells, and stimulate MHC class I-restricted CD8+ T cells to mediate cytotoxic T lymphocyte (CTL) responses.16–20 However, others21–23 report that antigens derived from apoptotic cells induce tolerance and that necrotic cells act as danger signals to promote cross priming.
As is the case for most solid tumours, the uveal melanoma cells investigated in this study lack costimulatory molecules and therefore cannot provide the second signal, resulting in anergy of the antigen-specific T cells.24 Introduction of genes for costimulatory molecules has elicited T-cell mediated antitumour responses in a wide variety of tumour models.14,25–28 Despite previous success using CD80 gene modification of tumour cells in animal models and clinical trials, this form of therapy has not advanced. The present study investigates a novel therapy that combines CD80 gene transfer to uveal melanoma cells with subsequent induction of apoptosis of these cells. Lymphocyte activation and proliferation in response to live or apoptotic tumour cells and cytokine release by these T cells after coculture was investigated. We report that although live CD80-gene-modified melanoma cells efficiently enhance immune responses, the use of apoptotic cells expressing CD80 markedly improves this effect, providing a potential adjuvant treatment for uveal melanoma.
Materials and methods
Cell lines and primary tumour cultures
The 174 CEM-T2 (T2) cell line (HLA-A2+, TAP-1 deficient) and K562 (human leucocyte antigen (HLA) class I negative) supplied by Cancer Research UK (London, UK) were cultured in RPMI-1640 (Life Technologies, Inc., Paisley, UK) + 10% fetal calf serum (FCS; Life Technologies). The uveal melanoma line SOM 157d (HLA-A2+) and primary tumour cultures, SOM 151, SOM 154 (HLA-A2−) and SOM 165 (HLA-A2−) were cultured in RPMI-1640 supplemented by 20% FCS, 10 ng/ml epidermal growth factor, 2 mm l-glutamine, 2 mg/ml glucose, 100 U/ml penicillin, 100 mg/ml streptomycin and 2·5 mg/ml fungizone. Cells were derived from intraocular melanomas obtained after ethical approval by the local ethics committee from consented patients attending the Department of Ophthalmology and Orthoptics, Royal Hallamshire Hospital, Sheffield, UK. The molecular HLA types of cell lines were determined by the Tissue Typing Department of the Blood Transfusion Service, Sheffield, UK.
Separation of peripheral blood mononuclear cells from whole blood and DC generation
Peripheral blood was obtained from healthy donors or from patients with uveal melanoma into heparin, and peripheral blood mononuclear cells (PBMC) isolated by standard gradient centrifugation in Lymphoprep (Nycomed, Pharmaceuticals Buckinghamshire, UK). The plasma layer was collected, heat inactivated at 56°, and stored at 4°. DCs were generated as previously described29 by resuspending in RPMI-1640 supplemented with 10% FCS and allowed to adhere to tissue culture flasks (Nalge Nunc Company, Rochester, NY) for 2 h at 37°. Non-adherent cells were removed and cryopreserved for future use as a source of T cells. The adherent cells were cultured for 6 days with 800 U/ml GM-CSF (Roche Diagnostics Ltd, Lewes, UK) and 500 U/ml IL-4 (Peprotech EC Ltd, London, UK).
Preparation of recombinant adenovirus
The recombinant adenoviral vectors (Ad) used in this study were derived from human adenovirus serotype 5, with deletions in the E1 region rendering them replication-defective. The recombinant AdCD80 was a kind gift from M. Gilligan (Institute for Cancer Studies, University of Birmingham, UK) and was constructed as described.30 AdGFP, which carries the green fluorescent protein gene (GFP, control vector), was a generous gift from V. Mautner (Institute for Cancer Studies, University of Birmingham, UK). Plaque-purified viruses were amplified in the 293 cell line (Microbix) and titrated according to previously established protocols31 to give high-titre virus stocks.
Genetic modification of uveal melanoma cells
Wild-type (WT) uveal melanoma cells (1 × 106) obtained from uveal melanoma cultures were seeded in T25 flasks and allowed to adhere overnight. Melanoma cells were infected with Ad at the indicated multiplicity of infection (MOI) for 90 min at 37° to achieve optimum gene transduction. Virus medium was replaced with growth medium and cells cultured for a further 48 hr in the presence of 500 U/ml interferon-γ (IFN-γ; Biogen, Cambridge, MA) prior to immunoassay. Phenotypic analysis of WT and gene-modified melanoma cells was determined by immunofluorescence staining using anti-CD80, anti-CD86, anti HLA-A, -B, -C and anti-HLA-DP, -DQ, -DR monoclonal antibodies (mAbs; Serotec, Oxford, UK). Briefly, cells were washed with fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline, 1% FCS), and incubated with fluoroscein isothiocyanate (FITC)-conjugated mAb for 30 min at 4°. After a subsequent wash in FACS buffer, flow cytometric acquisition was performed on a FACSort (Becton Dickinson, San Jose, CA) and data analysed using CellQuest™ software.
Determination of lymphocyte activation
Three-colour immunofluorescence staining using anti-CD4–FITC, anti-CD8–PerCP and phycoerythrin (PE)-conjugated anti-CD25 mAb (Becton Dickinson) determined the proportion of activated lymphocytes after coculture with WT and gene-modified melanoma cells, immunoglobulin G (IgG) isotype mAb conjugated to FITC, PE or PerCP (Becton Dickinson) were used as controls. Supernatants from cocultures were assayed for IFN-γ and interleukin-4 (IL-4) release using enzyme-linked immunosorbent assay (ELISA; R & D Systems, Oxon, UK) following the manufacturer's instructions.
Mixed lymphocyte tumour cell cultures
The T-cell stimulatory ability of melanoma cells was assessed in a mixed lymphocyte tumour cell culture (MLTC) where three donors/patients were assessed for each uveal melanoma culture. Melanoma cells (stimulators) were gene-modified and incubated with 500 U/ml IFN-γ during 48 hr as described above. Stimulators were mitomycin C (MMC; Sigma Aldrich, Poole, UK) treated (8 µg/ml) for 1·5 hr and resuspended in RPMI-1640 supplemented by 10% human AB serum (Sigma Aldrich), after extensive washing to remove any unbound MMC. The dose and incubation time of MMC was optimized and shown not to affect viability or surface molecule expression as assessed by flow cytometry (data not shown). Between 2 × 104 and 2·5 × 103 stimulators were cocultured with 2 × 105 responders per well for 5 days in a 96-well plate (Costar, Cambridge, MA). Where DCs were added, stimulators and DCs (2 × 104) were cultured for 1 hr at 37° prior to adding responders. [3H]thymidine (Amersham, Amersham, UK) was added at 0·37 MBq per well during the last 18 hr of culture, where after cells were collected and radioactive incorporation was measured as counts per minute (c.p.m.). For blocking studies, cytotoxic T-lymphocyte associated molecule (CTLA)-4 immunoglobulin fusion protein (Ansell, Alexis Corporation, Bingham, UK) or an IgG mAb (R & D Systems) control were added at 0·5 µg/ml to the stimulators 30 min before addition of responders and the remainder of the assay was carried out as described.
Induction of CTLs
WT or AdCD80 gene-modified SOM 157d cells were MMC treated and cocultured with 2 × 106 CD3+ effector cells, enriched using a T cell negative isolation kit (Dynal, Bromborough, UK). Cells were cultured in AIM-V (Life Technologies, Inc.) containing 10% autologous plasma and 20 U/ml rhIL-2 (Glaxo, Geneva, Switzerland) for a period of 7 days. A standard chromium release assay tested cytolytic activity of these cells. Tumour targets included WT and AdCD80-infected melanoma cells with T2 cells used as a control. Briefly, 106 target cells were labelled with 5·55 MBq of Na51CrO4 (Amersham) for 1 hr at 37°. Effectors were added at various concentrations to 2 × 103 target cells in the presence of 40× excess cold K562 to block non-specific lysis, in 96-well microtitre plates (Iwaki, Chiba, Japan). After a 4-hr incubation, 50 µl of supernatant was transferred to Lumaplates (Canberra Packard, Pangbowne, UK) and specific lysis was calculated as follows: % lysis = {[(c.p.m. test) − (c.p.m. spontaneous)]/[(c.p.m. maximum) − (c.p.m. spontaneous)]} × 100.
Induction and detection of apoptotic cell death
Melanoma cells were gene-modified as described and induction of apoptosis was achieved by exposure to 30 Gy using a caesium source (Gammacell 2000, Mølsgaard Medical, Ganløse, Denmark). Cells were thereafter left in culture until maximum apoptosis was achieved. Apoptosis was determined using annexin-V–FITC (annexin-V)–propidium iodide (PI) staining (ApoTarget; Biosource International, Camarillo, CA) following the manufacturer's instructions. Apoptotic cells were cocultured with DCs at a 1 : 1 ratio and thereafter used in MLTC to measure alloresponses of lymphocytes from healthy donors.
Statistical analysis
Using Microsoft Excel® a two-tailed Students t-test was employed to determine statistical significance of values where P < 0·05 was deemed significant and P < 0·005 highly significant.
Results
Adenoviral infection efficiency of uveal melanoma cells
Immunofluorescence staining of SOM 157d cells and primary uveal melanoma tissue revealed that expression of the costimulatory molecules CD80 and CD86 was negligible, MHC class I was expressed at high levels and MHC class II expression was not detected (Table 1 and Fig. 1a). Incubation for 48 hr in the presence of the immunostimulatory cytokine IFN-γ-induced MHC class II expression; however, expression levels of CD80 and CD86 did not alter. To achieve successful transduction of genes into tumour cells, a recombinant adenovirus was used. Adenoviruses have high transduction efficiency and can infect non-dividing cells, which is an important factor since primary tissue divides at a slow rate.32 Adenoviral gene transfer of the cDNA for CD80 and the GFP control into uveal melanoma cells was successful: after 48 hr culture SOM 157d cells and primary cultures readily expressed CD80 and GFP with greater than 90% positive cells, using an MOI of 30 and 25 plaque-forming units, respectively (Table 1 and Fig. 1b). Gene expression of all constructs was stable for 10 days, after which the number of cells expressing the gene decreased (data not shown).
| Untreated (% positive cells/MFI) | IFN-γ treated (% positive cells/MFI) | |
|---|---|---|
| Parental SOM 157d | ||
| CD80 | 3/24 | 0/28 |
| CD86 | 3/184 | 6/187 |
| HLA-A,-B,-C (MHC I) | 100/231 | 100/712 |
| HLA-DP,-DQ,-DR (MHC II) | 2/15 | 95/61 |
| AdCD80 transfected SOM 157d | ||
| CD80 (MOI 30) | 96/357 | 95/392 |
| AdGFP transfected SOM 157d | ||
| GFP (MOI 25) | 97/1547 | 97/1600 |
- SOM 157d cells were stained with FITC-conjugated anti-HLA-A, -B, -C (MHC I), anti-HLA-DP, -DQ, -DR (MHC II), anti-CD80 and anti-CD86 mAb and expression was determined by flow cytometry. A total of 105 cells were analysed for each sample. The table is a representative of three independent experiments and show percentage positive cells and mean fluorescence intensity (MFI).
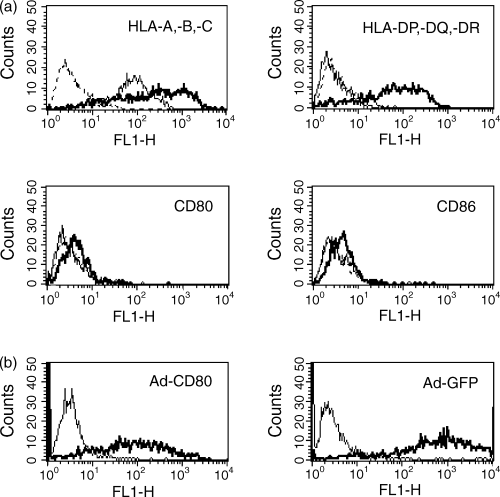
Adenoviral infection of SOM 157d cells was highly efficient. Overlay plots of untreated (solid line) and IFN-γ-treated (bold solid line) SOM 157d cells show staining of isotype control (broken line), HLA-A, -B, -C (MHC I), HLA-DP, -DQ, -DR (MHC II), CD80 and CD86 expression as determined by flow cytometry (a). IFN-γ-treated SOM 157d cells before (solid line) and after (bold line) infection with Ad-CD80 or Ad-GFP (b). The figure shows representative histogram plots from the data presented in Table 1.
CD80-transduced melanoma cells induced lymphocyte proliferation
WT and AdGFP-transduced SOM 157d melanoma cells were poor stimulators of lymphocyte proliferation (Fig. 2a) with no significant variation in responses (P > 0·3), indicating weak alloresponses. In addition, WT SOM157d cells demonstrated low stimulatory ability of uveal melanoma patient lymphocyte proliferation. However, SOM 157d cells expressing CD80 significantly stimulated (P < 0·005) T-cell proliferation of PBMC isolated from healthy donors (Fig. 2a) at all ratios, and an enhancement in the stimulation of uveal melanoma patient lymphocytes (Fig. 2b) was observed (P < 0·05), indicating that CD80 cells transfer successfully enhanced immune responses of both healthy and uveal melanoma patients' lymphocytes. To demonstrate an exclusive role for CD80, the MLTC was blocked by addition of CTLA-4 immunoglobulin, a high-affinity ligand for CD80. The stimulatory effect of CD80-expressing SOM 157d (Fig. 2c) was efficiently blocked in the presence of CTLA-4 immunoglobulin, demonstrating specificity for the CD80 costimulatory pathway (P < 0·05). Addition of an isotype control antibody to CD80-expressing uveal melanoma cell cocultures did not reduce the enhanced proliferation (data not shown).
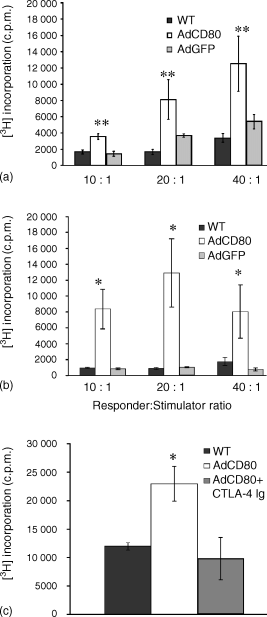
CD80 transduced uveal melanoma promote lymphocyte proliferation. CD80-transduced SOM 157d displayed enhanced allostimulatory ability of lymphocytes from healthy donors (a) and from uveal melanoma patients (b). Addition of CTLA-4 immunoglobulin fusion protein to SOM 157d cells (c) inhibited proliferation. Responders only were routinely less than 10% of WT counts (data not shown) and bars represent the mean (± SD) of three determinations with results typical of those found in three independent experiments (*P < 0·05; **P < 0·005).
Transduction of CD80 induces CTLs against uveal melanoma cells
To demonstrate that CD80 transduction of uveal melanoma cells enhanced production of CTLs, a chromium release assay was employed. WT and CD80-expressing SOM 157d cells cocultured with allogeneic matched HLA-A2+ CD3+ lymphocytes isolated from healthy donors for a period of 7 days demonstrated low levels of cytolytic activity against target cells expressing CD80 (Fig. 3a). AdCD80 gene-modified cells enhanced cytotoxicity of allogeneic CD3+ cells, as demonstrated by enhanced killing of WT and AdCD80 infected SOM 157d target cells (Fig. 3b). Cytolytic killing against the non-melanoma cell line T2 was minimal. These results show that in addition to lymphocyte activation of T cells cocultured with CD80-expressing melanoma cells, these cells are cytotoxic as they are able to lyse matched HLA-A2+ tumour targets. In contrast to other in vitro studies33 this data suggests that costimulation is important in the induction and the effector phase of the immune response. This is in agreement with a study by Allison et al. 34 They demonstrate that expression of CD80 on melanoma cells would enhance effector function by driving proliferation of activated cells and by enabling lower avidity cells to be triggered for effector function.
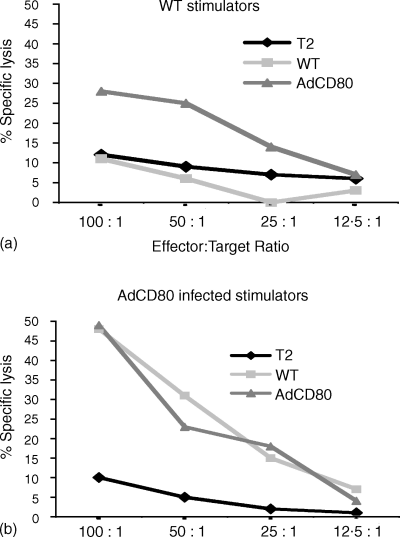
CD80 enhance CTL killing of SOM 157d by HLA-matched allogeneic CD3+ lymphocytes. CTL were generated from CD3+ cells isolated from healthy donors by in vitro stimulation using AdCD80-gene-modified (a) or WT SOM 157d (b) for 7 days before a 51Cr release assay was performed. Target cells were WT SOM157; AdCD80 infected SOM 157d or T2 cells. Data are representative of two identical experiments where data points represent the mean of triplicates.
Autologous lymphocytes proliferate after coculture with CD80-expressing cells
To mimic the in vivo situation of uveal melanoma this study also employed primary tumour material. Primary tumour cultures, SOM 151, SOM 154 and SOM 165, were gene-modified with AdCD80 and AdGFP at an early passage1–4 and their ability to induce proliferation of allogeneic and autologous lymphocytes in a MLTC was assessed. WT and AdGFP gene-modified primary melanoma cells cocultured with PBMC isolated from healthy donors yielded little response in all cultures investigated. This lack of response was overcome by AdCD80-transduction of short-term cultures, indicating that freshly resected tumour tissue can be employed for this form of therapy. SOM 151 cells were able to induce a weak proliferation of autologous lymphocytes, an effect that was enhanced significantly (P < 0·05) when cultured with gene-modified cells (Fig. 4a). In an autologous coculture using SOM 154 no response was observed after AdCD80 infection (Fig. 4b), however, CD80-expressing SOM 154 cells were able to induce lymphocyte proliferation of PBMC isolated from healthy donors (P < 0·005) at all ratios. A third short-term culture investigated, SOM 165, was able to induce autologous PBMC proliferation, which was significantly enhanced (P < 0·005) after coculture with AdCD80-expressing melanoma cells at the 10 : 1 ratio (Fig. 4c).
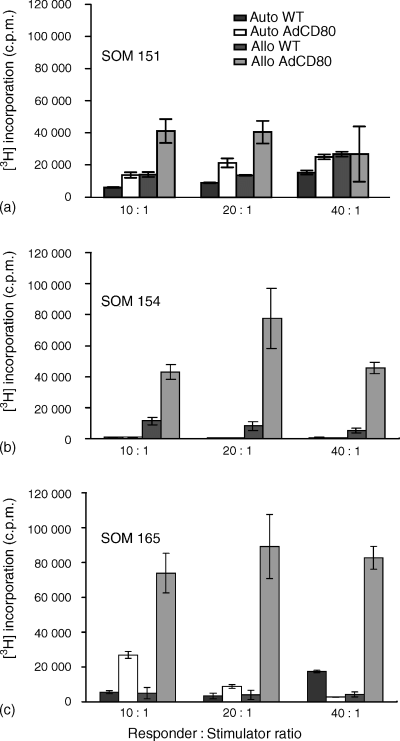
CD80 costimulation results in enhanced autologous lymphocyte response. Primary uveal melanoma cultures were gene-modified at an early passage (<5) and cocultured for 5 days with allogeneic lymphocytes from healthy donors or autologous lymphocytes and thymidine incorporation was determined. Proliferation of lymphocytes after coculture with WT and CD80-transduced SOM 151 (a), SOM 154 (b) and SOM 165 (c) are shown (auto = autologous; allo = allogeneic). Bars represent c.p.m. of triplicates (*P < 0·05; **P < 0·005).
Apoptotic CD80-expressing uveal melanoma cells induce efficient lymphocyte proliferation
A report from this laboratory19 demonstrated that DCs pulsed with apoptotic and necrotic SOM157d cells induced lymphocyte proliferation, however, non-replicating (NR) cells were unable to stimulate a comparable response using the SOM 157d cell line in a HLA-A2± matched setting. In the present study we set out to determine if inducing apoptosis of CD80-expressing SOM 157d cells has an effect on the lymphocyte response. MLTC was assessed using cocultures of lymphocytes combined with either MMC-treated (NR) or irradiated (apoptotic) WT or AdCD80-gene-modified melanoma cells in the presence or absence of immature DCs. Apoptosis was induced by irradiation at 30 Gy, with maximum apoptosis detected at day 9 (routinely > 40% annexin-V positive, PI-negative cells) following irradiation (data not shown). In agreement with the previous study, NR SOM 157d cells were poor stimulators, however, when induced to undergo apoptosis the proliferative response was significantly enhanced (P < 0·005) in the presence of DCs (Fig. 5a). Proliferation of lymphocytes in autologous DC and lymphocyte cocultures was consistently between than 20 000–40 000 c.p.m. (data not shown). As anticipated, the presence of CD80 on SOM 157d cells enhanced their stimulatory ability, similar to the enhancement seen after coculture with apoptotic cells (P < 0·005). However, when CD80-expressing melanoma cells were induced to undergo apoptosis and cocultured with lymphocytes and DCs, the proliferative response of these lymphocytes was significantly enhanced (P < 0·005). This enhanced response may result from the combination of direct presentation by the CD80-expressing melanoma cell and indirect presentation by DCs.
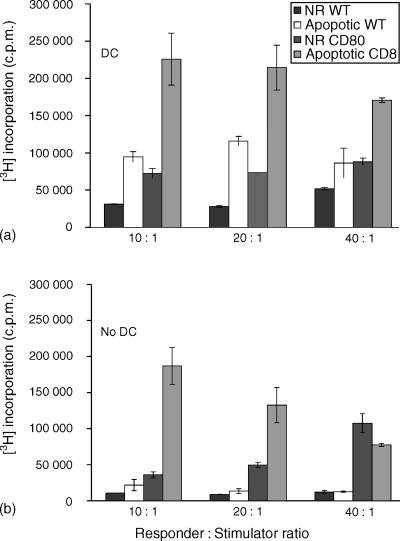
Apoptotic cell death and costimulation induce proliferation of allogeneic lymphocytes. NR and apoptotic SOM 157d cells expressing CD80 or not were cocultured with PBMC from healthy donors in the presence (a) or absence (b) of DCs for 5 days and subsequent thymidine incorporation was determined (c.p.m.). Results show a representative of three independent experiments where bars represent the mean (± SD) of quadruplicates (NR = non-replicating). Percentage apoptotic cells after MMC treatment < 5% and after irradiation treatment > 40%.
To assess the need for professional APCs to engulf dying tumour cells for efficient tumour antigen presentation, DCs were left out of the coculture. In this setting, apoptotic WT melanoma cells did not increase lymphocyte proliferation when compared with NR melanoma cells (Fig. 5b). However, as expected, NR CD80-expressing melanoma cells increased proliferation, which was improved further after coculture with apoptotic melanoma cells (P < 0·05) at high responder to stimulator ratio. Taken together these results suggest that the need for CD80 to be expressed by the melanoma cells and the presence of apoptotic bodies that can be engulfed by immature DCs are important for an efficient response to be mounted.
Immature DCs are required in conjunction with costimulation and cell death for an efficient pro-inflammatory immune response
Immunofluorescence staining of lymphocytes after coculture revealed that NR and apoptotic CD80-expressing SOM 157d cells significantly increased the proportion of CD4+ CD25+ T cells (P < 0·05) in the presence of DCs; however, only apoptotic CD80-expressing cells were able to significantly (P < 0·05) increase CD8+ CD25+ T cells (Fig. 6a). NR and apoptotic CD80-expressing SOM 157d cells significantly enhanced (P < 0·05) IFN-γ production by lymphocytes in the presence of DCs (Fig. 6b). In the absence of DCs no significant increase in the proportion of activated cells was observed after coculture with apoptotic or CD80-expressing SOM 157d cells (data not shown) and only NR CD80-expressing SOM 157d cells were able to significantly (P < 0·05) enhance IFN-γ production by these lymphocytes (Fig. 6b). IL-4 was undetected in all cocultures tested (unpublished data).
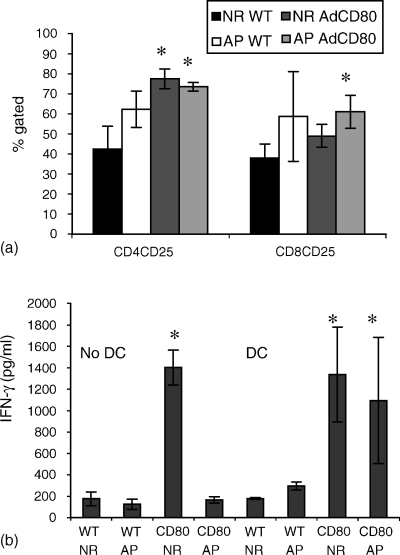
Apoptotic tumour cells expressing CD80 promotes T cell activation and IFN-γ production. Melanoma cells were cocultured in the presence of DCs (a), or with PBMC alone (not shown) for a period of 5 days and lymphocyte activation was detected by flow cytometry [apoptotic (AP) and non-replicating (NR)]. ELISA-determined expression levels of IFN-γ (b). Lymphocyte activation results are represented as a mean of two donors ± SD. IFN-γ ELISA results represent the mean ± SD from two donors (each of which was assayed in duplicate).
Discussion
A tumour can escape immune surveillance through different mechanisms and one of these is thought to be the failure to supply costimulation through the CD28 pathway. The two signals needed for efficient activation of T cells have been described previously35 and are now well recognized.36 A major consequence of the lack of costimulation is the failure to enhance T-cell survival in response to antigen receptor activation.37 The uveal melanoma cell line SOM 157d, as well as the primary short-term cultures investigated herein, lacked the CD80 and CD86 expression needed for T-cell activation. This study investigated the immunogenicity of gene-modified uveal melanoma cell lines and primary tumour tissue and its use as a novel adjuvant therapy.
In agreement with other studies14,30,38 when costimulation was provided by the tumour cell immune responses of cocultured PBMC isolated from healthy donors were repeatedly enhanced. A negative control adenovirus expressing GFP was used in all assays and although immune responses were slightly enhanced using some donors this was not statistically significant, indicating that the virus did not alter measured immune responses. In addition to this, cocultures in the presence of a CTLA-4 fusion protein demonstrated specificity to the CD28 costimulatory pathway. Allogeneic lymphocytes isolated from healthy donors, and from the blood of uveal melanoma patients proliferated more rapidly after coculture with CD80-expressing SOM 157d cells than with their parental counterpart. In addition, the ability of CD80 gene-modified SOM 157d cells to induce CTLs using matched HLA-A2+ lymphocytes suggests that costimulation by these tumour cells was sufficient to induce a killing response. When cultured in an autologous system, proliferative responses were weak. Although CD80 transfer enhanced responses at some responder to stimulator ratios, this was not as marked as in the allogeneic setting. There is a possibility that the autologous lymphocytes are tolerized to the tumour, as lymphocytes isolated from patients readily proliferated after culture with CD80 transduced SOM 157d cells. This would suggest a potential advantage of allogeneic vaccines.
Although uveal melanoma and skin melanoma originate from melanocytes, cytologically the tumours differ in mitotic and apoptotic rate, with less proliferation and cell death in uveal melanoma.39 Cell death induces an inflammatory response as dying cells contain potentially inflammatory factors and are therefore cleared by scavanger cells.40 The ability of DCs to phagocytose apoptotic tumour cells as a delivery vector of tumour antigens has been described previously.19,20 Additionally, it has been demonstrated that murine bone-marrow-derived DCs can be activated in vitro by exogenous signals from apoptotic leukaemia cells and stimulate in vivo a cell-mediated cytotoxic immune response protecting mice against leukaemia development.41 Moreover, large numbers of bystander apoptotic cells, which can be expected to yield secondary necrosis, have been found to promote DC maturation.42 The results described in the current study report for the first time that CD80 present on apoptotic tumour cells serves as an efficient immunogen for the initiation of a potent immune response. Apoptotic cells improved lymphocyte proliferation both in the absence and presence of immature DCs. However, the highest proliferative response was achieved with apoptotic melanoma cells expressing CD80 in the presence of DCs. IFN-γ production was increased considerably to twice that of NR cells after culture with CD80-expressing live cells in the presence of DCs. Moreover, the proportion of CD4 CD25 and CD8 CD25 positive cells was significantly higher after coculture with apoptotic cells expressing CD80. Taken together these results suggest that gene-modified uveal melanoma cells were acting as professional APCs and could interact with the immune cells directly. We have shown that addition of DC further enhances the response to CD80-infected SOM 157d cells as evidenced by an increased lymphocyte IFN-γ production. Taking into consideration an in vivo scenario, the injected melanoma cells would not only encounter T cells, but also DCs. These can efficiently cross-present tumour antigens from melanoma cells, thus maximizing the immune response. The need for professional APC to work together with tumour cells providing costimulation to induce effector T cells has been discussed previously15 and agrees with the findings in this study. Furthermore, cross-presentation of antigens to CTLs appears to have two critical features in that it is mediated by DCs and that apoptotic cells are the preferred source of antigen.17
In conclusion, these results suggest that successful immunotherapy of uveal melanoma could be mediated by CD80 gene-modification. Immunotherapy using irradiated tumour cells as a source of tumour antigen has been described19,20 and the results from the present study suggest that the use of irradiated CD80-expressing melanoma cells undergoing apoptosis would be a more suitable delivery system for efficient tumour antigen presentation, with the additional advantage that melanoma cells express the whole tumour antigen array. This may serve as a potential vaccine by enhancing the host's antitumour immunity after conventional therapy.
Acknowledgments
We would like to thank Dr Andrew Jackson (Cancer Research UK) for providing support and discussion and J. Bartholomew (Paterson Institute, Manchester, UK) for the generous gift of the 174 CEM-T2 cell line. This work was supported by Yorkshire Cancer Research, Harrogate, the Humane Research Trust and Sheffield Hospital Trustees.




