Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a meta-analytic test of current theories and perceptions
Summary
C4 plants contribute ≈ 20% of global gross primary productivity, and uncertainties regarding their responses to rising atmospheric CO2 concentrations may limit predictions of future global change impacts on C4-dominated ecosystems. These uncertainties have not yet been considered rigorously due to expectations of C4 low responsiveness based on photosynthetic theory and early experiments. We carried out a literature review (1980–97) and meta-analysis in order to identify emerging patterns of C4 grass responses to elevated CO2, as compared with those of C3 grasses. The focus was on nondomesticated Poaceae alone, to the exclusion of C4 dicotyledonous and C4 crop species. This provides a clear test, controlled for genotypic variability at family level, of differences between the CO2-responsiveness of these functional types. Eleven responses were considered, ranging from physiological behaviour at the leaf level to carbon allocation patterns at the whole plant level. Results were also assessed in the context of environmental stress conditions (light, temperature, water and nutrient stress), and experimental growing conditions (pot size, experimental duration and fumigation method).
Both C4 and C3 species increased total biomass significantly in elevated CO2, by 33% and 44%, respectively. Differing tendencies between types in shoot structural response were revealed: C3 species showed a greater increase in tillering, whereas C4 species showed a greater increase in leaf area in elevated CO2. At the leaf level, significant stomatal closure and increased leaf water use efficiency were confirmed in both types, and higher carbon assimilation rates were found in both C3 and C4 species (33% and 25%, respectively). Environmental stress did not alter the C4 CO2-response, except for the loss of a significant positive CO2-response for above-ground biomass and leaf area under water stress. In C3 species, stimulation of carbon assimilation rate was reduced by stress (overall), and nutrient stress tended to reduce the mean biomass response to elevated CO2. Leaf carbohydrate status increased and leaf nitrogen concentration decreased significantly in elevated CO2 only in C3 species.
We conclude that the relative responses of the C4 and C3 photosynthetic types to elevated CO2 concur only to some extent with expectations based on photosynthetic theory. The significant positive responses of C4 grass species at both the leaf and the whole plant level demand a re-evaluation of the assumption of low responsiveness in C4 plants at both levels, and not only with regard to water relations. The combined shoot structural and water use efficiency responses of these functional types will have consequential implications for the water balance of important catchments and range-lands throughout the world, especially in semiarid subtropical and temperate regions. It may be premature to predict that C4 grass species will lose their competitive advantage over C3 grass species in elevated CO2.
Introduction
The effects of atmospheric CO2 enrichment have been studied in great detail for agricultural crops ( Cure & Acock 1986), trees ( Ceulemans & Mousseau 1994), and other plant types ( Bazzaz 1990; Poorter 1993; Idso & Idso 1994). The great majority of these studies have been carried out on C3 species. Much of the early research into CO2-responses of C4 species focused on weedy and old-field dicotyledonous (dicot) species (e.g. Amaranthus sp., Bazzaz & Carlson 1984), or important planted C4 crop species (e.g. Zea mays and Sorghum sp., Morison & Gifford 1984). Despite the fact that about half of the world’s grass species possess the C4 photosynthetic pathway, fewer studies have tested the responses of wild temperate C4 grasses to elevated CO2, and only a handful have used tropical C4 grasses. These plants account for ≈ 18% of the total global productivity, mainly due to the extensive grasslands and savannas of the tropics ( Ehleringer et al. 1997 ), but they also play an important role in mixed temperate grasslands such as the North American prairies. Lloyd & Farquhar (1994), using a modelling approach based on 13C discrimination, estimated a contribution of 21% by C4 plants to global gross primary productivity (GPP) under current atmospheric conditions. Any changes in C4 productivity driven by CO2 and other climatic perturbations will, consequently, have a substantial impact on global GPP.
By far the greatest proportion of C4 species are monocotyledonous (monocot), whereas C4 dicots are relatively uncommon, both in terms of species representation and abundance ( Ehleringer et al. 1997 ). Many C4 dicots are noxious weeds and old-field invaders, and gain importance only in disturbed sites. Thus, studies using C4 dicots and bred crops may not represent the potential CO2-responsiveness of natural, relatively undisturbed ecosystems with a significant C4 monocot component, such as prairies, tropical grasslands, and savannas. Nevertheless, results obtained from many of these studies, and from the first field-based study of a C4-containing ecosystem, a salt marsh on Chesapeake Bay ( Curtis et al. 1989 ), appeared to confirm the theory that C4 plants should not show significant growth responses to elevated CO2, due to their CO2-concentrating mechanism in the bundle sheath cells ( Osmond et al. 1982 ; Pearcy & Ehleringer 1984; Bowes 1993). This mechanism increases the effective concentration of CO2 at the site of carboxylation, thereby masking photorespiration and apparently ensuring saturation of photosynthesis at current atmospheric CO2 concentrations. It follows, in theory, that C4 plants should not benefit from increased atmospheric CO2 availability, and may suffer reduced competitive advantage over C3 species ( Bazzaz 1990; Bowes 1993; Ehleringer & Monson 1993). As a result of this common perception, the potential contribution of C4-dominated ecosystems to the global carbon budget in a future high-CO2 environment, especially in the highly productive tropics, has been largely discounted or ignored.
It is now becoming increasingly clear that the response of C4 species to elevated CO2 is not as clearcut as previously thought ( Henderson et al. 1994 ), and that many C4 plants show significant photosynthetic and growth responses to CO2. In a recent review, Poorter (1993) found an average growth enhancement of 22% for C4 species. Owensby et al. (1993) have also reported significant above-ground biomass increases in the C4 component of a tall-grass prairie site exposed to elevated CO2. This was explained by the reduced water loss under high CO2 of C4 species relative to competing C3 species, especially during a dry year. However, there also appears to be a primary direct enhancement of photosynthetic activity in elevated CO2 in a number of C4 species ( Sionit & Patterson 1984; Knapp et al. 1993 ), suggesting that the assumption of photosynthesis saturation at current CO2 concentration may need to be re-evaluated.
Interacting environmental stresses can influence the response to elevated CO2 in plants ( Idso & Idso 1994; Curtis 1996; Lloyd & Farquhar 1996; Curtis & Wang 1998), and may do so differentially for different functional types. The literature of C4 responses to elevated CO2 shows that environmental factors, especially those known to be of importance to C4 productivity and biogeographic distributions (high minimum temperatures and high light levels), may influence the relative CO2-response. Responses of C4 species under stressful conditions may not emerge clearly from experiments employing growth conditions optimal for C3 plants. These factors could account for some of the poor responses to high CO2 previously reported for environmentally controlled experiments, as opposed to significant responses measured more recently under natural field conditions ( Owensby et al. 1993 ).
The purpose of this review is to assess critically from the literature, using meta-analytic methods (e.g. Curtis 1996; Curtis & Wang 1998), the physiological and growth responses of wild C4 grass species (family: Poaceae) to elevated atmospheric CO2. To enable a critical test of current theories and perceptions, a similar literature review was carried out for the CO2-responses of wild C3 grass species (Poaceae). This provides a clear comparison, controlled for genotypic and morphological variability. The influences of exposure and growth conditions were also analysed, in order to determine whether current understanding of the relative responses of C3 and C4 species, and resulting uncritical extrapolation to natural environments, may be biased by experimental conditions very different from natural conditions.
Materials and methods
Database compilation
The data analysed in this study were taken from published sources by investigators at the National Botanical Institute, South Africa, and the CO2 Meta-Analysis Project, Ohio State University, USA. In cases where the two individual databases overlapped, data were used from the CO2 Meta-Analysis Project only. Non-overlapping data were checked for consistency. The studies addressing C4 pathway grass species that were included in our analyses were as comprehensive as possible for all years (1980–97), while the studies addressing C3 pathway grass species were as comprehensive as possible for 1991–97, with most studies from 1980 to 1990 also included. The following criteria were used for incorporation of studies in the database:
(a) The species was wild or semiwild, a member of the family Poaceae, and the photosynthetic pathway (C3 or C4) was either clearly stated or otherwise unambiguous.
(b) Only data which included response means, sample sizes (N), and either standard deviation (SD) or standard error (SE) were used, since a weighted meta-analysis gives a more robust analysis than if resampling tests must be used to estimate variances or if an unweighted analysis is used ( Rosenberg et al. 1997 ).
(c) The paper was published between 1980 and 1997.
(d) The ambient CO2 treatment concentration was between 300 and 400 μmol mol–1, and the elevated CO2 treatment was between 550 and 750 μmol mol–1.
(e) Data were presented for individual plants, or for individual species where plants were grown in stands or in mixture with other species.
(f) Only absolute data were used, not relative data such as relative growth rates.
(g) At least one of the following parameters was measured:
· A: Leaf-based light-saturated net CO2 assimilation rates measured at the growth CO2 concentration
· GS: Leaf-based stomatal conductance measured at the growth CO2 concentration
· WUE: Instantaneous leaf water use efficiency at the growth CO2 concentration, either published as such or calculated from net CO2 assimilation rates and transpiration rates
· TOTWT: Total plant biomass, either presented as such or calculated as the sum of above-and below-ground biomass.
· AGWT: Above-ground biomass
· BGWT: Below-ground biomass
· INDLA: Individual leaf area
· TILLERS: Number of tillers
· SLA: Specific leaf area either presented as SLA, or calculated as the inverse of specific leaf mass
· TNC: Concentration of total nonstructural carbohydrates in leaves, either presented as such or calculated as the sum of total sugar and starch concentrations, and expressed on a dry mass basis
· N: Leaf total nitrogen concentration expressed on a dry mass basis
The responses at elevated and ambient CO2 were extracted either from tables, or manually digitized from figures. Where the interaction between CO2 treatments and deliberately imposed light, temperature, water, or nutrient stress treatments was reported, the CO2-response was entered separately under both levels of the stressful environmental factor. For those analyses testing a response to stress, all possible data in which plants were not stressed were included as controls for the meta-analysis (‘no stress’), rather than only the data for nonstressed plants in studies reporting the response under intentional factorial stress treatments (controls within those studies). This necessitated careful decisions about how to code some treatment responses. For example, we determined that ‘high nutrient levels’ or normal nutrient levels (comparable to the field situation) were equivalent to ‘no nutrient stress’, and that ‘high light levels’ or light levels which were deemed normal or sufficiently high, were similarly equivalent to ‘no light stress’. In these instances, we recorded ‘none’ for the level of stress. Furthermore, we utilized only the extreme levels of any given stress. That is, we included only ‘low nutrients’ (nutrient stress) and ‘high nutrients’ (no nutrient stress, or normal) in our analyses, and did not include intermediate levels (e.g. ‘medium nutrient levels’). Studies which provided data on interactions with environmental stresses are identified in Appendix 3 (C3) and Appendix 4 (C4).
Where additional environmental stresses (such as salinity or ozone treatments) were imposed factorially, only the CO2-response at the ambient, nonstressful level of this other factor was used. Unintentional stresses were not taken into account, except in the case of separately reported data for wet and dry years in some prairie studies. Where competition treatments were intentionally and differentially imposed, only the CO2 response at the lowest level of competition was used.
Response parameters were combined whenever appropriate in order to overcome the problem of low sample sizes. For example, rather than differentiate between what some authors termed root biomass and colleagues termed below-ground biomass, we pooled these data and report them as below-ground biomass. Thus, while we lost some potential detail in the analysis, we improved our ability to generalize and distinguish among effects ( Gurevitch & Hedges 1993).
In order to test for potential influences of exposure methodology on the responses to elevated CO2, the following categorical variables were assigned to each data entry:
(i) Pot size: ≤ 10 L, > 10 L, or in-ground. These size classes have been previously used in a similar meta-analytic review (e.g. Curtis 1996).
(ii) Duration of exposure (from treatment initiation until measurement): ≤ 60 days, 61–120 days, > 120 days. Where repeated measurements were taken, only the last measurement was used (usually at harvest). However, in some field studies showing marked seasonal responses, declining towards the end of the growth season, a single date at or just after the mid-season peak was chosen.
(iii) Exposure method: GC=indoor controlled-environment growth chamber, GH=outdoor enclosed mini-greenhouse or enclosed portion of greenhouse, OTC= open-top chamber in the field or greenhouse, FACE=free-air CO2 enrichment.
The database used for the meta-analysis comprised 62 papers ( Appendix 1). Other papers on C4 grass responses to elevated CO2 which did not meet the criteria for meta-analysis are given in Appendix 2 to provide a complete reference list.
Meta-analyses
Meta-analyses were conducted with MetaWin ( Rosenberg et al. 1997 ), using the natural log of the response ratio (response in elevated CO2/response in ambient CO2) as our metric ( Hedges et al. 1999 ). We used the mixed-effects model in our analyses, because of the large number of diverse studies examined and the assumption that there is random variation among studies in the effects in which we are interested. Consequently, the confidence intervals generated are larger than those of a fixed-effects model, and as such represent potentially more conservative interpretation. In general, means of single response variables were considered significantly different from zero (significant response to elevated CO2) if their 95% confidence intervals did not overlap zero. Similarly, means of two different response variables (e.g. stress treatment classes) were considered significantly different from each other if their 95% confidence intervals did not overlap. Some results are also discussed in terms of trends and tendencies in order to highlight interesting comparisons, even if they did not satisfy this statistical guideline. For a more detailed description of the statistical approach see Curtis & Wang (1998) and Hedges et al. (1999) .
Results
Sample sizes for all variables presented in the Figures are given in Table 1.
| CO2-responses ( Fig. 1) | Environmental stress ( Fig. 2) | Exposure methods/Growth conditions( 3, 5) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Photopathway | All stresses | No stress | Lightnone;low | Temp.none; low/high | Water none;low | Nutrients cat.1;2;3 | Pot size cat. 1;2;3 | Duration cat. 1;2;3;4 | Method |
| A | C4 C3 | 4868 | 158 | 38 ; 8 | 42 ; 6 | 41 ; 19 | 34 ; - ; 1227 ; 32 ; 9 | 29 ; 7 ; 1255 ; 8 ; 5 | 29 ; 6 ; 13 ; -50 ; 11 ; 3 ; 4 | |
| GS | C4 C3 | 477 | 163 | 40 ; 5 | 41 ; 6 | 44 ; 3 | 35 ; - ; 104 ; - ; - | 28 ; 7 ; 124 ; - ; 3 | 27 ; 7 ; 13 ; -4 ; - ; 3 ; - | |
| WUE | C4 C3 | 132 | 72 | |||||||
| TOTWT | C4 C3 | 2571 | 69 | 22 ; 3 | 54 ; 13 | 20 ; - ; 249 ; 17 ; 5 | 18 ; 2 ; 549 ; 13 ; 9 | |||
| AGWT | C4 C3 | 1940 | 1023 | 36 ; 3 | 16 ; 3 | 37 ; 3 | 19 ; - ; -18 ; 13 ; 9 | 19 ; - ; -7 ; 15 ; 18 | 18 ; - ; - ; -11 ; 10 ; 17 ; 2 | |
| BGWT | C4 C3 | 930 | 48 | 21 ; 9 | 9 ; - ; -19 ; 10 ; - | |||||
| INDLA | C4 C3 | 148 | 50 | 10 ; 4 | 9 ; - ; 26 ; - ; - | 9 ; - ; 52 ; 2 ; 4 | 10 ; 2 ; 2 ; -7 ; - ; - ; - | |||
| TILLERS | C4 C3 | 912 | 56 | 4 ; - ; 52 ; 7 ; 3 | 5 ; 2 ; 2 ; -8 ; - ; 4 ; - | |||||
| SLA | C4 C3 | 1921 | 65 | |||||||
| TNC | C4 C3 | 412 | 011 | |||||||
| N | C4 C3 | 1537 | 510 | 22 ; 11 | ||||||
Relative CO2-responses of C3 and C4 species
CO2 responses of the full data set, including responses under interacting stress variables, are presented as the mean percentage change in elevated CO2 ( Fig. 1a). Net CO2 assimilation rates (A) increased significantly in both C3 and C4 species, by 33% and 25%, respectively. Stomatal conductances (GS) decreased significantly by 24% and 29% for C3 and C4, respectively. Increases in instantaneous leaf water use efficiency (WUE) were significant only in C4 species (72%); the sample size for C3 was small and variability high. Total plant biomass (TOTWT) was enhanced in both C3 (44%) and C4 species (33%). C3 species showed greater CO2-induced increases in above-ground biomass (AGWT, 38%) and below-ground biomass (BGWT, 44%), where these were reported individually, than C4 species. This suggests a deficiency in data for C4 biomass partitioning into above-and below-ground components, as the smaller effect here does not concur with the larger positive result for TOTWT. Due to reporting shortcomings, the data set for above- and below-ground biomass was often drawn from a different set of publications than that for total plant biomass, likely contributing to the lack of correspondence between the results for the three variables. Individual leaf area (INDLA) increased by 15% and 25% and tiller numbers increased by 27% and 14% in C3 and C4 species, respectively. C3 species showed greater decreases in specific leaf area (SLA, 19%) and foliar total nitrogen (N) concentrations (21%) than C4 species. Only C3 species showed significantly increased foliar total nonstructural carbohydrate (TNC) concentrations (37%) in elevated CO2.
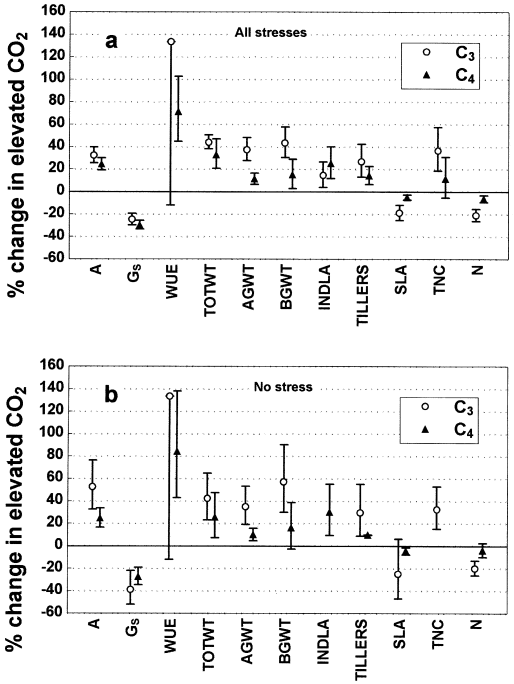
Comparative photosynthetic, growth, morphological, and chemical responses of wild C3 and C4 grass species to elevated atmospheric CO2 concentrations. (a) full data set including responses under all levels of environmental interactions other than CO2. (b) selected data set of CO2-responses under nonstressful environmental conditions. Abbreviations: A, net CO2 assimilation rate; GS, stomatal conductance; WUE, instantaneous leaf water use efficiency; TOTWT, total plant dry weight; AGWT, above-ground dry weight; BGWT, below-ground dry weight; INDLA, individual leaf area; TILLERS, tiller number; SLA, specific leaf area; TNC, leaf total nonstructural carbohydrate concentration; N, leaf total nitrogen concentration. No data were available for INDLA (C3 species) and TNC (C4 species) for nonstressful environmental conditions. Data represent percentage change in elevated CO2 with 95% confidence intervals.
Influence of environmental stress
Under nonstressful growth conditions ( Fig. 1b), the relative mean stimulation of photosynthetic rate in C3 species increased from 33% to 53%, and below-ground biomass enhancement rose from 44% to 57%. However, both changes were not significant according to the 95% confidence interval (CI) overlap test. The CO2-responses of all other variables remained similar compared to the ‘all stresses’ analysis ( Fig. 1a). By contrast, when interacting stresses were removed from the database for C4 species, the response of total biomass to elevated CO2 decreased from 33% to 26%, the tillering response was reduced (from a 14% increase to a 10% increase), but stimulation of individual leaf area rose from 25% to 30%. Again, these responses were not significant according to the CI overlap test.
The influence of environmental stresses was further explored by comparing the CO2-responses of deliberately stressed plants with the responses of all other plants (not deliberately stressed) for each stress variable individually ( Fig. 2). Only results which can be interpreted with reasonable confidence, taking into account the sample size (> 2), the confidence interval, and the power to draw robust statistical conclusions, are presented. For example, no studies addressing the interactions between elevated CO2 and light or water stress in C3 grass species existed or were suitable for use in the meta-analysis, and in many other cases the number of studies for a particular measurement category and stress factor were too small, or did not exist.
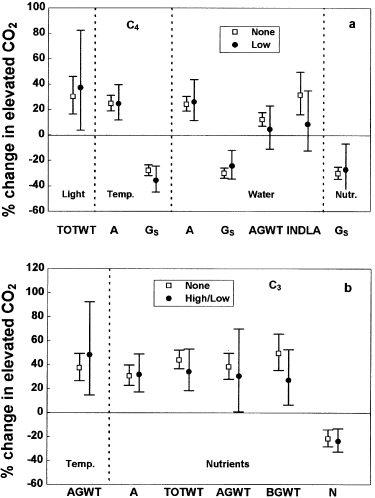
CO2-responses of wild C4 (a) and C3 (b) grass species as influenced by interaction with low light, low or high temperature, low water, or low nutrient stress. Abbreviations as for Fig. 1 Data represent percentage change in elevated CO2 with 95% confidence intervals.
In C4 species ( Fig. 2a), low light, low temperature, or low nutrient supply levels did not alter the mean responses to elevated CO2. Droughting treatments (low water supply) similarly did not alter the CO2-responses of gas exchange in C4 species, but resulted in the loss of a significant CO2-response for above-ground biomass and individual leaf area (95% confidence intervals overlap zero), compared to plants that were not water stressed. There were no data for C4 plants under high temperature stress, probably because high temperatures are not regarded as being potentially harmful to C4 plants as they are for C3 plants. In C3 species ( Fig. 2b), on the other hand, abnormally high temperatures increased the mean above-ground biomass response to elevated CO2, but this tendency was not significant. Low nutrient stress did not alter the mean CO2-response of photosynthesis and leaf nitrogen concentration in C3 species. By contrast, mean CO2-induced increases in total plant, above- and below-ground biomass tended to diminish under low nutrient supply levels, although these changes were not significant.
Exposure methods and growth conditions
The effects of exposure methods and growth conditions on the relative responses to elevated CO2 are presented in 3-5. Those variables for which data for at least two categories were available for either the C3 or C4 data set, and which had reasonable sample sizes and the potential for meaningful statistical inferences, are presented. These are matched with the results for the corresponding variable in the other (C3 or C4) data set, even if the sample sizes are small and categories missing. This was done in order to allow at least a rudimentary comparison between C3 and C4 species. Even though this comparison is largely fragmentary, it exposes gaps in the knowledge base, particularly with regard to the lack of information from long-term studies in the field, and could provide a guideline for future studies (and publication of existing data).
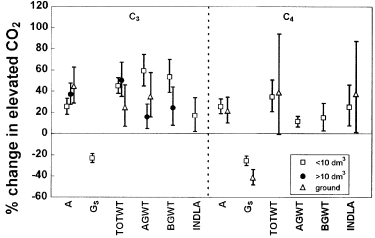
Effect of pot size on CO2-response of C3 and C4 grass species. Legend refers to pot size in dm3, or plants grown in-ground. Abbreviations as for Fig. 1 Data represent percentage change in elevated CO2 with 95% confidence intervals.
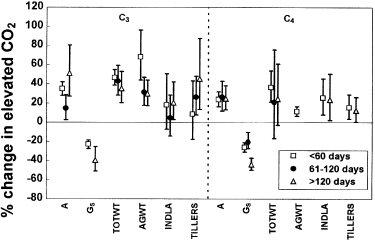
Effect of duration of exposure on the CO2-response of C3 and C4 grass species. Abbreviations as for Fig. 1 Data represent percentage change in elevated CO2 with 95% confidence intervals.
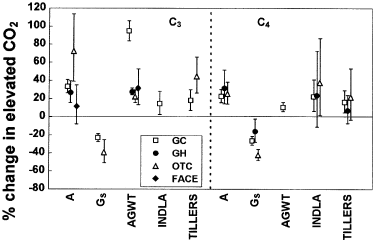
Effect of exposure method on the CO2-response of C3 and C4 grass species. Abbreviations as for Fig. 1 In the legend, GC, growth chamber; GH, greenhouse; OTC, open-top chamber; FACE, free-air CO2 enrichment. Data represent percentage change in elevated CO2 with 95% confidence intervals. Note that GC, GH and OTC treatments typically have 2× ambient [CO2] (650–700 μmol mol–1), and that FACE is typically about 550 μmol mol–1[CO2].
An increasing volume of available rooting space (‘pot size’, Fig. 3) allowed for slightly greater CO2-induced increases in photosynthetic rate in C3 species, although not significantly according to the CI overlap test. The mean photosynthetic CO2-response in C4 species was not altered. Stomatal conductances were reduced more strongly in C4 species growing in open ground than in small pots. Large positive responses in biomass in C3 species were favoured by growth in smaller rooting volumes, as supported by no or only minimal overlap in the confidence intervals between pot size classes. This effect on biomass was not discernible in C4 species, although interpretation here is strongly limited by insufficient sample sizes. Significant increases in individual leaf area in C4 species in high CO2 were measured in plants growing both in small pots and in the ground.
The mean CO2-responses for photosynthetic rate and conductance in C3 species were greater in the longer term (> 120 days, Fig. 4) than in the medium term (61–120 days). The mean above-ground biomass response, by contrast, was greatest in the short term (< 60 days). The positive tillering response to elevated CO2 achieved significance (95% confidence interval not overlapping zero) only after 60 days of exposure to high CO2, and continued to increase with experimental duration, although not significantly. In C4 species, relative decreases in conductance were significantly greater in the longer term (> 120 days). The biomass response to elevated CO2 appeared to decrease somewhat after 60 days (although small sample sizes and large confidence limits preclude a clear interpretation), so that mean biomass increases were no longer significant after 60 days (confidence intervals overlap zero). Similar increases in leaf area in elevated CO2 were found both in the short and longer term.
Increases in photosynthetic rates in elevated CO2 appeared to be greatest when C3 species were grown in open-top chambers (73%), and smallest in FACE systems (12%, Fig. 5), although the latter is probably attributable to the generally lower CO2 concentrations used in these systems than in the other types of growth facilities. In both C3 and C4 species, mean decreases in conductances were greatest in OTCs. Above-ground biomass increases were found for all exposure methods in C3 species, but responses were particularly high in growth chambers (95% increase in GC compared to 23–32% increases in other facilities). The mean tillering response was greater in OTCs (45% increase) than in growth chambers (18%). In C4 species, CO2-induced increases in photosynthetic rates were similar in all exposure facilities. Leaf area increases were found for most exposure methods (with the possible exception of greenhouses, where confidence intervals overlapped zero), and the tillering response to elevated CO2 was small for all methods and not significant in GHs and OTCs (CI overlapped zero), but in both cases interpretation was hampered by insufficient sample sizes.
Discussion
Relative CO2-responses of C3 and C4 species
The results of this meta-analysis confirm the widely held view that the relative responses of C4 species to elevated CO2 are usually smaller than those for C3 species, especially for growth under nonstressful environmental conditions. Nevertheless, differences in CO2 response between C3 and C4 grass species are not as large as current perceptions have it. A similar conclusion was drawn from a previous semiquantitative minireview of C3 vs. C4 responses ( Poorter 1993). The present analysis shows that C4 grasses are certainly responsive to elevated CO2 particularly with regard to gas exchange and leaf area development.
Photosynthetic stimulation of C4 species is, surprisingly, comparable to that of C3 species. This contradicts the general view that C4 photosynthesis does not increase in elevated CO2, due to the specialized CO2 concentrating mechanism in C4 leaves ( Bowes 1993). Many of the definitive early studies of C4 photosynthesis were performed on crop species, such as maize, which appears to be CO2-saturated at ambient CO2 levels and shows very low responsiveness to higher CO2 concentrations, compared to wild C4 species ( Ziska & Bunce 1997). A closer examination of gas exchange in other C4 grasses reveals that photosynthesis is not necessarily saturated at current CO2 levels and can increase at higher ci ( Sionit & Patterson 1984; Imai & Okamoto-Sato 1991). This simple explanation may account for the majority of cases of photosynthetic stimulation in C4 species. For example, LeCain & Morgan (1998) showed that photosynthesis was not saturated at ambient CO2 concentration in any of the six wild C4 grass species studied. Ziska & Bunce (1997) similarly measured higher photosynthetic rates at elevated CO2 in 8 out of 10 C4 species studied, due to the effect of increased ci. Another study has shown that under favourable growth conditions (high light), the operational ci of a tropical C4 grass was close to the inflection point of the A/ci response, so that increasing CO2 supply caused slight enhancements in the CO2 assimilation rates, and improved growth ( Ghannoum et al. 1997 ). On the other hand, under growth-limiting conditions (low light), the operational ci was well above the CO2 saturation level for photosynthesis, and no photosynthetic or growth response to elevated CO2 was measured. The relative ‘saturation level’ may therefore change with changing conditions, and this may play a role in photosynthetic responses to elevated CO2 in wild C4 grasses.
Stimulation of photosynthetic rates due to increases in ci represents a simple short-term effect. In addition, longer term biochemical changes, such as altered enzyme efficiencies, or altered regeneration rates of phosphoenolpyruvate (PEP) or ribulose-1,5-bisphosphate (RuBP), may develop in elevated CO2. These are termed ‘regulatory’ or ‘acclimatory’ responses and change the shape of the A/ci response. Unlike for many C3 species ( Wullschleger 1993), instances of up- or downregulation of photosynthetic capacity in C4 species have not been given much attention in the literature as they have appeared to be rare. Sage (1994) concluded that little adjustment is found in the A/ci response in C4 species under elevated CO2 (see also Ziska & Bunce 1997), except possibly downregulation under conditions of nutrient deficiency ( Wong 1979; Morgan et al. 1994; Ghannoum & Conroy 1998). Nevertheless, photosynthetic downregulation has also been measured under conditions not apparently stressful ( Read et al. 1997 ; LeCain & Morgan 1998). Many earlier reports of unchanged or reduced photosynthetic rates in C4 species in elevated CO2 may have been due to the acclimation phenomenon, but are not identifiable without full A/ci measurements (e.g. Wray & Strain 1986). The mechanisms of photosynthetic acclimation in C4 species are apparently not related to feedback inhibition resulting from carbohydrate (TNC) accumulation, or to reductions in leaf nitrogen (N) concentrations ( Read et al. 1997 ; LeCain & Morgan 1998), as they are in C3 species ( Stitt 1991; Sage 1994; Cotrufo et al. 1998 ). The meta-analysis confirmed that accumulation of TNC and reductions in leaf N in elevated CO2 are insignificantly low in C4 species. There is currently no available information on the possible mechanisms of photosynthetic acclimation in C4 species, and this warrants further attention.
Natural seasonal dynamics of photosynthetic capacity in C4 species may also influence the response to elevated CO2. In young Themeda triandra (red grass) plants with high assimilation rates, photosynthetic upregulation resulted in increased photosynthetic rates in high CO2 ( Ludwig 1996), but downregulation was measured in the same set of plants later in the season (when absolute rates were reduced), which led to similar or reduced photosynthetic rates at high compared to ambient CO2 (Wand, unpublished data). Growth enhancement, particularly of leaf area, was linked to this early response. Detailed measurements of A/ci responses in C4 and C3 grass species growing in elevated CO2 in a field experiment in South Africa (utilization of a natural CO2 spring) have also shown photosynthetic upregulation in Themeda triandra during the active growth season (Wand, unpublished data). Similarly, significant increases in CO2 assimilation rates in the salt marsh C4 species Spartina patens in high CO2 were confined to the early season, when absolute rates of assimilation were highest ( Ziska et al. 1990 ). Knapp et al. (1993) have also reported upregulation in Andropogon gerardii (big bluestem), and Chen et al. (1994) modelled this response. Recent reports indicate that C4 photosynthetic physiology may change with progressive developmental stages, showing more similarities with C3 physiology (lower CO2-concentrating ability) when leaves are young or senescent, compared to mature leaves ( Dai et al. 1995 ; He & Edwards 1996). This was tentatively proposed as an explanation for ontogenetic shifts in CO2-responsiveness ( Ghannoum et al. 1997 ), but subsequent work on C4 grass species does not support this explanation, as C4 photosynthetic characteristics were already fully developed in young leaves ( Ghannoum et al. 1998 ). In conclusion, this aspect of C4 response to elevated CO2 requires more attention, as conflicting evidence also exists. For example, photosynthetic downregulation was found in both young and older Bouteloua gracilis (blue grama) plants ( Read et al. 1997 ). Furthermore, seasonal dependencies of C4 growth responses, in contrast with responses of carbon assimilation, are not evident in many field-based elevated CO2 experiments ( Curtis et al. 1989 ; Kirkham et al. 1991 ; Hamerlynck et al. 1997 ).
The issue of whether biochemical differences between C4 photosynthetic subtypes may shed light on the reasons for interspecific differences in CO2 responsiveness ( Henderson et al. 1994 ) is beyond the scope of the present review, but we make some brief comments. The three C4 subtypes (NADP-ME (NADP-malic enzyme), PCK (phosphoenolpyruvate carboxykinase), and NAD-ME (NAD-malic enzyme)) exhibit increasing levels of ‘leakiness’ to CO2 from the bundle sheath to the mesophyll, in the above order ( Hattersley 1982; Furbank & Hatch 1987; Jenkins et al. 1989 ; Brown & Byrd 1993; but see Hatch et al. 1995 ). This amounts to a loss of between 10 and 40% of carbon fixed by PEP carboxylase, which could, conceivably, be counteracted by increased CO2 supply from the atmosphere. Recent studies investigating the relative responsiveness of the subtypes to elevated CO2 yielded counter-intuitive results, with the least ‘leaky’ NADP-ME showing the largest responses ( LeCain & Morgan 1998; Wand, unpublished data). Nevertheless, this line of investigation may well contribute to an improved understanding of photosynthetic responses of C4 plants to elevated CO2.
Elevated CO2 has significant positive effects on plant water relations in both C3 and C4 grass species, via reductions in stomatal conductance (GS). In fact, this response, coupled with reduced transpirational water loss and the corresponding increases in WUE, are probably the most ubiquitous responses to elevated CO2 for almost all plant functional types ( Gifford & Morison 1985; Chaves & Pereira 1992; Tyree & Alexander 1993). C4 and C3 responses in GS to elevated CO2 were similar ( Fig. 1a), but clear interpretation is hampered by the fact that fewer data are available for C3 Poaceae. The decrease in GS in C4 species is consistent across a range of environmental stresses, but greatest in plants grown in the ground and exposed to elevated CO2 for more than 120 days. This suggests that developmental changes in GS, possibly related to altered stomatal sizes or densities, may occur as leaves mature in high CO2. Information on long-term changes in GS (e.g. changing stomatal densities) is limited ( Ghannoum et al. 1997 ). It is generally thought that CO2-induced reductions in GS are primarily short-term effects, but some researchers have measured acclimatory responses in stomatal physiology in C4 species, as shown by the responses of conductance to increasing CO2 (GS/ci curves) (Morgan et al. 1994; Read et al. 1997 ; LeCain & Morgan 1998). This can take the form of either up-regulation (higher GS at equivalent ci for leaves grown in elevated CO2), or downregulation (reduced GS at low ci in elevated CO2). Studies in the greenhouse and field (Wand, unpubl. data) showed that GS was significantly reduced under increasing CO2 levels in the short term (changes in the cuvette CO2 concentration) in all seven C4 grass species studied. In addition, either upregulation or downregulation of GS was also found in the longer term (a treatment effect) in some species, and this developmental response appeared to depend on season or environmental conditions (e.g. water stress). Reduced transpirational water loss in elevated CO2 and the resulting improvement in soil water content over the course of the growing season, as reported for the tall-grass prairie ( Kirkham et al. 1993 ), are likely to be reflected in longer term changes in stomatal conductances.
The stimulation of C4 whole plant growth under elevated CO2 (mean of 33%, 95% confidence interval 21%-47%) is slightly higher than the 22% reported by Poorter (1993). Growth stimulation could be either a direct effect of greater carbon assimilation rates (discussed above), or an indirect effect of improved soil and leaf water relations resulting from reduced stomatal conductances and transpirational water loss ( Knapp et al. 1993 ; Owensby et al. 1993 ). Cell elongation and blade extension rates in developing grass leaves are positively correlated with leaf water potentials ( Boyer 1970; Toft et al. 1987 ). Although not included in the meta-analysis, the C4 literature database clearly showed a consistent and significant positive increase in shoot water potentials in grasses exposed to elevated CO2 (e.g. Kirkham et al. 1993 ; Hamerlynck et al. 1997 ).
The growth response of C3 species in this review is a little larger than that for C4 species (44%) and comparable to the C3 herbaceous monocot component of Poorter’s database (42%). We tentatively support Poorter’s conclusion that differences in growth stimulation between C3 and C4 plants are probably not as large as suggested by current perceptions. Unfortunately, the responses for above- and below-ground biomass in C4 species in this meta-analysis do not match those for whole-plant biomass, and care must be taken in interpretation. Also, many field studies using C4 species have not reported biomass responses adequately, probably due to logistical difficulties and an unwillingness to disturb the ecosystem in longer term experiments. We need more information on whether increased carbon assimilation rates will lead to sustained enhanced biomass production in C4-grass-dominated ecosystems such as prairies and savannas, which comprise a large percentage of productive land surface ( Hall et al. 1995 ). This would help to improve our models of global carbon dynamics. Currently, the potential of C4-grass-dominated ecosystems as significant carbon sinks is considered small, but this may need to be re-assessed.
An interesting contrast emerged regarding the morphological development of C3 and C4 species under elevated CO2. C3 species generally develop more tillers, with only small increases in leaf area, but decreased specific leaf areas (increased leaf density or thickness). C4 species, on the other hand, appeared to respond mainly with increased leaf areas, and smaller increases in tiller numbers. This contrast may indicate a greater sensitivity in C4 species to self-shading of the basal nodes from which tillers are initiated ( Deregibus et al. 1985 ; Everson et al. 1988 ), and may provide the mechanism for growth stimulation even under moderate photosynthetic enhancement. Gradually increasing canopy leaf areas, leading to a progressive increase in whole-canopy carbon assimilation rates, would result in a continuously greater supply of carbon products to support enhanced growth. Early increases in leaf area, leaf area duration (the cumulative leaf area over the growth period), leaf area ratio (the proportion of leaf area to plant biomass), plant height and total plant biomass of C4 species in elevated CO2 have been reported by Patterson & Flint (1980), Riechers & Strain (1988) and Ackerly et al. (1992) . Early responses in biomass and leaf area, which persist for the whole growth period, have also been found for some C3 species ( Bowler & Press 1993), but the stimulation of leaf area, in particular, appears to be characteristic of the CO2-response of C4 species. Coleman & Bazzaz (1992) and Ackerly et al. (1992) came to the conclusion that standing photosynthetically active leaf area (net leaf area production and loss) in a C4 species was the primary influence on growth responses in elevated CO2.
Influence of environmental stress and growth methodology
Environmental stresses tend to reduce (although not significantly) the potential CO2-response in C3 species, as evidenced by the suppression of mean photosynthetic and below-ground biomass responses when all stresses are included in the analysis ( Fig. 1a,b), as well as the reductions in mean growth responses when nutrients are limiting ( Fig. 2b). By contrast, C4 species were generally not negatively impacted by environmental stresses, although leaf area stimulation was sensitive to water stress ( Fig. 2a). The current view that CO2-responses in C4 graminoids are particularly marked under conditions of water stress ( Nie et al. 1992 ; Knapp et al. 1993 ; Owensby et al. 1993 ; Ham et al. 1995 ) was not borne out by the meta-analysis, possibly due to the lack of data suitable for inclusion in the database. Many of these studies do not present plant- and leaf-level responses, concentrating on canopy-level gas fluxes instead.
C4 graminoids may well benefit from increased CO2 supply under some stressful environmental conditions, such as low soil fertility. However, there is a lack of information about the changes in plant nitrogen use efficiency in C3 and C4 graminoids in elevated CO2. This understanding may be critical in predicting changes in their relative competitive abilities, as it has been suggested that competitive advantage, especially in grasslands, may be due to a greater ability to extract nitrogen from the soil ( Tilman 1990). This in turn may be associated with greater plant and photosynthetic nitrogen use efficiency ( Richardson et al. in press ).
Contrary to strong evidence for nongraminoid C3 species showing reduced CO2-responsiveness in small rooting volumes ( Arp 1991; McConnaughay et al. 1993 , 1996), C3 grass species were more responsive in small pots, with respect to above- and below-ground growth. In C4 species, rooting volume did not appear to play a role in the biomass response to CO2, but more data are needed to confirm this as the sample size was too small for a confident interpretation. Nevertheless, field studies with C3 species have not supported the expected significant productivity increases based on earlier pot studies, and predictions of competitive advantages over C4 species should be cautiously reviewed. In addition, above-ground biomass enhancement of C3 species diminished with increasing duration of exposure to elevated CO2, even though tiller number appeared to be stimulated, which suggests that individual tiller size is progressively reduced in this group.
In conclusion, responses to elevated CO2 in wild C4 and C3 Poaceae at the leaf and whole plant levels are summarised in Fig. 6, and demonstrate many trends common to both photosynthetic types. At the leaf level, the greater carbohydrate accumulation and greater reductions in leaf nitrogen concentration in the C3 type alone differentiated the types, and constituted the only evidence for so-called ‘sink limitation’ which is often invoked in elevated CO2 studies on C3 dicots. Average photosynthetic responsiveness did not concur with predictions based solely on photosynthetic theory. However, at the shoot level, there were clearcut differences between types resulting from disparate effects on above-ground morphologies. These, rather than photosynthetic differences between the types, might be of greater importance when evaluating responses to elevated CO2.
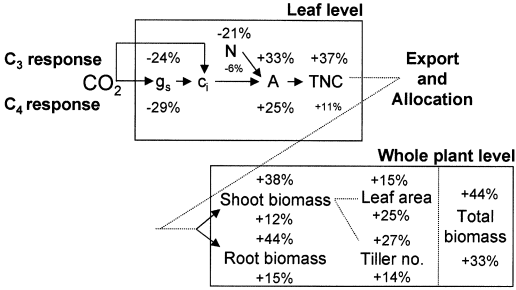
Summary scheme of the CO2- response levels (% change) and their relationships under all conditions (full data set). C3 responses are given above and C4 responses given below the stated parameter. Response levels printed in small font are not significantly different from zero. Abbreviations as for Fig. 1, and ci=intercellular CO2 concentration.
The combined shoot structural and water use efficiency responses of these functional types are likely to have consequential implications for the water balance of important catchments and rangelands throughout the world, especially in semiarid subtropical and temperate regions. Improved water relations would be highly beneficial to C4 grasses growing in marginal semiarid sites where growing season may be limited by soil water availability, such as over much of southern Africa and parts of North America. The results of this meta-analysis suggest that it may be premature to predict that the C4 type will lose its competitive advantage in certain regions as CO2 levels rise, based solely only on differential photosynthetic mechanisms (Collatz et al. 1998).
Acknowledgements
We thank B.G. Drake of the Smithsonian Environmental Research Centre, Edgewater, USA, for stimulating our interest in C4 plant responses to elevated CO2. We are also grateful to W.D. Stock, O. Ghannoum and J.A. Morgan for suggestions and valuable discussion. P. Moran-Palma, X. Wang, N. Ceja, E. Lai, T. Goodwin and M. Rheinhardt are thanked for their assistance. M.B. Kirkham and two anonymous referees made valuable comments that helped improve the manuscript.
Appendices
Appendix 1
References included in database for meta-analysis
Baxter R, Ashenden TW, Farrar JF (1997) Effect of elevated CO2 and nutrient status on growth, dry matter partitioning and nutrient content of Poa alpina var vivipara L. Journal of Experimental Botany, 48, 1477–1486.
Baxter R, Ashenden TW, Sparks TH, Farrar JF (1994a) Effects of elevated carbon dioxide on three montane grass species. I. Growth and dry matter partitioning. Journal of Experimental Botany, 45, 305–315.
Baxter R, Bell SA, Sparks TH, Ashenden TW, Farrar JF (1995) Effects of elevated CO2 concentrations on three montane grass species. III. Source leaf metabolism and whole plant carbon partitioning. Journal of Experimental Botany, 46, 917–929.
Baxter R, Gantley M, Ashenden TW, Farrar JF (1994b) Effects of elevated carbon dioxide on three grass species from montane pasture. Journal of Experimental Botany, 45, 1267–1287.
Bowler JM, Press MC (1993) Growth responses of two contrasting upland grass species to elevated CO2 and nitrogen concentration. New Phytologist, 124, 515–522.
Bowler JM, Press MC (1996) Effects of elevated CO2, nitrogen form and concentration on growth and photosynthesis of a fast- and slow-growing grass. New Phytologist, 132, 391–401.
Bowman WD, Strain BR (1987) Interaction between CO2 enrichment and salinity stress in the C4 non-halophyte Andropogon glomeratus (Walter) BSP. Plant, Cell and Environment, 10, 267–270.
Campbell BD, Laing WA, Greer DH, Crush JR, Clark H, Williamson DY, Given MDJ (1995) Variation in grassland populations and species and the implications for community responses to elevated CO2. Journal of Biogeography, 22, 315–322.
Carlson RW, Bazzaz FA (1982) Photosynthetic and growth response to fumigation with SO2 at elevated CO2 for C3 and C4 plants. Oecologia, 54, 50–54.
Casella E, Soussana JF, Loiseau P (1996) Long-term effects of CO2 enrichment and temperature increase on a temperate grass sward. 1. Productivity and water use. Plant and Soil, 182, 83–99.
Curtis PS, Balduman LM, Drake BG, Whigham DF (1990) Elevated atmospheric CO2 effects on belowground processes in C3 and C4 estuarine marsh communities. Ecology, 71, 2001–2006.
Curtis PS, Drake BG, Leadley PW, Arp WJ, Whigham DF (1989) Growth and senescence in plant communities exposed to elevated CO2 concentrations on an estuarine marsh. Oecologia, 78, 20–26.
Ferris R, Nijs I, Behaeghe T, Impens I (1996) Contrasting CO2 and temperature effects on leaf growth of perennial rye grass in spring and summer. Journal of Experimental Botany, 47, 1033–1043.
Fischer BU, Frehner M, Hebeisen T, Zanetti S, Stadelmann F, Luscher A, Hartwig UA, Hendrey GR, Blum H, Nösberger J (1997) Source-sink relations in Lolium perenne L. as reflected by carbohydrate concentrations in leaves and pseudo-stems during regrowth in a free air carbon dioxide enrichment (FACE) experiment. Plant, Cell and Environment, 20, 945–952.
Fitter AH, Self GK, Wolfenden J, vanVuuren MMI, Brown TK, Williamson L, Graves JD, Robinson D (1996) Root production and mortality under elevated atmospheric carbon dioxide. Plant and Soil, 187, 299–306.
Garbutt K, Williams WE, Bazzaz FA (1990) Analysis of the differential response of five annuals to elevated CO2 during growth. Ecology, 71, 1185–1194.
Ghannoum O, von Caemmerer S, Barlow EWR, Conroy J (1997) The effect of CO2 enrichment and irradiance on the growth, morphology and gas exchange of a C3 (Panicum laxum) and a C4 (Panicum antidotale) grass. Australian Journal of Plant Physiology, 24, 227–237.
Gifford RM, Morison JIL (1985) Photosynthesis, water use and growth of a C4 grass stand at high CO2 concentration. Photosynthesis Research, 7, 77–90.
Gloser J, M et al. (1994 ) Net photosynthesis, growth rate and biomass allocation in a rhizomatous grass Calamagrostis epigejos grown at elevated CO2 concentration. Photosynthetica, 30, 143–150.
Greer DH, Laing WA, Campbell BD (1995) Photosynthetic responses of thirteen pasture species to elevated CO2 and temperature. Australian Journal of Plant Physiology, 22, 713–722.
Hakala K, Mela T (1996) The effects of prolonged exposure to elevated temperatures and elevated CO2 levels on the growth, yield and dry matter partitioning of field-sown meadow fescue. Agricultural and Food Science in Finland, 5, 285–298.
Hamerlynck EP, McAllister CA, Knapp AK, Ham JM, Owensby CE (1997) Photosynthetic gas exchange and water relation responses of three tallgrass prairie species to elevated carbon dioxide and moderate drought. International Journal of Plant Sciences, 158, 608–616.
Jackson RB, Luo Y, Cardon ZG, Sala OE, Field CB, Mooney HA (1995) Photosynthesis, growth and density for the dominant species in a CO2-enriched grassland. Journal of Biogeography, 22, 221–225.
Jackson RB, Reynolds AL (1996) Nitrate and ammonium uptake for single- and mixed species communitites grown at elevated CO2. Oecologia, 105, 74–80.
Jackson RB, Sala OE, Field CB, Mooney HA (1994) CO2 alters water use, carbon gain, and yield for the dominant species in a natural grassland. Oecologia, 98, 257–262.
Jones MB, Jongen M, Doyle T (1996) Effects of elevated carbon dioxide concentrations on agricultural grassland production. Agricultural and Forest Meteorology, 79, 243–252.
Kirkham MB, He H, Bolger TP, Lawlor DJ, Kanemasu ET (1991) Leaf photosynthesis and water use of big bluestem under elevated carbon dioxide. Crop Science, 31, 1589–1594.
Knapp AK, Hamerlynck EP, Owensby CE (1993) Photosynthetic and water relations responses to elevated CO2 in the C4 grass Andropogon gerardii. International Journal of Plant Sciences, 154, 459–466.
Larigauderie A, Hilbert DW, Oechel WC (1988) Effects of CO2 enrichment and nitrogen availability on resource acquisition and resource allocation in a grass, Bromus mollis. Oecologia, 77, 544–549.
Leadley PW, Stoecklin J (1996) Effects of elevated CO2 on model calcareous grasslands: community, species, and genotype level responses. Global Change Biology, 2, 389–397.
Lenssen GM, Vandium WE, Jak P, Rozema J (1995) The response of Aster tripolium and Puccinellia maritima to atmospheric carbon dioxide enrichment and their interaction with flooding and salinity. Aquatic Botany, 50, 181–192.
Marks S, Clay K (1990) Effects of CO2 enrichment, nutrient addition, and fungal endophyte-infection on the growth of two grasses. Oecologia, 84, 207–214.
Marks S, Strain BR (1989) Effects of drought and CO2 enrichment on competition between two old-field perennials. New Phytologist, 111, 181–186.
Morgan JA, Hunt HW, Monz CA, LeCain DR (1994a) Consequences of growth at two carbon dioxide concentrations and two temperatures for leaf gas exchange in Pascopyrum smithii (C3) and Bouteloua gracilis (C4). Plant, Cell and Environment, 17, 1023–1033.
Morgan JA, Knight WG, Dudley LM, Hunt HW (1994b) Enhanced root system C-sink activity, water relations and aspects of nutrient acquisition in mycotrophic Bouteloua gracilis subjected to CO2 enrichment. Plant and Soil, 165, 139–146.
Newton PCD, Clark H, Bell CC, Glasgow EM, Tate KR, Ross DJ, Yeates GW, Saggar S (1995) Plant growth and soil processes in temperate grassland communities at elevated CO2. Journal of Biogeography, 22, 235–240.
Nie D, He H, Kirkham MB, Kanemasu ET (1992) Photosynthesis of a C3 grass and a C4 grass under elevated CO2. Photosynthetica, 26, 189–198.
Nijs I, Ferris R, Blum H, Hendrey G, Impens I (1997) Stomatal regulation in a changing climate: a field study using Free Air Temperature Increase (FATI) and Free Air CO2 enrichment (FACE). Plant, Cell and Environment, 20, 1041–1050.
Nijs I, Teughels H, Blum H, Hendrey G, Impens I (1996) Simulation of climate change with infra-red heaters reduces the productivity of Lolium perenne L. in summer. Environmental and Experimental Botany, 36, 271–280.
Polley HW, Johnson HB, Mayeux HS, Brown DA, White JWC (1996) Leaf and plant water use efficiency of C4 species grown at glacial to elevated CO2 concentrations. International Journal of Plant Sciences, 157, 164–170.
Potvin C, Strain BR (1985) Photosynthetic response to growth temperature and CO2 enrichment in two species of C4 grasses. Canadian Journal of Botany, 63, 483–487.
Read JJ, Morgan JA, Chatterton NJ, Harrison PA (1997) Gas exchange and carbohydrate and nitrogen concentrations in leaves of Pascopyrum smithii (C3) and Bouteloua gracilis (C4) at different carbon dioxide concentrations and temperatures. Annals of Botany, 79, 197–206.
Ryle GJA, Powell CE, Tewson V (1992) Effects of elevated CO2 on the photosynthesis, respiration and growth of perennial ryegrass. Journal of Experimental Botany, 43, 811–818.
Saebo A, Mortensen LM (1995) Growth and regrowth of Phleum pratense, Lolium perenne, Trifolium repens and Trifolium pratense at normal and elevated CO2 concentration. Agriculture, Ecosystems and Environment, 55, 29–35.
Saebo A, Mortensen LM (1996) The influence of elevated CO2 concentration on growth of seven grasses and one clover species in a cool maritime climate. Acta Agriculturae Scandinavica Section B, Soil and Plant Sciences, 46, 49–54.
Schäppi B, Körner Chr (1996) Growth responses of an alpine grassland to elevated CO2. Oecologia, 105, 43–52.
Sionit N, Patterson DT (1984) Responses of C4 grasses to atmospheric CO2 enrichment. I. Effect of irradiance. Oecologia, 65, 30–34.
Sionit N, Patterson DT (1985) Responses of C4 grasses to atmospheric CO2 enrichment. II. Effect of water stress. Crop Science, 25, 533–537.
van de Staaij JWM, Lenssen GM, Stroetenga M, Rozema J (1993) The combined effects of elevated CO2 levels and UV-B radiation on growth characteristics of Elymus athericus (= E. pycnanathus). Vegetatio, 104/105, 433–439.
Stewart J, Potvin C (1996) Effects of elevated CO2 on an artificial grassland community: competition, invasion and neighbourhood growth. Functional Ecology, 10, 157–166.
Stirling CM, Davey PA, Williams TG, Long SP (1997) Acclimation of photosynthesis to elevated CO2 and temperature in five British native species of contrasting functional type. Global Change Biology, 3, 237–246.
Teughels H, Nijs I, van Hecke P, Impens I (1995) Competition in a global change environment: The importance of different plant traits for competitive success. Journal of Biogeography, 22, 297–305.
Thompson GB, Drake BG (1994) Insects and fungi on a C3 sedge and a C4 grass exposed to elevated atmospheric CO2 concentrations in open-top chambers in the field. Plant, Cell and Environment, 17, 1161–1167.
Volin JC, Reich PB (1996) Interaction of elevated CO2 and O3 on growth, photosynthesis and respiration of three perennial species grown in low and high nitrogen. Physiologia Plantarum, 97, 674–684.
Wand SJE, Midgley GF, Musil CF (1996) Physiological and growth responses of two African species, Acacia karroo and Themeda triandra, to combined increases in CO2 and UV-B radiation. Physiologia Plantarum, 98, 882–890.
Wilsey BJ (1996) Urea additions and defoliation affect plant responses to elevated CO2 in a C3 grass from Yellowstone National Park. Oecologia, 108, 321–327.
Wilsey BJ, Coleman JS, McNaughton SJ (1997) Effects of elevated CO2 and defoliation of grasses: a comparative ecosystem approach. Ecological Applications, 7, 844–853.
Wilsey BJ, McNaughton SJ, Coleman JS (1994) Will increases in atmospheric CO2 affect regrowth following grazing in C4 grasses from tropical grasslands? A test with Sporobolus kentrophyllus. Oecologia, 99, 141–144.
Wray SM, Strain BR (1986) Response of two old field perennials to interactions of CO2 enrichment and drought stress. American Journal of Botany, 73, 1486–1491.
Wray SM, Strain BR (1987) Competition in old-field perennials under CO2 enrichment. Ecology, 68, 1116–1120.
Zanetti S, Hartwig UA, van Kessel C, Lüscher A, Hebeisen T, Frehner M, Fischer BU, Hendrey GR, Blum H, Nösberger J (1997) Does nitrogen nutrition restrict the CO2 response of fertile grassland lacking legumes? Oecologia, 112, 17–25.
Ziska LH, Hogan KP, Smith AP, Drake BG (1991) Growth and photosynthetic response of nine tropical species with long-term exposure to elevated carbon dioxide. Oecologia, 86, 383–389.
Appendix 2
Other References for C4 grass species not included in database for meta-analysis)
Alberto AMP, Ziska LH, Cervancia CR, Manalo PA (1996) The influence of increasing carbon dioxide and temperature on competitive interactions between a C3 crop, rice (Oryza sativa) and a C4 weed (Echinochloa glabrescens). Australian Journal of Plant Physiology, 23, 795–802.
Arp WJ, Drake BG, Pockman WT, Curtis PS, Whigham DF (1993) Interactions between C3 and C4 salt marsh plant species during four years of exposure to elevated atmospheric CO2. Vegetatio, 104/105, 133–143.
Bremer DJ, Ham JM, Owensby CE (1996) Effect of elevated atmospheric carbon dioxide and open-top chambers on transpiration in a tallgrass prairie. Journal of Environmental Quality, 25, 691–701.
Carter DR, Peterson KM (1983) Effects of a CO2-enriched atmosphere on the growth and competitive interaction of a C3 and a C4 grass. Oecologia, 58, 188–193.
Curtis PS, Drake BG, Whigham DF (1989) Nitrogen and carbon dynamics in C3 and C4 estuarine marsh plants grown under elevated CO2 in situ. Oecologia, 78, 297–301.
Hunt HW, Elliott ET, Detling JK, Morgan JA, Chen D-X (1996) Responses of a C3 and a C4 perennial grass to elevated CO2 and temperature under different water regimes. Global Change Biology, 2, 35–47.
Knapp AK, Fahnestock JT, Owensby CE (1994) Elevated atmospheric CO2 alters stomatal responses to variable sunlight in a C4 grass. Plant, Cell and Environment, 17, 189–195.
Knapp AK, Hamerlynck EP, Ham JM, Owensby CE (1996) Responses in stomatal conductance to elevated CO2 in 12 grassland species that differ in growth form. Vegetatio, 125, 31–41.
Lenssen GM, Lamers J, Stroetenga M, Rozema J (1993) Interactive effects of atmospheric CO2 enrichment, salinity and flooding on growth of C3 (Elymus athericus) and C4 (Spartina anglica) salt marsh species. Vegetatio, 104/105, 379–388.
Owensby CE, Coyne PI, Auen LM (1993a) Nitrogen and phosphorus dynamics of a tallgrass prairie ecosystem exposed to elevated carbon dioxide. Plant, Cell and Environment, 16, 843–850.
Owensby CE, Coyne PI, Ham JM, Auen LM, Knapp AK (1993b) Biomass production in a tallgrass prairie ecosystem exposed to ambient and elevated CO2. Ecological Applications, 3, 644–653.
Patterson DT (1986) Responses of soybean (Glycine max) and three C4 grass weeds to CO2 enrichment during drought. Weed Science, 34, 203–210.
Patterson DT, Flint EP (1980) Potential effects of global atmospheric CO2 enrichment on the growth and competitiveness of C3 and C4 weed and crop plants. Weed Science, 28, 71–75.
Potvin C, Strain BR (1985) Effects of CO2 enrichment and temperature on growth in two C4 weeds, Echinochloa crus-galli and Eleusine indica. Canadian Journal of Botany, 63, 1495–1499.
Read JJ, Morgan JA (1996) Growth and partitioning in Pascopyrum smithii (C3) and Bouteloua gracilis (C4) as influenced by carbon dioxide and temperature. Annals of Botany, 77, 487–496.
Riechers GH, Strain BR (1988) Growth of blue grama (Bouteloua gracilis) in response to atmospheric CO2 enrichment. Canadian Journal of Botany, 66, 1570–1573.
Smith SD, Strain BR, Sharkey TD (1987) Effects of CO2 enrichment on four Great Basin grasses. Functional Ecology, 1, 139–143.
Zangerl AR, Bazzaz FA (1984) The response of plants to elevated CO2. II. Competitive interactions among annual plants under varying light and nutrients. Oecologia, 62, 412–417.
Ziska LH, Drake BG, Chamberlain S (1990) Long-term photosynthetic response in single leaves of a C3 and C4 salt marsh species grown at elevated atmospheric CO2 in situ. Oecologia, 83, 469–472
References
Appendix 3
| Interacting stresses | |||||||
|---|---|---|---|---|---|---|---|
| Reference | C3 species | Exposure facility | Pot size(L) | Duration(days) | Nutr. | Temp. | Water |
| Baxter et al. (1994a) | Agrostis capillaris Festuca vivipara Poa alpina | OTC OTC OTC | 0.70.70.7 | 79189105 | |||
| Baxter et al. (1994b) | Agrostis capillaris Festuca vivipara Poa alpina | OTC OTC OTC | 0.40.40.4 | 43189105 | |||
| Baxter et al. (1995) | Agrostis capillaris Festuca vivipara Poa alpina | OTC OTC OTC | 0.70.70.7 | 79189105 | |||
| Baxter et al. (1997) | Poa alpina | GC | 2.5 | 50 | |||
| Bowler & Press (1993) | Agrostis capillaris Nardus stricta | GC GC | 3.83.8 | 5863 | ** | ||
| Bowler & Press (1996) | Agrostis capillaris Nardus stricta | GC GC | 2525 | 4249 | ** | ||
| Campbell et al. (1995) | Agrostis capillaris Bromus willdenowii Dactylis glomerata Festuca arundinacea Lolium multiflorum Lolium perenne Phalaris aquatica | GC GC GC GC GC GC GC | 0.80.80.80.80.80.80.8 | 28422842284228 | ******* | ||
| Casella et al. (1996) | Lolium perenne | GH | 220 | 720 | * | ||
| Ferris et al. (1996) | Lolium perenne | GH | 3.7 | 133 | * | ||
| Fischer et al. (1997) | Lolium perenne | FACE | G | 426 | * | ||
| Fitter et al. (1996) | Festuca ovina | OTC | G | 730 | |||
| Ghannoum et al. (1997) | Panicum laxum | GH | 7.0 | 49 | |||
| Gloser & Bartak (1994) | Calamagrostis epigejos | GC | 0.5 | 21 | |||
| Greer et al. (1995) | Lolium perenne Agrostis capillaris | GC GC | 1.21.2 | 28/5628/56 | ** | ||
| Hakala & Mela (1996) | Festuca pratensis | OTC | G | 510 | * | ||
| Jackson & Reynolds (1996) | Avena fatua Bromus hordeaceus Lolium multiflorum Vulpia microstachys | OTC OTC OTC OTC | 30303030 | 135135135135 | **** | ||
| Jackson et al. (1994) | Avena barbata | OTC | G | 430 | |||
| Jackson et al. (1995) | Avena barbata Avena sativa | OTC GH | G3.1 | 79030 | |||
| Jones et al. (1996) | Lolium perenne | OTC | G | 735 | |||
| Larigauderie et al. (1988) | Bromus mollis | GC | 3 | 129 | * | ||
| Leadley & Stöcklin (1996) | Bromus erectus Festuca ovina | GC GC | 24.324.3 | 126126 | |||
| Lenssen et al. (1995) | Puccinellia maritima | GH | 1.8 | 28 | |||
| Marks & Clay (1990) | Lolium perenne | GC | 0.5 | 70 | * | ||
| Morgan et al. (1994a) | Pascopyrum smithii | GC | 20.4 | 460 | * | ||
| Newton et al. (1995) | Lolium perenne | GC | 150 | 340 | |||
| Nie et al. (1992) | Poa pratensis | GH | G | 61 | * | ||
| Nijs et al. (1996) | Lolium perenne | FACE | G | 23 | * | ||
| Nijs et al. (1997) | Lolium perenne | FACE | G | 143 | * | ||
| Read et al. (1997) | Pascopyrum smithii | GC | 6 | 49 | * | ||
| Ryle et al. (1992) | Lolium perenne | GC | 2 | 49 | |||
| Saebo & Mortensen (1995) | Lolium perenne Phleum pratense | OTC OTC | 4848 | 147147 | |||
| Saebo & Mortensen (1996) | Agrostis capillaris Dactylis glomerata Festuca arundinaceae Festuca duruiscula Festuca pratensis Festuca rubra Poa pratensis | OTC OTC OTC OTC OTC OTC OTC | 48484848484848 | 60646472727172 | |||
| Schäppi & Körner (1996) | Poa alpina | OTC | G | 310 | * | ||
| Stewart & Potvin (1996) | Poa pratensis Poa pratensis Elymus athericus | GCOTC GH | 27.4G1.8 | 616165 | |||
| Stirling et al. (1997) | Poa alpina Poa annua | GH GH | 4.74.7 | 7575 | ** | ||
| Teughels et al. (1995) | Lolium perenne Festuca arundinaceae | GH GH | 6.26.2 | 6030 | |||
| van de Staaij et al. (1993) | Elymus athericus | GH | 1.8 | 65 | |||
| Volin & Reich (1996) | Agropyron smithii | GC | 2.5 | 58 | * | ||
| Wilsey (1996) | Stipa occidentalis | GC | 2 | 86 | * | ||
| Wilsey et al. (1997) | Agropyron caninum Festuca idahoensis Briza subaristata Stipa occidentalis | GC GC GC GC | 2.02.02.02.0 | 75757575 | |||
| Zanetti et al. (1997) | Lolium perenne | FACE | G | 913 | |||
| Ziska et al. (1991) | Pharus latifolius | OTC | 12.5 | 100 | |||
Appendix 4
| Interacting stresses | |||||||
|---|---|---|---|---|---|---|---|
| Reference | C4 species | Exposure facility | Pot size(L) | Duration(days) | Temp. | Water | Light |
| Bowman & Strain (1987) | Andropogon glomeratus | GC | 1 | 56 | |||
| Campbell et al. (1995) | Digitaria sanguinalis Paspalum dilatatum | GC GC | 0.80.8 | 4242 | ** | ||
| Carlson & Bazzaz (1982) | Setaria faberii Setaria lutescens | GH GH | 11 | 3232 | |||
| Curtis et al. (1989) | Spartina patens | OTC | G | 124 | |||
| Curtis et al. (1990) | Spartina patens | OTC | G | 580 | |||
| Garbutt et al. (1990) | Setaria faberii | GH | 1 | 78 | |||
| Ghannoum et al. (1997) | Panicum antidotale | GH | 7 | 49 | * | ||
| Gifford & Morison (1985) | Paspalum plicatulum | GC | 3.2 | 131 | * | ||
| Hamerlynck et al. (1997) | Andropogon gerardii | OTC | G | 1255 | * | ||
| Kirkham et al. (1991) | Andropogon gerardii | OTC | G | 214 | * | ||
| Knapp et al. (1993) | Andropogon gerardii | OTC | G | 480 | * | ||
| Marks & Clay (1990) | Tridens flavus | GC | 0.5 | 70 | |||
| Marks & Strain (1989) | Andropogon virginicus | GC | 0.5 | 16 | * | ||
| Morgan et al. (1994a) | Bouteloua gracilis | GC | 8 | 76 | * | ||
| Morgan et al. (1994b) | Bouteloua gracilis | GC | 20 | 190 | |||
| Newton et al. (1995) | Paspalum dilatatum | GC | 150 | 340 | |||
| Nie et al. (1992) | Andropogon gerardii | GH | G | 61 | * | ||
| Polley et al. (1996) | Schizachyrium scoparium | GH | 30 | 480 | |||
| Potvin & Strain (1985a) | Echinochloa crus-galli Eleusine indica | GC GC | 11 | 4848 | * * | ||
| Read et al. (1997) | Bouteloua gracilis | GC | 6 | 49 | * | ||
| Sionit & Patterson (1984) | Digitaria sanguinalis | GC | 1 | 22 | * | ||
| Echinochloa crus-galli | GC | 1 | 22 | * | |||
| Eleusine indica | GC | 1 | 22 | * | |||
| Setaria faberii | GC | 1 | 22 | * | |||
| Sionit & Patterson (1985) | Digitaria sanguinalis Echinochloa crus-galli Eleusine indica Setaria faberii | GC GC GC GC | 2222 | 43434343 | * *** | ||
| Thompson & Drake (1994) | Spartina patens | OTC | G | 1600 | |||
| Volin & Reich (1996) | Bouteloua curtipendula | GC | 2.5 | 58 | |||
| Wand et al. (1996) | Themeda triandra | OTC | 3.9 | 210 | |||
| Wilsey et al. (1994) | Sporobolus kentrophyllus | GC | 4.2 | 42 | |||
| Wilsey et al. (1997) | Sporobolus kentrophyllus Paspalum dilatum Digitaria macroblephara Themeda triandra | GC GC GC GC | 2222 | 75757575 | |||
| Wray & Strain (1986) | Andropogon virginicus | GC | 0.5 | 56 | * | ||
| Wray & Strain (1987) | Andropogon virginicus | GC | 0.5 | 63 | |||
| Ziska et al. (1990) | Spartina patens | OTC | G | 500 | |||




