Soybean leaf growth and gas exchange response to drought under carbon dioxide enrichment
Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the US Department of Agriculture and does not imply approval or the exclusion of other products that may also be suitable.
Abstract
This study was conducted to determine the response in leaf growth and gas exchange of soybean (Glycine max Merr.) to the combined effects of water deficits and carbon dioxide (CO2) enrichment. Plants grown in pots were allowed to develop initially in a glasshouse under ambient CO2 and well-watered conditions. Four-week old plants were transferred into two different glasshouses with either ambient (360 μmol mol-1) or elevated (700 μmol mol-1) CO2. Following a 2-day acclimation period, the soil of the drought-stressed pots was allowed to dry slowly over a 2-week period. The stressed pots were watered daily so that the soil dried at an equivalent rate under the two CO2 levels. Elevated [CO2] decreased water loss rate and increased leaf area development and photosynthetic rate under both well-watered and drought-stressed conditions. There was, however, no significant effect of [CO2] in the response relative to soil water content of normalized leaf gas exchange and leaf area. The drought response based on soil water content for transpiration, leaf area, and photosynthesis provide an effective method for describing the responses of soybean physiological processes to the available soil water, independent of [CO2].
Abbreviations
-
- FTSW
-
- Fraction Transpiration Soil Water
-
- NLA
-
- Normalized Leaf Area
-
- NPn
-
- Normalized Photosynthesis
-
- NTR
-
- Normalized Transpiration Rate
Introduction
The rise in global atmospheric carbon dioxide concentration ([CO2]) and its potential impacts on climate change have been well documented ( Tans et al. 1990 ; Allen et al. 1994 ; Keeling et al. 1995 ). The current [CO2] (about 360 μmol mol–1) has been projected to double by the middle of the next century (e.g. Watson et al. 1990 ). According to climate models, increases in atmospheric [CO2] and other greenhouse gases, such as methane, nitrous oxide and CFCs, have been predicted to cause corresponding rises in global air temperatures and shifts in precipitation patterns (e.g. Manabe & Wetherald 1987; Wilson & Mitchell 1987; Hansen et al. 1988 ). Although model results indicate that increasing [CO2] may result in a slight increase in global mean precipitation, large uncertainties exist at the regional scale due to the large spatial-temporal variability of precipitation events and intensity ( Wigley et al. 1986 ).
A general circulation model coupled with a time-dependent climate-change experiment predicted recently that precipitation and soil moisture in southern Europe and central North America would be reduced in a climate of greater atmospheric [CO2] ( Gregory et al. 1997 ). Because soil water availability is the environmental factor most limiting for crop growth ( Boyer 1982), it is crucial to analyse the possible interactions of water deficits and [CO2] upon major crops such as soybean. If there is a fundamental change in plant responses to soil water content, then plant growth under climate changes associated with less precipitation might be either aggravated or lessened as compared to what is expected using response functions developed for current CO2 levels.
Under well-watered conditions, increases in soybean growth, development, and yield resulting from [CO2] enrichment have been well documented ( Ackerson et al. 1984 ; Havelka et al. 1984 ; Rogers et al. 1984 ; Allen et al. 1991 ). The stimulatory effect of elevated atmospheric [CO2] on net photosynthesis, which is temperature-dependent, is primarily caused by increased [CO2] in the chloroplast. Ribulose-1,5-bisphosphate carboxylase/oxygenase is not saturated by CO2 under current atmospheric concentrations, so an increase in [CO2] increases the rate of carboxylation in C3 plants such as soybean (e.g. Vu et al. 1997 ). In addition, carbon dioxide enrichment causes partial stomatal closure, decreased stomatal conductance, and results in reduced canopy or single leaflet transpiration rate ( Morison & Gifford 1984; Rogers et al. 1984 ; Jones et al. 1985 ). However, leaf area is also increased with [CO2] enrichment, so that the surface area available for transpiration increases, which offsets some of the water savings from [CO2] enrichment ( Morison & Gifford 1984; Allen et al. 1985 ; Jones et al. 1985 ). Also, the stomatal closure caused by [CO2] enrichment results in increased leaf temperature, and thus an increase in the vapour pressure gradient between leaf and air. This also counterbalances some of the water savings from [CO2] enrichment ( Allen et al. 1985 ).
There have been, however, only a few investigations that have considered the direct consequences of soil water deficits imposed on plants that are exposed to [CO2]-enriched atmospheres. Sionit et al. (1980) found in wheat (Triticum aestivum L.) that leaf water potential decreased more slowly and did not reach as low a value in plants subjected to 1000 μmol CO2 mol–1 atmosphere as for those in 300 μmol CO2 mol–1. Similarly, whole canopy CO2 assimilation and transpiration of soybean ( Allen et al. 1994 ) and rice (Oryza sativa L.) ( Baker et al. 1997a , b) decreased more slowly and to a lesser extent when exposed to elevated [CO2] as compared to ambient [CO2]. In none of these studies was attention given to determining whether the observed responses to soil drying were simply because of soil water conservation as a result of decreased transpiration under elevated [CO2], or because CO2 enrichment resulted in an inherent change in plant response to decreasing soil water content.
Morison & Gifford (1984) grew 16 plant species from seeds with no rewatering of the pots under an ambient CO2 atmosphere and under an atmosphere with CO2 elevated by 340 μmol CO2 mol–1. These experiments, which lasted for 50 d or more, showed no difference in the rate of soil moisture decline for plants subjected to the two CO2 treatments. This resulted because the rate of water loss between the CO2 treatments was compensated between changes in leaf area development and in stomatal conductance. The long-term nature of these experiments during exponential growth make it difficult to resolve the response of individual processes to changes in soil water content. Although leaf area development and transpiration seemed to decline at approximately the same soil water content in the two CO2 treatments, the confounding effects of the two processes and the variability in the data precluded a critical examination of any variation in their response to soil water content.
An approach that is especially useful in comparing plant responses to water deficits is to express responses as a function of available, volumetric soil water content ( Ritchie 1981). By expressing response in terms of relative available soil water content, it is possible to resolve the critical issue of whether there is any difference in plant response at equivalent levels of soil water deficit. This approach has proved effective in making comparisons among plant species ( Sinclair & Ludlow 1986) and cultivars ( Ray & Sinclair 1997).
The objective of this research was to determine if there was any change in plant response to soil water content as a result of plant exposure to increased [CO2]. The possibility for such a change in response to soil water may result from changes in the regulatory processes in the plant as a result of increased [CO2] and greater levels of photosynthate in the plant ( Allen et al. 1988 ). For example, there is a possibility that enhanced photosynthate may result in physiological changes in the plant that result in a shift in the threshold soil water content at which stomatal conductance decreases, as compared to the threshold obtained under current atmospheric [CO2]. In these comparisons, plant response was characterized by leaf gas exchange and leaf area development as a function of relative soil water content. An important feature of this study was to subject plants of similar size to soil drying at equivalent, but realistic rates of soil drying, so that the responses of specific traits could be isolated.
Materials and methods
Plant culture
Two nearly identical experiments were performed in which soybean plants were exposed to differing atmospheres of [CO2] while being subjected to drying soil. All plants were initially grown in a common glasshouse under well-watered conditions and ambient [CO2], before initiating the experimental treatments. Therefore, differences among plants that might develop in the initial phases of growth and confound treatment response to drought were eliminated. Seeds of soybean cv Braxton were sown in pots (10 cm diameter × 30 cm tall) filled with a mixture of 2/3 potting soil (Vitagreen, Inc., Clermont, FL) and 1/3 vegetable plug mix (W.R. Grace and Co., Cambridge, MA). Each pot contained about 3 kg of soil mixture inoculated with a commercial preparation of Bradyrhizobium japonicum (Nitragin, Milwaukee, WI). Natural light was supplemented with incandescent lamps to give a 16 h photoperiod so that the plants remained in vegetative development throughout the experiment. Day/night temperatures were ≈ 28 °C/20 °C. Pots were overseeded and thinned to one plant per pot after emergence.
When the plants were 4 weeks old and had reached the V4 to V5 growth stage ( Fehr et al. 1971 ), the plants in each of the experiments were split into two groups and transferred to adjacent glasshouses for the experimental treatments. The daytime CO2 concentration of one glasshouse was at ambient levels (≈ 360 μmol mol–1) and the other was maintained above ambient, at a concentration of 700 μmol mol–1. For experiment 1, 23 plants were transferred to the ambient [CO2] glasshouse and 20 plants were transferred to the 700 μmol mol–1 glasshouse on 20 October 1995. More plants were used in experiment 2 with 37 plants placed in the ambient [CO2] glasshouse and 30 plants placed in the high [CO2] glasshouse on 17 April 1996. Plants were separated so as to be positioned as individuals and not as a closed canopy.
The elongated glasshouses (27.4 m) had a semicircular cross-section with maximum width (4.3 m) at the base. Air was drawn from the outside atmosphere at the open end of the glasshouse and through the glasshouse by computer-controlled variable-speed fans at one end. Carbon dioxide was injected through a controlled valve into a pre-diluted air ductwork arch located 1.2 m inside the entry end of the house. Jets of enriched air from the duct provided uniformly enriched air to the glasshouse. Carbon dioxide injection rate was computer-controlled based on ventilation fan speed and a proportionalintegral-differential algorithm. Continuous air sampling at 7 m downstream from the injection provided data for the algorithm. The soybean plants were located 2.4 m downstream from the CO2 injection point. In each glasshouse, no plants were positioned between the glasshouse entry and the experimental plants so that the atmospheric vapour pressure in the two glasshouses was equivalent.
Water deficit treatment
Following transfer of plants to the CO2-exposure glasshouses, the plants were allowed to acclimate for 2 days before beginning the soil drying experiment. After acclimation, a two-piece lid was attached to the top of each pot and sealed around the plant stem to prevent direct soil evaporation. There was a small port in each lid through which the pots could be rewatered as needed. On the afternoon preceding the initiation of the experiment, all pots were fully watered and allowed to drain overnight. After draining, the pots were weighed to determine the hydrated weight of the pot. This first day when the initial weight of the pot was measured was defined as day one of the experiment (1 DOE).
In the following days the well-watered pots were allowed to dry to a weight 200 g less than the fully hydrated weight, and then watered daily to return the pots to this weight. The drought-stressed pots were allowed to dry throughout the duration of the experiment. Because [CO2] can affect soil water depletion rate and timing, the pots in the drought-stressed treatments were watered daily so that the daily net water loss was held to 70 g. Consequently, the daily rate in the development of soil water deficit was equivalent under the two [CO2] treatments.
The experiment was terminated when all the soil water in the drought-stressed pots had decreased to a level where there was no longer soil water available to support transpiration. This endpoint, as defined by Sinclair & Ludlow (1986), was identified when the daily transpiration rate of a drought-stressed plant had decreased to less than 10% of the well-watered plants. The dehydration of the pots took 17 d in both experiments. There was no significant difference between CO2 treatments in the total amount of stored transpirable water available from the soil in each of the experiments.
The severity of drought stress in each pot on each day was calculated based on the decrease in pot weight below that of the hydrated weight. To facilitate comparison of the results of these experiments with other experiments that have reported plant response to fraction of available soil water, the soil water content on each day was expressed as a fraction of the total available soil water. In this case, the concept of fraction of transpirable soil water (FTSW) proposed by Sinclair & Ludlow (1986) was used. The daily FTSW was calculated as the ratio between the amount of transpirable soil water remaining in the pot on each day and the total amount of stored transpirable soil water determined for that pot. The calculation of FTSW, based only on soil water content, has been fully described by Sinclair & Ludlow (1986) and Ray & Sinclair (1997).
Plant gas exchange
Daily transpiration rate for each plant was obtained from the difference in weight of each pot measured on successive afternoons. Because of daily variations in the environment, daily values of plant transpiration rates were variable. To reduce the level of variability, the daily transpiration rate of each drought-stressed plant was normalized against the mean rate of the well-watered plants subjected to the same CO2 treatments. Therefore, a normalized transpiration rate (NTR) for each drought-stressed plant was calculated for each day. Five plants in each [CO2] treatment and water regime were measured for transpiration rate through the complete 17-d dehydration cycle.
In addition to the determination of plant transpiration rate on each day, leaf photosynthetic rate was measured in experiment 1 on each day when PAR flux density exceeded 1000 μmol m–2 s–1. The photosynthetic measures were made on the central leaflet of the first uppermost fully developed leaf using a LI-6200 (LI-COR, Inc. Lincoln, NE). These measurements were taken between 12.00 and 13.00 hours EST and expressed per unit leaf area. One leaflet for each replicate plant of each treatment was measured.
Leaf area expansion
Leaf area was estimated on each day for the five plants in each [CO2] and water regime treatments except for the last 2 d of the dehydration cycle when the leaves were shrivelled, making accurate measurement difficult. The plant leaf area was estimated from measures of the length of the central leaflet of each leaf on the plant. The area of the individual leaves was calculated from leaflet length measurements using the following equation, which was based on measurements of 75 leaves of Braxton soybean using a LI-3000 portable area meter and a LI-3050 A transparent belt conveyer (Li-COR, Lincoln, NE):
Leaf area (cm2) = 1.324 * (leaflet length, cm)2 (r2 = 0.97**).
Daily leaf area expansion was calculated as the difference between current leaf area and leaf area on the previous day. Normalized leaf area expansion (NLA) for each drought-stressed plant was calculated each day as described above, for NTR.
Plant sampling
Plants were destructively sampled during the experiments to determine dry matter accumulation in various components of the plant. An initial harvest was carried out only on plants from the ambient [CO2] glasshouse. Three plants were harvested on 2 DOE in experiment 1 and seven plants were harvested on 1 DOE in experiment 2. All subsequent harvests were of five plants from each [CO2] treatment and watering regime. The additional harvests were on 10 and 17 DOE of experiment 1 and on 8, 13, 18 DOE of experiment 2. Each plant was detached at ground level and separated into shoots, roots and nodules in experiment 1, and leaves, stems, petioles, roots and nodules in experiment 2. Dry weights were determined after oven drying at 70 °C for 48 h.
Biomass data were statistically analysed using the general linear model procedure (GLM) of SAS Institute (1988), with models appropriate to the experimental design. Individual plants were the replicates in this analysis among treatments.
Results
Exposure of soybean plants to 700 μmol mol–1[CO2] significantly decreased daily transpiration compared to the 360 μmol mol–1[CO2] treatment, regardless of the water treatment. The curve of cumulative transpiration vs. time was represented as a linear regression model for the well-watered plants and a polynomial regression for the drought-stressed ones ( Fig. 1). Comparison (t-test, P < 0.05) of the slopes of the linear regressions obtained for well-watered plants showed that the elevated [CO2] resulted in a significant 25% decrease of the average water loss, compared to the ambient [CO2] treatment. The total amount of water lost in transpiration under drought over the 17-d experiment period averaged 55 and 67% of the well-watered plants for the 360 and 700 μmol mol–1[CO2] treatments, respectively.
Cumulative total transpiration per plant vs. time for soybean exposed to two atmospheric CO2 concentrations and two watering treatments in experiment 1. Each data point is the mean of five plants (n = 5). Error bars are ± SE of the mean.
The soil water content as reflected in the FTSW data decreased gradually during the 17-d stress period, for both experiments and both CO2 levels ( Fig. 2). As soil water content was adjusted daily in the drought-stressed pots by adding water, there was no significant difference (t-test, P < 0.05) among [CO2] treatments in the progressive decrease in soil water content (FTSW) against time ( Fig. 1). The well-watered treatments were maintained daily at a FTSW value close to 0.75 during the experiments.
Fraction of transpirable soil water (FTSW) plotted as a function of time in two soybean glasshouse experiments, during a dehydration cycle in the presence of two atmospheric CO2 concentrations. Each value is derived from the mean of five pots.
Biomass accumulation in different plant tissues for experiments 1 and 2 are given in Tables 1 and 2, respectively. There was no significant effect of drought or [CO2] treatments on dry matter partitioning before 10 DOE in experiment 1 and 13 DOE in experiment 2. Even at the final harvest, there were no significant effects of [CO2] increase on DW in well-watered plants, except for an increase in leaf DW at 18 DOE in experiment 2. By contrast, the elevated [CO2] resulted in plants under drought having significant DW increases by the final harvest in shoot, root and nodule mass relative to those under ambient CO2. For drought-stressed treatments, total plant biomass at the final harvest was 37 and 30% higher for elevated [CO2] than for the ambient [CO2], in experiments 1 and 2, respectively. The beneficial effect of elevated [CO2] on biomass accumulation under drought was slightly greater in nodules, where it averaged 59 and 32% in experiments 1 and 2, respectively ( Tables 1 and 2). The proportion of root DW relative to total plant biomass increased notably under drought. In both experiments, root DW did not show any drought-induced decline under elevated [CO2], whereas a significant decline was observed under ambient [CO2] ( Tables 1 and 2).
| Treatment (μmol mol–1 CO2) | ||||
|---|---|---|---|---|
| 360 | 700 | |||
| WW | DS | WW | DS | |
| 2 DOE (n = 3) | ||||
| Shoot | 1.95 | – | – | – |
| Root | 0.68 | – | – | – |
| Nodule | 0.13 | – | – | – |
| Total | 2.76 | – | – | – |
| 10 DOE (n = 5) | ||||
| Shoot | 4.02a † | 3.84a | 5.46a | 4.84a |
| Root | 1.18b | 1.36b | 1.65ab | 2.51a |
| Nodule | 0.46a | 0.41a | 0.54a | 0.50a |
| Total | 5.66a | 5.61a | 7.65a | 7.85a |
| 17 DAT (n = 5) | ||||
| Shoot | 8.91a | 4.81c | 9.79a | 6.55b |
| Root | 2.73a | 1.96b | 2.62a | 2.61a |
| Nodule | 1.04ab | 0.56c | 1.09a | 0.89b |
| Total | 12.67a | 7.34c | 13.50a | 10.04b |
- † Means followed by the same letter within a row were not significantly different as determined by LSD (P≤ 0.05).
| Treatment (μmol mol–1 CO2) | ||||
|---|---|---|---|---|
| 360 | 700 | |||
| WW | DS | WW | DS | |
| 1 DOE (n = 7) | ||||
| Leaf | 0.96 | – | – | – |
| Petiole | 0.17 | – | – | – |
| Stem | 0.43 | – | – | – |
| Root | 0.73 | – | – | – |
| Nodule | 0.10 | – | – | – |
| Total | 2.40 | |||
| 8 DOE (n = 5) | ||||
| Leaf | 3.92a † | 3.91a | 4.21a | 4.71a |
| Petiole | 0.67a | 0.64a | 0.71a | 0.68a |
| Stem | 1.78a | 2.15a | 1.85a | 2.10a |
| Root | 1.72a | 2.11a | 1.92a | 1.97a |
| Nodule | 0.61a | 0.73a | 0.66a | 0.68a |
| Total | 8.70a | 9.53a | 9.36a | 10.15a |
| 13 DOE (n = 5) | ||||
| Leaf | 5.70a | 3.59b | 5.79a | 4.70a |
| Petiole | 1.11a | 0.78b | 1.03ab | 0.83b |
| Stem | 3.10a | 2.07b | 2.75ab | 2.59ab |
| Root | 2.78ab | 2.31ab | 2.19b | 2.83a |
| Nodule | 1.17a | 0.77b | 0.96b | 0.91b |
| Total | 13.86a | 9.53b | 12.72a | 11.85ab |
| 18 DOE (n = 5) | ||||
| Leaf | 7.44b | 3.59c | 9.25a | 4.24c |
| Petiole | 1.76a | 0.85b | 1.86a | 0.98b |
| Stem | 4.55a | 2.15c | 5.02a | 3.20b |
| Root | 3.63a | 2.80b | 3.35ab | 3.73a |
| Nodule | 1.69a | 0.98c | 1.63a | 1.29b |
| Total | 19.06a | 10.38c | 21.10a | 13.44b |
- † Means followed by the same letter within a row were not significantly different as determined by LSD (P≤ 0.05).
Similar to transpiration, the curves of cumulative leaf area vs. time fitted a linear regression model for the well-watered plants and a polynomial regression for the drought-stressed ones. The comparison of these models showed that exposure to 700 μmol mol–1[CO2] increased leaf area significantly (t-test, P < 0.05) compared to the 360 μmol mol–1[CO2] treatment, regardless of the water treatment ( Fig. 3).The total gain of leaf area due to [CO2] increase over the 17-d experiment period averaged 12 and 10% in well-watered and drought-stressed conditions, respectively.
Total leaf area development per plant vs. time for soybean exposed to two atmospheric CO2 concentrations and two watering treatments in experiment 1. Each data point is the mean of five plants (n = 5). Error bars are ± SE of the mean.
Figure 4 shows the response of photosynthetic rate to drought and [CO2]. Regardless of water treatment, Pn was consistently higher in the 700 than the 360 μmol mol–1[CO2] treatments.
Leaf net photosynthesis (Pn) vs. time for soybean exposed to two atmospheric CO2 concentrations and two watering treatments in experiment 1. Each data point is the mean of five plants (n = 5). Error bars are ± SE of the mean.
For both [CO2] conditions, drought stress induced a significant decrease in leaf gas exchange and growth as the soil dried. There was, however, no significant effect of CO2 treatment on the relative response of leaf gas exchange or leaf growth to FTSW. For example, in experiment 1 ( Fig. 5a,b,c) the patterns of NTR, NLA, and normalized photosynthesis rate (NPn) plotted against FTSW for both CO2 treatments were similar.
Experiment 1 results for normalized transpiration (a) normalized leaf area (b), and normalized leaf net photosynthesis (c) as a function of fraction of transpirable soil water (FTSW) for soybean exposed to two atmospheric CO2 concentrations during a dehydration cycle.
The regression of NTR and NLA against FTSW for data combined from both experiments and both [CO2] treatments gives the following logistic equations:
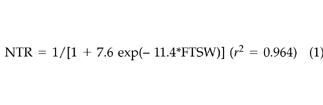 (1)
(1)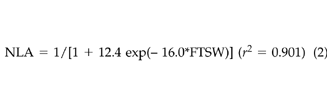 (2)
(2)These results indicated that for both [CO2] treatments little change in NTR and NLA occurred with FTSW values greater than 0.4. As soil dried to FTSW values less than 0.3, NTR, NLA, and NPn decreased almost linearly.
Discussion
The short-term exposure of soybean plants to elevated CO2 in these experiments resulted in decreased water loss ( Fig. 2) and increased leaf area development ( Fig. 3) and photosynthesis ( Fig. 4) under both well-watered and drought-stressed conditions. These results are similar to those of Morison & Gifford (1984), who studied 16 plant species and found decreased transpiration rate per unit leaf area and increased leaf area development for plants that were subjected to elevated CO2. Similarly, Jones et al. (1985) found for soybean that [CO2] enrichment caused increased stomatal resistance and reduced leaf transpiration. Probably as a consequence of exposing the plants in our experiments to enriched CO2 for only a relatively short time, there was no statistically significant effect of [CO2] increase on biomass accumulation in well-watered plants ( Tables 1 and 2) except for a slight increase in leaf DW at 18 DOE in experiment 2. By contrast, the elevated CO2 allowed the plants under drought to sustain significant increases in shoot, root and nodule mass relative to those under ambient CO2.
The stimulatory effect of [CO2] on plant growth response to drought in previous experiments could result from both a reduction of water loss and an increase in carbon assimilation. An obvious effect of elevated [CO2] is that the reduction in water loss by leaf transpiration ( Fig. 2) could lead to a delay in the onset of drought stress under high CO2 ( Morison 1993; Baker et al. 1997a , b). Importantly, in the current study soil water content was adjusted daily in the drought-stressed treatments so that the CO2-effect of differences in the development of soil water deficits as a result of CO2 treatments were eliminated ( Fig. 1).
The results of these two experiments showed that there was no significant effect of changes in atmospheric [CO2] on the relative response to soil water content of normalized transpiration, leaf area and photosynthetic rate ( Fig. 5). The pattern of NTR, NLA and NPn to FTSW observed for both [CO2] treatments was similar to results obtained with other crop species and experimental conditions carried out under ambient CO2 ( Meyer & Green 1981; Ritchie 1981; Sinclair & Ludlow 1986; Rosenthal et al. 1987 ). In particular, little change in the behaviour of processes sensitive to water deficit were observed with soil drying across a wide range of FTSW. In soybean, NTR, NLA and NPn fitted similar logistic curves for both [CO2] treatments and started to decrease for FTSW between 0.3 and 0.4.
In summary, the results of these experiments confirmed the beneficial effects of high [CO2] on soybean exposed to drought stress. Virtually all the benefit of elevated [CO2] for the drought-stressed plants resulted from soil water conservation associated with decreased transpiration rate. This decrease in the rate of loss of soil water under elevated CO2 will be particularly important for crop production under global environment change. Without an increase in temperature, this means that crops will be able to withstand less frequent rains without being subjected to drought stress. If temperatures do increase, the increased transpiration rates driven by the higher temperatures will be offset to some extent by water conservation under elevated CO2. The important original finding of this research, however, is that no fundamental change in the plant response of transpiration, leaf area development, or photosynthesis as functions of soil water content results from plant exposure to elevated CO2. Therefore, past investigations on anticipated responses of crops to climate changes involving precipitation changes need not be rejected because of inappropriate drought response functions. Our results indicated a strong consistency in the relationship of NTR, NLA and NPn to FTSW independent of [CO2] treatments.
Acknowledgements
Partial support for this research was provided by United Soybean Board project #6010P. We thank Bertrand Parisseaux and Patrice Mahieu (ENSA, Montpellier, France) for their valuable technical help.




