Enforced cytokinesis without complete nuclear division in embryonic cells depleting the activity of DNA topoisomerase IIα
Communicated by: Hiroshi Handa
Abstract
Background: There are two distinct DNA topoisomerase II (topo II) isoforms, designated topo IIα and topo IIβ, in mammalian cells. The function of topo IIα in the development of mammalian cells has not been elucidated because of a lack of topo IIα mutants.
Results: We generated mice with a targeted disruption of the topo IIα gene. The development of topo IIα−/– embryos was terminated at the 4- or 8-cell stage. When wild-type embryos at the 2- or 4-cell stage were treated with ICRF-193, a catalytic inhibitor of topo II, nuclear division occurred followed by cytokinesis to form 4 or 8 cells, respectively, then development was terminated. Microscope analysis of 4,6-diamidino-2-phenylindole (DAPI)-stained nuclei of both topo IIα−/– and ICFR-193-treated embryonic cells revealed a droplet-like structure connecting the terminals of two adjacent nuclei forming a bridge-like structure. Phosphorylated histone H3, a marker for the M phases, disappeared from the nuclei of the topo IIα-depleted embryonic cells. Laser scanning cytometry of the topo IIα-depleted cells revealed the presence of 2N DNA cells.
Conclusions: Our results indicate that topo IIα has an essential role in the early stages of mouse development and that depletion of topo IIα from the embryonic cells causes incomplete nuclear division followed by enforced cytokinesis.
Introduction
DNA topoisomerases (topo) are enzymes that alter the topological structure of chromosomal DNA and perform essential functions in DNA replication, chromosome condensation, transcription and recombination (Wang 1996). They are classified into two categories, topo I and topo II, based on the mode of the enzymatic reaction. Topo I and topo II change the linking numbers by catalysing transient single-stranded and double-stranded breaks in DNA, respectively, followed by re-ligation to reproduce the intact helix.
Studies with yeast temperature-sensitive topo II mutants indicate that this enzyme is essential for the condensation and segregation of chromosomal DNA (DiNardo et al. 1984; Holm et al. 1985; Uemura & Yanagida 1984). Studies with specific topo II inhibitors demonstrate the requirement for the enzyme at the G2/M phases in mammalian cells. ICRF-193, a derivative of bisdioxopiperazine, is a catalytic inhibitor of topo II that does not stabilize cleavable complexes, unlike other topo II inhibitors (Andoh & Ishida 1998; Roca et al. 1994). Cultured mammalian cells treated with ICRF-193 have entangled chromosomes with immature condensation (Anderson & Roberge 1996; Ishida et al. 1994) and arrest at the G2/M phase by a topo IIα-dependent checkpoint control (Downes et al. 1994).
There are two distinct topo II isoforms, designated topo IIα and topo IIβ, in mammalian cells (Chung et al. 1989; Nitiss 1998). There is no significant difference between the two isoforms with respect to biochemical properties (Austin et al. 1995; Dereuddre et al. 1995). Whereas topo IIβ expression is maintained at a relatively constant level throughout the cell cycle, the topo IIα level is significantly increased at the G2/M phases, suggesting that it is necessary at the G2/M phases (Adachi et al. 1997, 2000; Isaacs et al. 1998; Meyer et al. 1997; Woessner et al. 1991). Yang et al. (2000) reported that topo IIβ homozygous knockout mice develop to the neonatal stage, after which they die due to abnormalities in neural development. This finding suggests that topo IIβ is not essential for cell division. The function of topo IIα in the development of mammalian cells, however, has not been elucidated because of a lack of topo IIα mutants. In the present study, we attempted to generate mice with a disrupted topo IIα gene, and examined abnormalities in the cell cycle at the early stages of development. We also examined the effects of ICRF-193 on cell cycle progression in wild-type embryos.
Results
DNA topoisomerase IIα−/– mice have an embryonic lethal phenotype
We generated chimera mice by injecting topo IIα+/– embryonic stem (ES) cells (Kobayashi et al. 2001) into C57BL/6 blastocysts, and chimeric males that transmitted the mutant allele to the germ-line were obtained. Heterozygotes were identified by Southern blot analysis of chromosomal DNA isolated from the tails (Fig. 1B). There was no difference between the wild-type mice and heterozygous mutant mice in terms of growth, health and fertility, even after 1 year (data not shown).
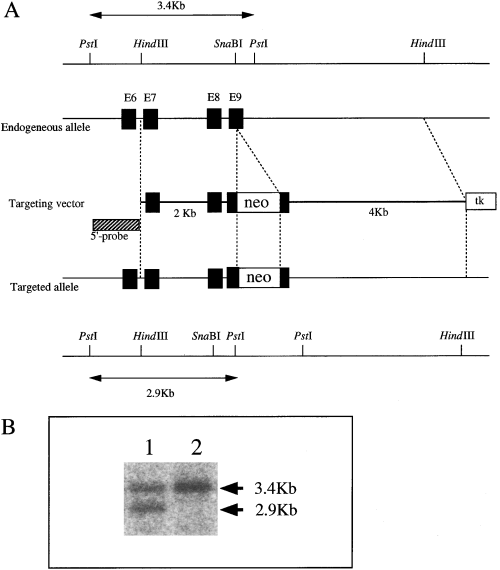
Targeted disruption of the mouse topo IIα gene. (A) Black boxes indicate exons of the mouse topo IIα gene. The neomycin resistance gene (neo) was introduced into exon 9. tk is the herpes simplex virus thymidine kinase gene. (B) Genotyping by Southern blot analysis of representative progeny mice yielded by intercross of topo IIα+/– heterozygotes. DNA extracted from the tails of pups was digested with PstI and hybridized with the 5′-probe. Lane 1, genotype of topo IIα+/– offspring; lane 2, genotype of topo IIα+/+ offspring.
To determine whether the topo IIα−/– mice were viable, we analysed the genotypes of F1 progenies obtained by intercrossing topo IIα+/– heterozygotes. A total of 99 progenies at 4 weeks of age were genotyped, and there were no topo IIα−/– progeny (Table 1). There were also no topo IIα−/– homozygous mutants in the blastocysts obtained by natural mating between heterozygous males and females (Table 1). The ratio of wild-type to heterozygous progeny was 14/25 at 3.5 dpc and 37/62 at 4 weeks, which was close to the expected ratio of 1 : 2. This result indicates that both sperm and oocytes containing the inactive topo ΙΙα allele were derived from a productive gametogenesis and maintained their viability through fertilization, indicating that the gene expression of topo IIα is not required for viability of sperm or oocytes until fertilization.
| Age of progenies | Genotype | ||
|---|---|---|---|
| +/+ | +/– | –/– | |
| 3.5 dpc (blastocyst) | 14 | 25 | 0 |
| 4 weeks | 37 | 62 | 0 |
- For the genomic DNA isolation, blastocysts were frozen, thawed, and incubated at 95 °C for 10 min, followed by treatment with 0.1 mg/mL proteinase K at 55 °C for 6 h. Genomic DNA was isolated from the tail of 4-week-old mice by phenol–chloroform extraction, and the genotype was analysed. Genotypes were determined either by PCR or by Southern blotting: +/+, wild type; +/–, heterozygote; –/–, homozygote.
Among the 90 embryos at the 2- or 4-cell stage obtained by natural mating between heterozygous males and females, 72 (80%) developed to the morula stage in vitro. The rest of the embryos (20%) terminated development at the 4- or 8-cell stage. Immunostaining analysis with anti-topo IIα monoclonal antibody revealed that there was no detectable topo IIα in the nuclei of the embryos that terminated development at the 4- or 8-cell stages (Fig. 2). On the other hand, topo IIα was detected in all nuclei of embryos that developed to the blastocyst stage (data not shown). These results indicated that the development of topo IIα−/– embryos terminated at the 4- or 8-cell stages.
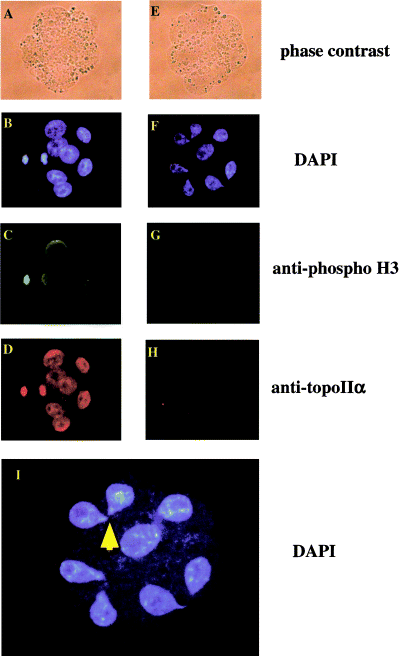
Abnormal shape of the nuclei in topo IIα−/– embryonic cells, and phosphorylation of histone H3 in embryos obtained by intercross of topo IIα+/– heterozygotes. Embryos at the 2- or 4-cell stage, obtained by intercross of topo IIα+/– heterozygotes, were incubated in vitro for 26 h. They were fixed with 1% formaldehyde and stained with DAPI (B, F and I), anti-phosphorylated histone H3 antibody (C and G), or anti-topo IIα antibody (D and H). In the topo IIα-negative cells, histone H3 was not phosphorylated. Panels (A–D), wild-type embryonic cells; panels (E–H), topo IIα−/– embryonic cells. Panel I represents a large magnification (2.5-fold) of panel (F).
4,6-Diamidino-2-phenylindole (DAPI) staining revealed droplet-shaped nuclei in topo IIα−/– embryos at the 4- or 8-cell stage that were strikingly different from the oval-shaped nuclei of wild-type embryos (Fig. 2B,F,I). More than 80% of the topo IIα−/– embryonic cells contained droplet-shaped nuclei. Detailed observation of the droplet-shaped nuclei under a fluorescence microscope revealed that two nuclei were interconnected by their ends, suggesting that cytokinesis proceeds without completion of nuclear division in topo ΙΙα−/– embryos.
Effects of ICRF-193, a topo II inhibitor, on early development of wild-type mouse embryos
Unlike other topo II inhibitors, known as topoisomerase poisons, ICRF-193 does not stabilize cleavable complexes, and does not therefore induce DNA damage (Andoh & Ishida 1998; Clarke et al. 1993; Downes et al. 1994). We examined the effects of 10 µm ICRF-193 on the development of wild-type embryos in vitro. When 2-cell stage embryos were treated with ICRF-193, they developed to the 4-cell stage, although it took longer than embryos that were not treated with the drug (Fig. 3); then development was terminated. When 4-cell stage embryos were treated with ICRF-193, they developed to the 8-cell stage, but further cell division did not take place. The concentration of ICRF-193 needed to block embryo development was 1 µm (data not shown), similar to that reported to inhibit the G2/M phase transition in various mammalian cell lines (Clarke et al. 1993; Downes et al. 1994; Ishida et al. 1994). DAPI staining revealed that more than 80% of the ICRF-193-treated embryonic cells contained droplet-shaped nuclei (Fig. 4B), similar to that observed in topo IIα−/– embryos (Fig. 2F). The droplet-shaped nuclei were connected to each other. The results indicate that cytokinesis occurs in the presence of ICRF-193, even though nuclear division is not completed.

Delaying effect of ICRF-193 on the development of wild-type 2-cell stage embryo. Wild-type 2-cell stage embryos were treated with 10 µm ICRF-193. The ratio between the numbers of divided blastomeres and undivided blastomeres was determined at the times indicated. Open circle; 10 µm ICRF-193. Closed circle; control without ICRF-193. In the development of early embryos, ICRF-193 delayed, but did not inhibit cleavage.
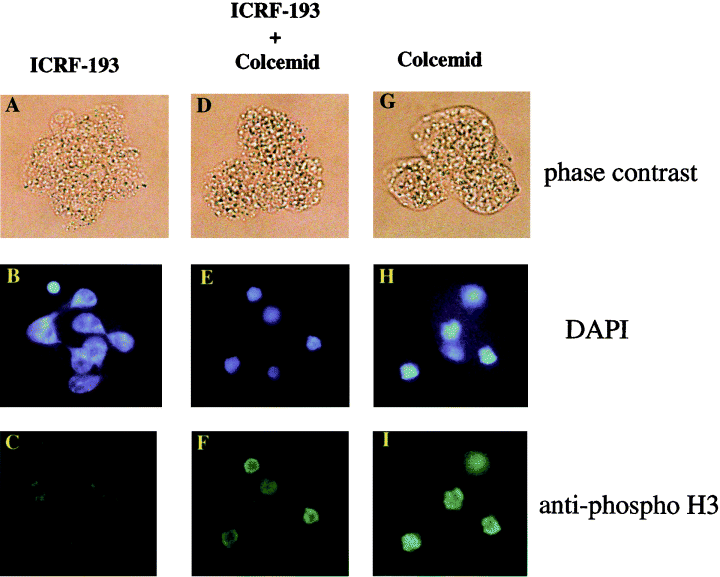
Characterization of wild-type embryonic cells treated with ICRF-193. Four-cell stage embryos at the S phase were incubated for 20 h with 10 µm ICRF-193 (A, B and C), 10 µm ICRF-193 and 100 ng/mL colcemid (D, E and F), or 100 ng/mL colcemid (G, H and I). After the removal of the zonae pellucidae, embryos were fixed with 1% formaldehyde, and stained with DAPI (B, F and H), and anti-phosphorylated histone H3 antibody (C, F and I). The small, round object stained by DAPI in the lower part of panel E is a polar body. The arrows in panel B indicate the points of connection of two adjacent nuclei.
We further examined the presence of phosphorylated histone H3 in the nuclei of topo IIα−/– and ICRF-193-treated embryos. Phosphorylation of histone H3 occurs in late G2 and M phases of the mammalian cell cycle, and is widely used as a marker of cell cycle progression. Phosphorylation of histone H3 starts in the late G2 phase, and appears to spread throughout the condensing chromatin during the M phase. Dephosphorylation of H3 begins in anaphase, and completes prior to chromosome decondensation in telophase cells (Hendzel et al. 1997). When 4-cell stage embryos were treated with colcemid, a tubulin-polymerization inhibitor, cells were arrested at the M phase with highly condensed DNA. We detected the presence of phosphorylated histone H3 in the embryonic cells at this stage (Fig. 4H,I). On the other hand, 4-cell stage embryo nuclei treated with hydroxyurea, a DNA synthesis inhibitor, were not stained with anti-phosphorylated histone H3 antibody (data not shown). These results indicate that the phosphorylation of histone H3 occurs in a cell cycle-dependent manner in embryonic cells. Phosphorylated histone H3 disappeared from the nuclei of topo IIα−/– embryos (Fig. 2G) and ICRF-193-treated embryos (Fig. 4C), suggesting that embryonic cells finish telophase without topo IIα activity. Moreover, the simultaneous treatment of ICRF-193 with colcemid produced results indistinguishable to those of colcemid only (compare Fig. 4E,H, or F,I), indicating that ICRF-193 does not inhibit the condensation of chromosomal DNA and phosphorylation of histone H3 in the embryonic cells at the G2/M phase.
Next, we examined whether the contractile ring appeared at the bridge-like point of the nuclei of the ICRF-193-treated embryos. Two-cell stage embryos were treated with 10 µm ICRF-193 for 24 h and stained with FITC-phalloidin. The ring-like structure was not observed around the bridge-like DNA (data not shown), indicating that the contractile ring is not formed at this stage.
Analyses of DNA content and chromosome condensation by laser-scanning cytometry (LSC) of topo IIα−/– and ICRF-193-treated embryos
To reveal the presence of cells in which cytokinesis was completed in topo IIα−/– or ICRF-193-treated embryonic cells, we examined the DNA content in cells using laser scanning cytometry (LSC) (Darzynkiewicz et al. 1999). As an internal standard for LSC analysis, we prepared embryonic cells containing 2N- and 4N-DNA using hydroxyurea and colcemid treatment, respectively. The zonae pellucidae of the embryos were removed using acidic tyrode solution, and blastomeres were dismembered with trypsin/EDTA. Blastomeres stained by propidium iodide (PI) and anti-topo IIα antibody were spread on glass slides and analysed by LSC. A cytogram of LSC analysis for the mixture of these 2N and 4N control cells is shown in Fig. 5A. There were two distinct groups of cells on the cytogram (Fig. 5A, frames 1 and 2). One group had a low DNA content (2N) with low chromosome condensation (Fig. 5A, frame1), and the other group had high DNA content (4N) with high chromosome condensation (Fig. 5A, frame 2). When stained with PI, cells in the group with low DNA content had the typical shape of interphase nuclei with decondensed chromosomes (Fig. 5C-1), while the high DNA content cells had highly condensed chromosomes characteristic of mitotic nuclei (Fig. 5C-2). Thus, embryonic cells with 2N and 4N DNA are distinguishable by LSC.
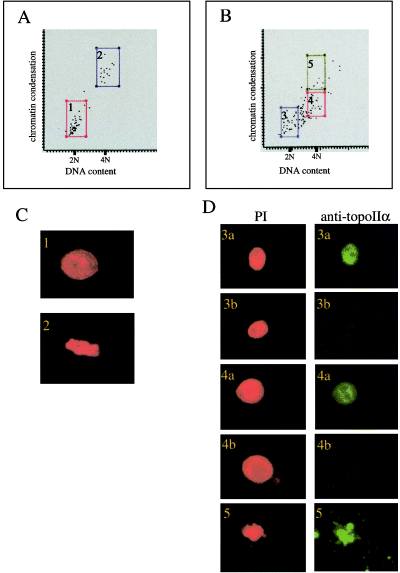
Measurement of DNA content and immunostaining analysis of mouse embryonic cells by laser-scanning cytometry (LSC). (A and B) Two-parameter-cytogram of DNA content and chromosome condensation of the nuclei of embryonic cells. Cells with 2N DNA content were prepared by treatment of late-2-cell stage embryos with 2 mm hydroxyurea for 12 h followed by harvesting the resulting 4-cell embryos arrested at the G1/S boundary. Cells with 4N DNA content were prepared by treatment of 4-cell embryos with 100 ng/mL colcemid for 12 h. The horizontal axis of the cytogram indicates fluorescence intensity of nuclei stained with PI, which corresponds to DNA content. The vertical axis indicates chromosome condensation. (A) Cytogram for the mixture of cells containing 2N- and 4N DNA. (B) Cytogram for the mixture of control cells containing 4N DNA and cells derived from the intercross between topo IIα+/– heterozygotes. (C and D) Images of the nuclei stained with PI (red) and anti-topo IIα antibody (green). The numbers shown in the panels correspond to those in the frames in the cytograms of (A) or (B). In frame 4 of panel (B), none of the cells contain the droplet-shaped nuclei whose terminals are connected.
We then performed an LSC analysis of topo IIα−/– embryonic cells. We cultured 2- or 4-cell stage embryos obtained by intercrosses between topo IIα+/– heterozygotes until embryos that had terminated development appeared. Mitotic cells obtained by treatment with colcemid were added as an internal standard. The cytogram of the LSC analysis is shown in Fig. 5B (a mixture of topo IIα+/+, topo IIα+/– and topo IIα−/–). The dots on the cytogram were divided into three groups based on DNA content and chromosome condensation indices. The dots in frame 5 in Fig. 5B represent mitotic cells which were added as an internal standard, confirmed by staining with PI and anti-topo IIα antibody (Fig. 5D-5). The dots in frames 3 and 4 of Fig. 5B correspond to cells with 2N and 4N DNA, respectively. Immunostaining with anti-topo IIα antibody indicated that there was a mixture of topo IIα-positive and -negative cells in both frames 3 and 4 (Fig. 5D-3a,b, 4a,b). There was a substantial number of cells between 2N DNA (flame 3) and 4N DNA (flame4; Fig. 5B). Over 95% of these cells contained topo IIα.
The majority of topo IIα−/– (13 of 21) and ICRF-193-treated (29 of 56) cells contained 2N DNA. Because the majority of the topo IIα-defective embryo cells contained the droplet-shaped nuclei as described above, these 2N cells might be produced by destroying the thin connective structure between the two droplet-shaped nuclei by either mechanical, chemical (acid tyrode), or enzymatic (trypsin/EDTA) treatment of embryonic cells during preparation of samples for LSC analysis.
The results of the LSC analysis indicated that the presence of 4N cells in topo IIα-depleted cells were caused by either gene knockout or ICRF-193-treatment. In the case of topo IIα−/– cells that were negative for staining by anti-topo IIα antibody, 5 of 21 cells contained 4N DNA (Fig. 5D-4b). In ICRF-193-treated embryonic cells at the 4-cell stage, approximately 10% of the cells contained single nuclei with 4N DNA (data not shown). It might be that a majority of ICRF-193-treated embryonic cells passes through a checkpoint resulting in enforced cytokinesis to form cells with 2N DNA (see Discussion).
Discussion
Topo IIα is essential for early embryogenesis
We attempted to generate mice with a disrupted topo IIα gene. The development of the topo IIα−/– embryos terminated at the 4- or 8-cell stage. This is the first genetic evidence that topo IIα is essential for the early embryogenesis of mice. We also demonstrated that the development of wild-type embryos was terminated by ICRF-193, a catalytic topo II inhibitor. The result supports the idea that topo IIα is essential for early embryogenesis.
None of the cells with a disrupted topo IIα gene terminated development before the 2-cell stage. The development of mice lacking the topo I gene terminates at the 4- or 8-cell stage (Morham et al. 1996), as in case of topo IIα−/– embryos. Maternal stocks of topo I might be present until the 2- or 4-cell stage in the topo I−/– embryos (Morham et al. 1996). We assume that embryonic cells lacking topo IIα develop until the 4- or 8-cell stage by using maternal stocks of the enzyme, as in case of topo I−/– embryos.
Abnormally-shaped nuclei of topo IIα−/– embryonic cells
Phase contrast microscopic analyses indicated that topo IIα−/– embryonic cells produced apparently normal blastomeres, and then stopped developing. DAPI staining of the nuclei revealed that most of the nuclei had an abnormal shape, resembling a droplet that connects the two nuclei at their end-points (Fig. 2I). When 2- or 4-cell stage embryos were treated with ICRF-193, the cells divided into the 4- or 8-cell stage, respectively, and then development terminated. Most of the nuclei in these ICRF-193-treated embryonic cells had a droplet shape with two adjacent nuclei being interconnected to each other (Fig. 4B), as observed in topo IIα−/– embryos. LSC analyses of topo ΙΙα-depleted embryonic cells, either by gene knockout or treatment with ICRF-193, revealed cells containing 2N DNA. The droplet-nuclei might interconnect due to the presence of unresolved catenates after a DNA replication that disturbs the adequate separation of the sister chromatid. Thus, depletion of topo IIα in the embryonic cells might have induced an incomplete nuclear division followed by enforced cytokinesis. Cells in which development was terminated would then undergo apoptosis. The system might function to eliminate embryos with problems during the segregation process of replicated DNA at the G2 phase.
Uemura & Yanagida (1984) reported abnormally shaped nuclei in yeast temperature-sensitive topo II mutants at a restrictive temperature. They demonstrated that the compaction and/or segregation of the nuclear chromatin region failed in the topo II mutants, but a cell plate was formed, which cut across the undivided nucleus. This results in the production of cells split in two and halved cells with a damaged nucleus at the ends. As in case of the yeast mutant, the incomplete segregation of replicated chromosomes might be the reason behind the abnormal shape of the nuclei in embryonic cells of mice with depleted topo IIα.
Previous reports indicated that inhibition of topo II function leads to the cut phenotype in mammalian cells (Gorbsky 1994; Wheatley et al. 1998). Because these inhibitors act on both topo IIα and topo IIβ, it remained uncertain which enzyme was responsible for this phenotype. The present study demonstrates that inhibition of topo IIα, but not topo IIβ, induces the cut phenotype in mammalian cells. Sakaguchi and Kikuchi observed that the expression of small interfering topo IIα RNAs in HeLa cells produced interconnected nuclei (A. Sakaguchi and A. Kikuchi, personal communication); we observed a very similar phenotype in the mouse embryo.
Ishida et al. (1994) demonstrated that when randomly growing mammalian cells were treated with ICRF-193, cells with polyploid nuclei appeared. Using synchronized cells, they showed that the late stages of chromosome condensation and segregation were blocked by ICRF-193. Other mitotic events, such as the activation of cdc2, spindle apparatus reorganization, and disassembly and reassembly of nuclear envelopes, occurred normally. Thus, the cells treated with ICRF-193 traversed an unusual M phase that was termed ‘absence of chromosome segregation’ (ACS)-M phase (Ishida et al. 1994). Cells then continued through further cell cycle rounds, becoming polyploid and losing viability (Andoh et al. 1993; Ishida et al. 1994). In addition, a minor popu-lation of ICRF-193-treated embryonic cells contained two nuclei (data not shown). This observation was similar to that made by Ishida et al. (1994). The reason for the difference in the response to topo ΙΙα inactivation between cell populations remains to be elucidated. It is possible that the role(s) of the enzyme in early embryos is not limited to the M phase, and that other types of DNA topoisomerases, such as topo I and topo IIβ, might compensate for the role of topo IIα depending on the cell cycle stage. We recently reported that topo IIα is essential for the G0-to-S phase transition in mammalian cells (Hossain et al. 2002). Topo IIα is required for transcription by RNA polymerase II on chromatin templates in vitro (Mondal & Parvin 2001). Thus, topo IIα might be required for the expression of genes whose products are essential for the progression of cell cycle of embryonic cells. Further studies are needed to elucidate various aspects of topo IIα for regulation of the cell cycle.
Experimental procedures
Generation of topo IIα homo-knockout mouse embryos
Topo IIα+/– heterozygous embryonic stem (ES) cells (Kobayashi et al. 2001) were microinjected into C57BL/6 blastocysts and the resulting male chimeras were mated with female C57BL/6 mice, followed by selection of the germ-line transmitters. The mice genotype was determined either by Southern blotting using the 5′ probe (Fig. 1B) or by polymerase chain reaction analysis using the following set of primers: wild-type allele, 5′-GTGAAAGGATTCCGCAGTTACGTG-3′ and 5′-CTGATGAGCCTTCACTGCAACC-3′; mutant allele, 5′-GTCAAGAAGGCGATAGAAGG-3′ and 5′-TCCTGCCGAGAAAGTATCCA-3′. All mice were maintained in a specific pathogen-free animal facility at the Graduate School of Pharmaceutical Sciences of the University of Tokyo.
Chemicals
ICRF-193, hydroxyurea, colcemid, and BrdU were purchased from Zenyaku Kogyo Co. Ltd (Tokyo, Japan), Gibco-BRL (Rockville, MD), Wako Pure Chemicals (Osaka, Japan), and Roche Diagnostics GmbH (Mannheim Germany), respectively. Phosphate-buffered saline without divalent cations (PBS(–)) was obtained from Gibco-BRL (Rockville, MD). All other chemicals were reagent-grade.
Collection and genotyping of embryos
Embryos at the 2- and 4-cell stage were collected from oviducts of mice that were mated after superovulation. The embryos were incubated in M16 medium at 37 °C in 5% CO2. Genotyping of embryos was performed by polymerase chain reaction using the following set of primers: wild-type allele, 5′-CGGTGGGAAGTGTGCTTAACAATGAGCGAG-3′ and 5′-CTGATGAGCCTTCACTGCAACC-3′; mutant allele, 5′-TGCATACGCTTGATCCGGCTAC-3′ and 5′-ATCCCCTCAGAAGAACTCGTC-3′.
Immunofluorescence microscopy
Immunocytochemical analysis was performed as previously described, with some modifications (Morham et al. 1996). Briefly, the embryos were fixed with 1% formaldehyde at room temperature for 20 min followed by incubation with 0.5% Triton X-100 for 20 min. After immersing in PBS(–) with 1% bovine serum albumin at room temperature for 10 min, the embryos were incubated with the first antibody at 37 °C for 30 min. Embryos were washed with PBS(–), followed by incubation with fluorescence-labelled secondary antibody at 37 °C for 30 min, and observed under fluorescence. Rabbit anti-phosphorylated histone H3 antibody was purchased from Upstate Biotechnology (Lake Placid, NY). Mouse anti-topo IIα antibody was as previously described (Cobb et al. 1999; Sakaguchi et al. 2002). Immune complexes were detected with either Alexa488-conjugated goat anti-rabbit IgG (Green) or with Alexa594-conjugated goat anti-mouse IgG (Red), both of which were purchased from Molecular Probes (Eugene, OR). Nuclei were stained either with (DAPI) (Blue) or with propidium iodide (PI) (Red).
DNA content measurement and immunostaining analysis by laser scanning cytometry (LSC)
Cells with 2N DNA content were prepared by incubating late 2-cell embryos with 2 mm hydroxyurea until the 4-cell stage. Hydroxyurea arrests cells at the G1/S boundary. Cells with 4N DNA content were prepared by incubating 4-cell stage embryos with colcemid, a microtubule-polymerization inhibitor. Colcemid arrests cells at the late M phase. DNA content was analysed by laser scanning cytometry (Olympus Optical Co., Japan). For immunostaining, the zonae pellucidae of embryos was removed using acidic tyrode solution, and blastomeres were dismembered with trypsin/EDTA solution. The dismembered blastomeres were fixed with 0.3% formaldehyde at room temperature for 20 min, followed by incubation with 200 µg/mL digitonin for 20 min. Cells were immersed in PBS(–) containing 1 mg/mL RNaseA and 0.1% bovine serum albumin at 37 °C for 30 min, followed by incubation with anti-topo IIα antibody at 37 °C for 30 min. The cells were then washed with PBS(–), incubated with PI and fluorescence-labelled secondary antibody at 37 °C for 30 min. Immunostained cells were further analysed by LSC (Darzynkiewicz et al. 1999).
Acknowledgements
We gratefully acknowledge the contributions of M. Katsuki, A. Aiba, K. Nakamura, K. Nakao, K.-i. Nakayama, K. Nakayama and T. Tsukiyama in generating knockout mouse embryos. We thank T. Katada, H. Nishina, H. Kurosu, H. Masai and J.M. Kim for helpful discussions. We also acknowledge the help of E. Kage and A. Shimizu at the initial stages of this study.




