‘Arm-reaching’ neurons in the parietal area V6A of the macaque monkey
Abstract
In previous experiments we have found that several cells of area V6A in the macaque superior parietal lobule were activated by small and stereotyped movements of the arms (C. Galletti, P. Fattori, D. F. Kutz & P. P. Battaglini, Eur. J. Neurosci., 1997, 9, 410). This behaviour was not accounted for by retinal information, nor by somatosensory inputs from the arms. We now want to investigate whether V6A neurons are modulated by purposeful movements aimed at reaching visual targets or targets located outside the field of view. V6A neuronal activity was collected while monkeys performed arm movements during an instructed-delay reaching task in darkness. The task required the animal to reach out for a visual target in the peripersonal space and to bring the hand back to its body. Quantitative analysis of neuronal activity carried out on 55 V6A neurons showed that: (i) the great majority of neurons (71%) was significantly modulated during the execution of arm movements; (ii) 30% of neurons were significantly modulated during preparation of reaching; and (iii) modulations during both execution and preparation of reaching occurred in the absence of any visual feedback and were not due to eye movements. V6A reach-related neurons could be useful in guiding the hand to reach its target with or without visual feedback.
Introduction
The caudal portion of the superior parietal lobule (SPL) has recently been demonstrated to be involved in the analysis of visual, as well as extraretinal, information (Galletti et al., 1991, 1995, 1996, 1999; Ferraina et al., 1997, 2001; Snyder et al., 1997, 2000; Nakamura et al., 1999; Battaglia-Mayer et al., 2000). We have demonstrated that area V6A, a visual area located in the caudalmost portion of the SPL (Galletti et al., 1996, 1999), contains cells the activity of which was modulated by pushing or pulling a lever located outside the visual field (Galletti et al., 1997). We now want to study the activity of V6A neurons during large arm movements aimed at reaching targets in the peripersonal space without any visual feedback. We recorded from area V6A during an instructed-delay reaching task performed in darkness, and quantitatively analysed V6A neuronal activity during different time epochs. The majority of V6A neurons were active during execution and/or preparation of reaching. Preliminary results have appeared in abstract form (Fattori et al., 1999).
Materials and methods
Experiments were carried out in accordance with National and European laws on care and use of laboratory animals and were approved by the University of Bologna Bioethical Committee.
Three Macaca fascicularis were trained to sit in a primate chair with the head restrained and to perform arm movements under controlled conditions. Single cell activity was recorded extracellularly from the anterior bank of the parietooccipital sulcus using glass-coated metal microelectrodes with a tip impedance of 0.8–2 MΩ at 1 kHz. Eye movements were recorded simultaneously using an infrared oculometer (Dr Bouis, Karlsruhe, Germany) and sampled at 100 Hz. Button presses were recorded with 1 ms resolution. The surgery to implant the recording apparatus was performed under general anaesthesia (sodium thiopenthal, 8 mg/kg/h, i.v.); adequate measures were taken to minimize pain and discomfort. Extracellular recording techniques and procedures for reconstructing microelectrode penetrations were similar to those described in recent reports (e.g. Galletti et al., 1999).
Area V6A was recognized on functional grounds following the criteria exposed in Galletti et al. (1999). Once a V6A neuron was isolated, we studied the correlation of neuronal activity with behavioural events by presenting objects of interest (e.g. food) within arm's reach. In order to assess whether cell's activity was related to arm movements, the animal was monitored using video-recording techniques and the recorded material was analysed off-line.
The ‘body out/in’ reaching task sketched in Fig. 1A was used to quantitatively study the neuronal modulation due to arm movements. The typical trial, always performed with the arm contralateral to the recording side, began when the monkey pressed a button near its chest, outside its field of view (Fig. 1A, a). After 200–500 ms, a light emitting diode (LED, mounted on a microswitch on a panel in front of the animal) lit green (Fig. 1A, b). The monkey had to wait for LED change in colour, without performing any arm movement (Fig. 1A, c). After a delay period of 0.5–2 s, the LED turned to red (Fig. 1A, d). This was the go-signal for the monkey to release the button and perform a forward arm movement to reach the LED (Fig. 1A, e), and to press it within given reaction and movement times (350 and 700 ms upper limits, respectively). The animal maintained the LED pushed (Fig. 1A, f) till it switched off (generally after 500 ms). This was the go-signal for the monkey to perform a backward arm movement towards the starting position (Fig. 1A, g). The trial finished with the button pressing (Fig. 1A, h), that in turn started another trial.
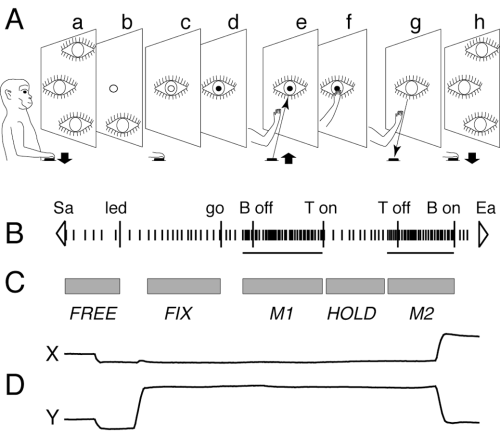
The ‘body out/in’ reaching task. (A) Behavioural events during the reaching task are shown from left to right (a–h). Reaching movements were performed between a push-button (black rectangle) located 5 cm from the monkey chest and a LED placed in front of the animal, in the straight-ahead direction (14 cm from the eyes, 20 cm from the button). Symbols: eye, gaze direction; open circle, target lit green; closed circle, target lit red. (B) Typical example of neural activity during a trial, and behavioural markers. Short vertical thick lines are spikes. Horizontal lines below spikes are neuronal response duration, as calculated by CP algorithm (see text). White triangles: start acquisition (Sa) and end acquisition (Ea). Long vertical thick lines are behavioural markers labelled as follows: led, target on; go, target change in colour; B off, release of the button; T on, touch of the LED; T off, release of the LED; B on, touch of the button. (C) Grey rectangles show time epochs (see text). (D) X and Y components of eye movement traces during a single trial.
We tried to correlate, trial by trial, the neural discharge with arm and eye movement and position. The quantitative analysis was carried out on blocks of at least 15 repetitions. As shown in Fig. 1C, each trial was subdivided into time epochs primarily based not on physical events, but on neuronal (Fig. 1B) or ocular (Fig. 1D) events. These ‘functional’ time epochs were:
FREE: from the beginning of the trial to the lighting of the LED.
FIX: steady fixation on the LED during delay period; it was calculated on single trial basis and had variable duration.
M1 (forward reach movement) and M2 (backward reach movement): periods of neural modulation related to arm movements towards the LED and towards the button, respectively; both could start before the onset of arm movement.
HOLD: from the end of the forward reach (LED pressing) to the onset of neuronal response related to the backward arm movement.
When neuronal activity did not change significantly during M1, M2 or HOLD, fixed durations were set for these three epochs, as follows: M1, M2: from 200 ms before movement onset to movement end; HOLD: from the end of forward reach to the LED switching off.
Cell responses were assessed, trial by trial, by estimating the onset of neuronal-response using the maximum likelihood estimator of a change point (CP) in a sequence of random variables (Seal et al., 1983). For each unit, the mean firing rate was calculated during each of these ‘functional’ epochs, and statistically compared with FREE (two-tailed student t-test; significance level P < 0.01). FREE was chosen as reference because in this epoch no visual stimuli were present, the monkey was not gazing at a fixed position, and was not executing nor preparing any arm movement.
Results
A total of 223 single neurons were recorded from three behaving monkeys in the anterior bank of the parietooccipital sulcus, within the limits of the physiologically defined area V6A (see Galletti et al., 1999) while the animal reached out for visual objects in peripersonal space. About 90% of V6A neurons were activated during arm movements towards objects of interest under normal light conditions. The same movements in darkness abolished or greatly reduced the neural modulation in 104 cells, that were presumably activated in light by visual stimulations of objects present in the visual field and/or by the sight of monkey arm. As we wanted to study the effect on neural activity of arm movement per se, the task we used (‘body out/in’ reaching task, see Methods) was performed in darkness. Great care was taken in reducing the luminance of the LED, which was the target of reaching. It was barely visible by the experimenter, who could not see the monkey hand approaching the target, even in dark-adapted conditions.
Among the V6A neurons that underwent the ‘body out/in’ task, 55 cells were recorded in blocks of at least 15 trial repetitions and were used for quantitative analysis. Forty units were collected from one monkey and 15 from a second one: no functional differences were observed between animals.
The activity of none of the tested cells was significantly modulated by target appearance, nor by its change in colour. Sixteen out of 55 neurons were modulated during FIX, 13 excited and 3 inhibited with respect to FREE. The majority of V6A neurons (39/55; 71%) was significantly modulated during the execution of arm-reaching movements: 69% of them during the forward movement (M1), 56% during the backward one (M2). The modulation was inhibitory in 21% of cases during M1 and in 36% of neurons during M2. Twenty-six per cent of reach-sensitive neurons were modulated by reaches in both directions; half of them showed significant quantitative difference between the two modulations. One-third (33%) of our population showed a modulation during the HOLD period.
Figure 2 shows two V6A units the activity of which was modulated during arm-reaching execution. The first example (Fig. 2A) shows sharp activation during M1 and no significant change of activity during M2. The second unit (Fig. 2B) shows inhibition during M1 and activation during M2. During M1, the eye position was remarkably constant in both units, while during M2 the animal often made saccades. We checked for the possibility that M2 activation in the unit of Fig. 2B was due to changes in gaze direction at the end of trials by correlating neural activity with eye traces, on a trial-by-trial basis. This analysis showed that the neural discharge was not dependent on oculomotor activity as it was equally present in all trials, even in those without saccades during M2 (see trials marked with stars). All neurons were checked in this way before being classified as modulated during reaching execution.
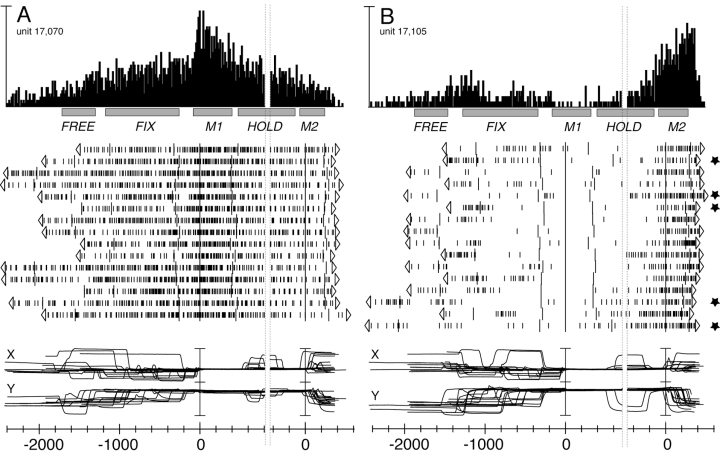
Two V6A neurons modulated during the ‘body out/in’ reaching task. From top to bottom: peri-event time histograms, time epochs, raster displays of impulse activity and recordings of X and Y components of eye positions. Neural activity and eye traces are aligned twice, in both (A) and (B), with the onset of forward and backward movements. Stars in (B) show trials without changes in gaze direction during M2. Vertical scale bar on histogram, 140 (A) and 75 (B) spikes/s; bin size, 15 ms; eye traces, 60 degrees per division. Other details as in Fig. 1.
Figure 3 shows the behaviour of another V6A arm-reaching cell. The unit shown in Fig. 3A, besides a strong modulation during M1, also showed a sudden change in activity during the delay period between target presentation and go-signal. The change in activity is apparently contemporary to the change in gaze direction when the animal decided to fixate on the target of reaching. However, a trial-by-trial comparison between neuronal and oculomotor activities demonstrated that this was not the case. An example of this analysis is shown in Fig. 3B for the three trials indicated by arrows in Fig. 3A. In the first trial, the onset of neuronal discharge was almost coincident with the saccade bringing the eye on the target. In the second trial, showing a saccade almost identical to that in the first one, the discharge was delayed more than 400 ms with respect to the saccade onset. The third trial confirms that the cell discharge during the delay was not due to oculomotor activity, as the eyes maintained fixation on the target from the beginning of the trial, and nevertheless there was a sudden and strong increase of neural discharge during the delay. Note that this discharge was maintained despite the two saccades made before the go-signal and, conversely, saccades at the end of trials did not evoke any discharge at all. In conclusion, in this as well as in many other cells of V6A, the neural modulation during FIX cannot be ascribed to oculomotor behaviour.
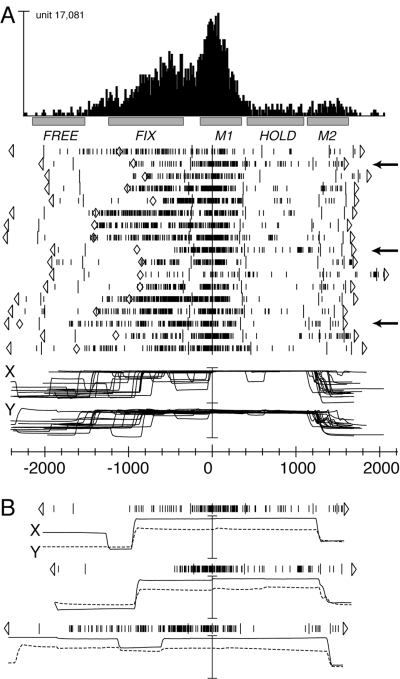
V6A arm-reaching neuron with strong activation during the delay period preceding the arm movement. (A) Data formats are the same as in Fig. 2. Cell activity and eye traces are aligned with the onset of forward arm movement. Diamonds on rasters indicate the onset of the saccadic eye movement bringing the eye to fixate on the reaching target. Arrows indicate the three trials shown separately in B. Vertical scale bar on histogram, 140 spikes/s; other scales as in Fig. 2. (B) Neural activity and eye traces of the three trials indicated by arrows in A. The trials are aligned with the onset of forward arm movement, as in A.
Our data also show that the change in cell activity after target onset cannot be the result of the visual stimulation produced by the lighting of the LED, because the latency of this modulation, as well as its variance, largely exceeded that of visual responses. For the unit shown in Fig. 3, for instance, the increase of activity during the delay period had a mean latency of 675 ms (± 168 SD) with respect to target onset (first behavioural marker on the left in Fig. 3). In the 16 V6A units modulated significantly during FIX, the latency of discharge with respect to target onset was 530 ms (weighted mean), whereas the latency for visual responses in V6A was 60 ms (weighted mean; C. Galletti, unpublished data).
In the hypothesis that the cell's discharge during the delay period could signal the preparation of the impending arm movement, we looked for a correlation between the activity during FIX and that during M1. This analysis showed that in all V6A neurons modulated during FIX (16/55), FIX modulation was of the same sign of M1 modulation, as in the example of Fig. 3.
Discussion
Almost all V6A neurons are modulated during the execution of arm-reaching movements in light, and the majority of them continue to be modulated in darkness. As V6A is a visual area (Galletti et al., 1999), before concluding that the modulation in darkness reflects an encoding of arm reaching-movement, it is important to rule out the possibility that residual visual stimulations or oculomotor signals are responsible for it. Our reaching task was performed in circumstances that minimize the role of vision in reaching: the forward reach movement was performed in darkness with a very dim and small visual target; the backward one was performed in complete darkness, towards an invisible target located outside the visual field. Considering this fact, and that the correlation of neuronal activity with eye movements was carefully checked trial by trial, we are confident that the observed modulation is not dependent on visual input, nor on eye-movement signals.
Possible sources of neuronal modulation during arm movement in our experimental conditions could be somatosensory signals coming from the moving arm (as suggested by somatosensory responses found in V6A, see Galletti et al., 1997) and/or motor-like signals delivered to V6A by motor centres, such as the premotor areas F2 and F7, to which V6A is connected reciprocally (Matelli et al., 1998; Shipp et al., 1998; C. Galletti, unpublished data).
The modulation during the execution of reaching reported here is a new finding for the caudal part of SPL. In a recent study carried out on the dorsal surface of the caudal part of the SPL, a clear neuronal modulation was found for arm reaching in light, but the modulation, if any, was very weak in a darkened environment (see figs 4–7 in Battaglia-Mayer et al., 2000). In another electrophysiological study carried out in the caudal part of SPL, a strong neural modulation during the delay preceding the reaching was observed, but a modulation during the movement of the arm was not reported (Snyder et al., 1997). The recording site of the latter study (parietal reach region, or PRR) included the dorsal surface of SPL and the medial wall of the intraparietal sulcus. The modulation during arm movement execution reported here for V6A, but not in the studies mentioned earlier, could be ascribed to differences in recording site (V6A occupies the anterior bank of the parietooccipital sulcus; see Galletti et al., 1999) or to differences in the tasks used. Concerning this latter point, in the studies mentioned earlier, the task was always a translation of the hand on a coronal plane, whereas in the present study we used a reaching-in-depth task: it has been demonstrated that the two tasks affect differently neuronal activity in other parietal areas, as areas 5 and 7a (MacKay, 1992).
The activity during the delay period before reaching execution was modulated in about 30% of cells in our task. This modulation could, in principle, reflect gaze-related activity as well as movement preparation, as the monkey was fixating on the target of reaching and was planning the ongoing reach movement with the hand motionless near its chest. Our results show that in many cases the modulation observed during the delay was apparently correlated to the saccades, but was actually not time-locked to them (as shown in Fig. 3). In other words, the modulation was not due to a change in gaze direction nor to the fixation on the target of reaching. It could reflect the motor preparation of the forthcoming arm movement, or the ‘intention’ to perform the arm movement, as suggested recently for area PRR (Snyder et al., 1997).
Our data show that the neural activity in V6A is modulated during reaching towards a visual target (from the body to the visual target), as well as during reaching towards a nonvisual, memorized target (from a position in the peripersonal space to the button near the body). Many cells show activity modulated during both reaches, often in different ways. This suggests that V6A reach-related neurons may encode the direction of arm movement. They could be able to guide the arm during reaching movement, no matter whether this action is visually guided or not. These neurons could evaluate whether a match exists between the programmed movement (signalled by corollary discharges from premotor cortex) and the ongoing movement (signalled by somatosensory inputs), and could interact with premotor cortex in order to correct the actually occurring arm movement. When the reaching movement is performed in light, many of these neurons could also use the visual feedback to monitor the actual state and activity of the arm, thus increasing their efficiency in the on-line control of arm movement execution.
Acknowledgements
We thank L. Sabattini and G. Mancinelli for mechanical and electronic assistance, and Dr R. Breveglieri for preparing the figures. This work was supported by grants from MURST, Italy.
Abbreviations
-
- CP
-
- change point
-
- LED
-
- light-emitting diode
-
- SPL
-
- superior parietal lobule.




