Sound lateralization during passive whole-body rotation
Abstract
The effect of passive whole-body rotation about the earth-vertical axis on the lateralization of dichotic sound was investigated in human subjects. Pure-tone pulses (1 kHz; 0.1 s duration) with various interaural time differences were presented via headphones during brief, low-amplitude rotation (angular acceleration 400°/s2; maximum velocity 90°/s; maximum displacement 194°). Subjects made two-alternative forced-choice (left/right) judgements on the acoustic stimuli. The auditory median plane of the head was shifted opposite to the direction of rotation, indicating a shift of the intracranial auditory percept in the direction of rotation. The mean magnitude of the shift was 10.7 µs. This result demonstrates a slight, but significant, influence of rotation on sound lateralization, suggesting that vestibular information is taken into account by the brain for accurate localization of stationary sound sources during natural head and body motion.
Introduction
Peripheral coordinates of auditory spatial information are centred to the head. To achieve accurate sound localization during motion, the auditory system thus must take into account changes of head position in space, particularly when a sound signal is rather short so that orientating responses toward that signal cannot be executed during its presence. Most probably, such a head-in-space-displacement signal used for sound localization is derived from vestibular information (Wallach, 1940).
In accordance with this view, several studies have reported slightly improved rather than deteriorated sound localization with both active head movements and passive head rotations of low angular-displacement amplitude (Thurlow & Runge, 1967; Perrett & Noble, 1997). Studies that employed passive whole-body rotation of high amplitude (with multiple revolutions) around the earth-vertical body axis have, on the other hand, reported large systematic errors in sound localization (termed ‘audiogyral illusion’; Münsterberg & Pierce, 1894; Pierce, 1901; Frey, 1912; Clark & Graybiel, 1949; Arnoult, 1950; Jongkees & Van de Veer, 1958; Lester & Morant, 1970), which may be due to the unnaturally strong vestibular stimulus, and its presentation in the absence of neck-proprioceptive signals (see Lewald et al., 1999) and neck-motor efference copies. Up to now, it is unclear whether this ‘audiogyral illusion’ reflects a systematic shift in sound localization with respect to the actual body coordinates or a systematic shift in the spatial frame of reference, particularly the subjective straight-ahead, with respect to actual sound location (Lester & Morant, 1970).
In a recent approach to this problem, Lewald & Karnath (2000) employed a more direct method of vestibular stimulation by cold-water irrigation of the external auditory canal in combination with a psychophysical task of sound lateralization. That study showed a strong shift of the auditory percept which was toward the nonstimulated side. Both whole-body rotation and cold-caloric stimulation thus generally demonstrated the involvement of vestibular afferent information in spatial hearing. However, the direction of the systematic error in sound localization found with whole-body rotation was opposite to that to be expected from the systematic deviations induced by cold-caloric stimulation. This diametrical opposition suggests that the results yielded by both methods reflect quite distinct phenomena (see Lewald & Karnath, 2000).
The present study aims at testing this hypothesis, starting from the assumption that corresponding shifts in sound lateralization must occur when vestibular input is provided by cold-caloric vestibular stimulation and rotational movement. The main experimental conditions of auditory stimulation and visual fixation were as in our preceding study (Lewald & Karnath, 2000), but were now combined with passive whole-body rotation about the earth-vertical axis. Because we focused on genuine vestibular–auditory interactions, we counteracted potential influences of kinesthetic illusions by presenting intracranially perceived dichotic sound stimuli. Unlike previous studies, that used long-lasting high-amplitude rotation producing quite artificial vestibular signals, here we employed brief low-amplitude rotation with values of angular acceleration, velocity and displacement that are rather similar to slow active head movements (Thurtell et al., 1999). These experiments may therefore allow more reliable conclusions about sound localization under natural conditions of motion.
Materials and methods
Experiments were conducted with 12 volunteers, six female and six male, aged from 21 to 46 years (mean 28.8). All subjects were without any known hearing deficiencies and all except one (one of the authors) were naive with respect to the purpose of the experiment.
The whole investigation was conducted in complete darkness. Subjects were seated in a light-bulb-shaped cabin with the head in the centre of the spherical part of the bulb (diameter 1.9 m). The subject sat, secured by belts and shoulder straps, in an upright position in a chair, which was located in the centre of the cabin and provided an adjustable support for the trunk. The subject's head and trunk were aligned. The whole cabin could be rotated by a four-axis gimbal system with two motor-driven rings. The system's outer ring rotated about an earth-horizontal axis. Mounted inside the inner ring was a frame supporting the cabin housing the subject (for details, see Koenig et al., 1996). The present study employed rotations to the left and right around the earth-vertical body axis coinciding with the longitudinal axis of the subject's trunk and the centre of the head.
Dichotic 1-kHz pure tones with variable interaural time differences (ITDs) were used as acoustic stimuli (duration 0.1 s, rise and fall time 10 ms, sound pressure level 70 dB re 20 µPa; identical ITDs of stimulus envelopes and carrier waves). These stimuli were presented to the subject via headphones (Sennheiser HD580). It is a crucial point for the interpretation of our data that the dichotic stimuli used were actually perceived as intracranial sound images between the two ears, as is generally known from previous psychoacoustical research (cf. Blauert, 1997; see Discussion). Therefore, after completing the experiments we asked our subjects about the perceived location of the sound. All subjects reported the sound to appear inside the head.
A dim laser-light spot was projected onto the inner surface of the cabin at eye level in the midsagittal plane of the subject's head/trunk and served as the visual fixation target. The subject's head was fixed by stabilizing rests for the left and right side of the head and occiput. A layer of plastic foam was clamped between the head and the head restraint such that the headphones were firmly embedded in the plastic-foam layer.
Two experimental conditions were employed by alternating rotation and reference trials. Each rotation trial began with a linear angular acceleration of 400°/s2 for 0.23 s until a velocity of 90°/s was reached. This maximum angular velocity of 90°/s was kept constant for 1 s. After that, the rotation slowed down at −45°/s over a period of 2 s until it stopped after an overall displacement of 194°. As illustrated by Fig. 1A, the acoustic stimulus started 0.9 s after the onset of the rotation, i.e. at the moment when the rotation had covered an angular displacement of ≈ 70°. The subject was instructed to fixate the central light spot and to press a ‘left’ or ‘right’ key according to whether the stimulus was perceived to the left or right of the midsagittal plane of the head. The key had to be pressed within the period from stimulus onset up to 1 s after its offset. Otherwise, the trial was invalid and repeated at the end of the sequence. On average, the key was pressed 0.39 s after sound offset.
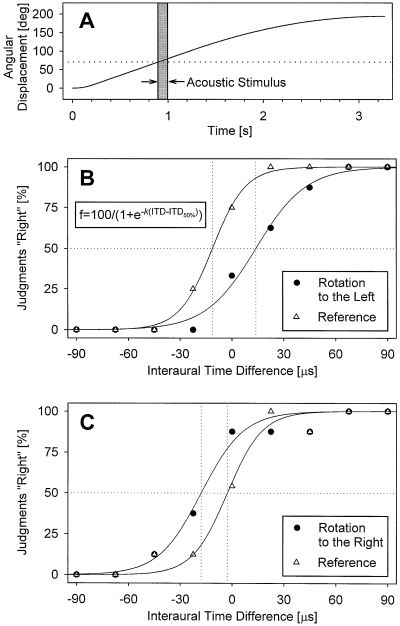
Shift of the auditory median plane (AMP), induced by whole-body rotation. (A) Temporal relation of angular displacement and presentation of the acoustic stimulus (shaded area). The dotted line indicates the angle of sound onset. (B and C) Frequency of the judgements ‘right’, plotted as a function of the interaural time difference (ITD) of the acoustic stimulus in two subjects, who were rotated to the left (B) and right (C), respectively. The original data (symbols) were fitted to a sigmoid equation (as given in the inset of B; solid lines). Data for both the reference condition (no rotation; open symbols) and the test condition (rotation to either the left or right; closed symbols), the ITD at the 50% level of the fitted function was determined (dotted lines) and defined as the AMP. Negative values of ITD indicate sound stimuli leading in time at the left ear, positive values stimuli leading at the right ear.
Ten seconds after the offset of the rotation, the next acoustic stimulus was presented without rotation (reference trial). Twelve seconds later, the next rotation trial began as described above, starting from the point where the preceeding rotation had stopped. The ITD of the stimulus was varied between trials following a quasi-random order over a range from −90 µs (stimulus leading at the left ear) to +90 µs (leading at the right ear), in steps of 22.5 µs. Each ITD was presented in eight rotation trials and eight reference trials, with the exception of the critical value of 0 µs which was presented in 24 rotation and 24 reference trials. One experimental session thus comprised 176 trials in all. After a block of 88 trials, the subject was allowed a rest of ≈ 5 min. One session was conducted with each subject. The direction of rotation (to the left or right) remained unchanged during each session. Six of the 12 subjects were rotated to the left (termed group L) and the remaining 6 were rotated to the right (group R). Some initial practice trials were given prior to data collection.
The analysis of the data was essentially the same as described previously (Lewald & Ehrenstein, 1996). The proportion of the subject's judgements ‘left’ or ‘right’ was determined as a function of stimulus ITD for rotation and reference conditions, and the resulting data were fitted to the sigmoid equation f(ITD)[%] = 100/(1 + e–k(ITD–ITD50%)) where f is the frequency of judgements ‘right’, given as percentage; ITD50% is that ITD where f is 50%; k is the slope of the function at ITD50%; e the base of the natural logarithm (see Fig. 1B). The fit was significant in each case (square of the multiple correlation coefficient R2 > 0.85; P < 0.0001). That ITD at which the left and right choices were 50% was defined as the ‘auditory median plane’ (AMP). All AMPs were normalized such that the AMPs for the reference condition (no rotation) was assigned a value of 0 µs. Negative values of ITD indicate acoustic stimuli leading in time at the left ear; positive ITDs indicate stimuli leading in time at the right ear.
Results
Figure 1B and C show individual results of two subjects. With rotation to the left, the psychometric function was typically shifted to the right with respect to the reference condition (Fig. 1B) while an opposite shift occurred with rotation to the right (Fig. 1C). The corresponding mean curves for all subjects are shown in Fig. 2A and B, and the normalized shifts of the AMP in Fig. 2C. The mean (± SEM) position of the AMP measured with the reference condition (no rotation) was −3.0 ± 2.7 µs. In five of the six group L subjects, body rotation to the left induced a shift of the AMP (with respect to the reference condition) to the right, whilst the shift was to the left in all six subjects rotated to the right (group R); i.e. in 11 of our 12 subjects, the AMP was shifted opposite to the direction of rotation. In group L subjects, the mean (± SEM) magnitude of the AMP shift was +6.8 ± 5.8 µs, range from −15.2 to +25.2 µs; group R subjects exhibited a mean shift of −14.7 ± 5.3 µs, range from −40.1 µs to −6.0 µs; Fig. 2C. The difference between the normalized AMP shifts measured in the two groups was significant (Fisher–Pitman randomization test for two independent samples; P = 0.006). When combining data for rotations to the left and right, there was a significant mean shift of the AMP by 10.7 ± 3.9 µs opposite to the direction of rotation (Fisher randomization test for matched pairs; P = 0.007; Fig. 2D), corresponding to a shift of the intracranial auditory percept that is in the direction of rotation (Fig. 2D, inset).
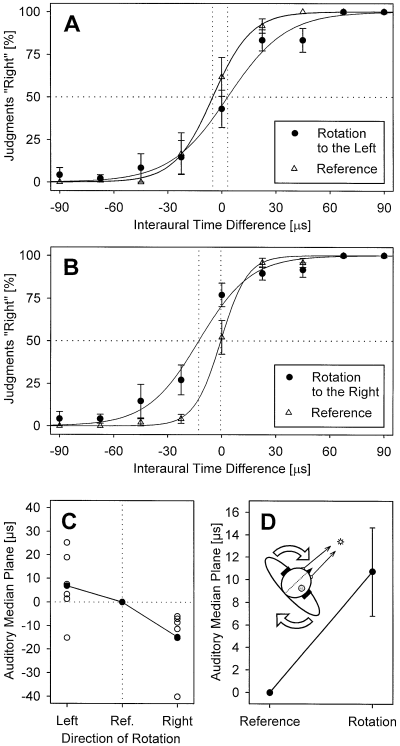
Summarized results of all 12 subjects. (A and B) Mean frequency (± SEM) of the judgements ‘right’, plotted as a function of the interaural time difference (ITD) of the acoustic stimulus for the six subjects who were rotated to the left (A) and the six subjects rotated to the right (B). Conventions are as in Fig. 1B and C. (C) Normalized individual (open symbols) and mean (closed symbols) shifts of the AMPs measured during rotation to the left and right, with the reference condition (no rotation) assigned zero. (D) Mean shift (± SEM) calculated from the nomalized data shown in C by combining left and right rotations. The positive value indicates a shift of the AMP opposite to the direction of rotation or, as illustrated by the inset, a shift of the intracranial auditory percept (shaded circle) in the direction of rotation (open arrows) with respect to the median plane of the head (dotted line). During rotation, visual fixation direction (solid arrows) remained stable with respect to the head.
Discussion
The present shift in sound lateralization during body rotation is in line with our previous results on auditory–vestibular interaction employing cold-water irrigation of the auditory canal as the vestibular stimulus (Lewald & Karnath, 2000). Because corresponding endolymph currents in the horizontal semicircular canal, induced by angular acceleration and cold-caloric stimulation, both resulted in shifts of auditory lateralization toward the same direction, both methods may demonstrate the same phenomenon of genuine vestibulo–auditory interaction. On the other hand, our data are in seeming contradiction to all previous studies that employed whole-body rotation as the vestibular stimulus. The so-called ‘audiogyral illusion’ demonstrated in those studies is characterized by a deviation of perceived sound location with respect to subjective body coordinates opposite to the deviation found here: the shift in localization of a sound stimulus presented during body rotation was opposite to the direction of rotation; immediately after abrupt termination of the rotation, there was also a systematic shift of sound localization, now in the direction of the former rotation (e.g. Münsterberg & Pierce, 1894). We hypothesize that the kind of interaction shown here is distinct from the ‘audiogyral illusion’, which may rather reflect merely kinesthetic factors such that the body appears to be shifting with respect to external space or the head shifting with respect to the trunk (Lester & Morant, 1970). In contrast to previous studies, such kinesthetic illusions were unlikely to play a role with the present experiments. With the method of presenting dichotic sound stimuli, the AMP values measured correspond to ITDs generating a virtual sound source heard in the centre of the head. The subject's left/right judgements on the intracranial auditory percept were, consequently, with respect to a frame of reference that was clearly defined by the perceived dimensions of the head. Thus, the subject's judgements are hardly affected by kinesthetic factors. Moreover, rotatory nystagmus, which may also be a source of the ‘audiogyral illusion’, was suppressed by presenting a fixation target (cf. Gemelli, 1951; Thurlow & Kerr, 1970). On the other hand, a potential illusory motion of this fixation target, induced by rotation, cannot be excluded with the present experiments (cf. Lackner, 1978; see also below). For the reasons mentioned above, it is, however, unlikely that the lateralization of an intracranial sound image can be influenced by a slight visual displacement perceived in external space.
Although the present vestibulo-auditory effect was statistically significant, its mean magnitude was considerably lower than that found with caloric stimulation (7.3 dB interaural level difference; Lewald & Karnath, 2000). This may be due to the more subtle vestibular stimulus yielded by the present low-amplitude angular motion. The shift of the auditory percept was in the range of the lowest ITD thresholds (≈ 10 µs for 1-kHz tones; Klumpp & Eady, 1956; Zwislocki & Feldman, 1956). Converting this shift into an angular value by using the equation of Kuhn (1977), the predicted azimuthal shift in free-field-sound localization is 0.8°. One may thus conclude that, during natural body motion, directional hearing is almost unaffected by such a systematic error. Moreover, it has been shown that neck-proprioceptive influence, induced by eccentric head-to-trunk position, also leads to a slight systematic shift in sound lateralization or localization which is opposite to the direction of the present shift (i.e. opposite to the direction of eccentric head position) and of similar magnitude (maximum 1.5 dB interaural level difference or 6° azimuth; Lewald & Ehrenstein, 1998; Lewald et al., 1999, 2000). When the head moves rapidly with respect to the trunk, the errors due to vestibular and proprioceptive influences may thus (at least partially) neutralize each other. These considerations are in agreement with several studies showing that sound localization is only inconsiderably deteriorated by active head motion or, under certain conditions, is even improved (e.g. Wallach, 1940; Pollack & Rose, 1967; Thurlow & Runge, 1967; Thurlow & Mergener, 1971; Perrett & Noble, 1997).
Despite the obvious functional insignificance of the systematic error shown here, the demonstration of significant vestibulo-auditory interaction strongly suggests that the auditory system uses vestibular information on head-in-space displacement. Moreover, taking together the present results and those cited above, it seems that the vestibular signal and the neck-proprioceptive information on head-to-trunk position are combined for accurate sound localization and maintaining perceptual stability of auditory space during motion. As has recently been hypothesized, the head-centred spatial coordinates of the auditory periphery may be centrally transformed into a trunk-centred system representing the decisive frame of reference for sound localization (Lewald et al., 2000). The integration of vestibular (head-in-space displacement) and proprioceptive (head-to-trunk position) signals into a signal of trunk-in-space displacement may provide an accurate relation of this trunk-centred frame of reference to auditory space even during simultaneous head and whole-body motion. This seems to afford a world-centred representation of auditory space (Lewald & Karnath, 2000). Neurophysiological studies on auditory neurons of the hippocampus (Tamura et al., 1990) and multimodal neurons of the posterior parietal cortex (for review, see Andersen et al., 1993; Thier & Karnath, 1997) support this view, even though the exact mechanisms of such interaction are yet unclear. Moreover, psychophysical studies on visual localization during vestibular stimulation (e.g. Mergner et al., 1991; Karnath et al., 1994; Maurer et al., 1997) have shown quite similar systematic errors as those shown in the present study and those of Lewald & Karnath (2000). Vestibular influences are thus not restricted to the auditory modality, but are integrated in a more general process of space perception for relating own body position to the surrounding auditory and visual world.
Acknowledgements
We thank K. Beykirch for his helpful support with the experiments and his software to stear the gimbal system, P. Dillmann for preparing the software and parts of the electronic equipment used for acoustic stimulation, and W. H. Ehrenstein for critical discussion of the results and valuable comments on the manuscript. This work was supported by grants of the Deutsche Forschungsgemeinschaft (Eh 91/4–2; Ka 1258/2–1; Ka 1258/6–1).
Abbreviations
-
- AMP
-
- auditory median plane
-
- ITD
-
- interaural time difference.




