Neuropeptide Y alters sedation through a hypothalamic Y1-mediated mechanism
Present address: Department de Biologica Cellular i Anatomia Patologica, Facultat de Medicina, Universitat de Barcelona, IDIBAPS, Casanova 143, 08036 Barcelona, Spain.
Abstract
Neuropeptide Y (NPY) has been reported to profoundly influence and regulate brain circuits involved in a number of behaviours, like anxiety, alcohol intake, pain and energy homeostasis. Here we show that NPY increases sedation induced by different types of anaesthetics through interactions with the Y1 receptor. Consistently, in Y1–/– (homozygote knockout) mice NPY does not potentiate the pentobarbital-induced sedation. Similar results were obtained for avertin but not for ketalar- (NMDA antagonist) induced sedation. Local microinjection of NPY exhibited the strongest potentiating effect on pentobarbital-induced sedation in the posterior hypothalamic area and Y1 expression was found in the dorsal-premammillary and medial part of medial mammillary nuclei. These results show that Y1 is essential for NPY-induced enhancement of sedation and place this activity of NPY in the posterior hypothalamic area, a region of the brain previously implicated in the regulation of the wake–sleep cycle.
Introduction
In recent years, a large number of NPY Y receptors have been pharmacologically characterized and their cDNAs have been cloned. The NPY Y receptor family is now composed of Y1, Y2, Y5 and Y6 (Eva et al., 1990; Gerald et al., 1995, 1996; Weinberg et al., 1996). All receptors bind NPY and the peptide YY with similar kinetics. NPY Y receptors are expressed in specific structures of the brain in an overlapping or nonoverlapping manner according to the subtype. Y1 and Y5 receptor expression is largely overlapping, whereas, the Y2 receptor is expressed in a complementary pattern (Naveilhan et al., 1998). NPY has been shown to elicit a broad range of pharmacological effects, like antinociceptive, anxiolytic and orexigenic actions (White, 1993; Wettstein et al., 1995) as well as modulation of the circadian rhythm (Gribkoff et al., 1998). The establishment of NPY and Y receptor knockout mice has demonstrated the physiological importance of these receptors in functions like energy homeostasis, pain, susceptibility to seizure and alcohol intake (Erickson et al., 1996a, 1996b; Kushi et al., 1998; Marsh et al., 1998, 1999; Pedrazzini et al., 1998; Thiele et al., 1998; Naveilhan et al., 1999, 2001).
At the cellular level, NPY has inhibitory actions and can act both pre- and postsynaptically. NPY is able to depress the GABA-mediated calcium transient in developing suprachiasmatic nucleus neurons (Obrietan & van den Pol, 1996) and to modulate NMDA-induced neuronal activation and dopamine release (Monnet et al., 1992, 1994; Ault & Werling, 1997). GABA agonists, like pentobarbital, as well as NMDA antagonists, like ketamin (ketalar), are used as general anaesthetics. NPY injections share many of the properties of GABAA agonists and alcohols in sleep, including shortening of sleep latency, stimulating non-rapid eye movement (NREM) sleep as well as blunting hypothalamic–pituitary–adrenocortical (HPA) hormones that are associated with increased REM sleep (Ehlers et al., 1997a, 1997b; Steiger & Holsboer, 1997). However, the direct link between NPY, its receptors and GABA as well as the brain circuits involved are uncertain. We have studied the physiological influence of the Y1 receptor as well as the pharmacological action of NPY on sedation by different types of anaesthetics. The results show that NPY modulates sedation through the GABAergic system by interacting with the Y1 receptor in the posterior hypothalamus.
Materials and methods
Chemicals
Pentobarbital (60 mg/kg, Apoteksbolaget, Umeå, Sweden) and ketalar (ketamin, 50 mg/kg, Parke-Davis Scandinavia AB, Solna, Sweden) were diluted in saline at concentrations of 3 mg/mL and 7.5 mg/mL, respectively. Avertin stock consisted of a mix of 1 g of 2,2,2-tribromomethanol (Aldrich, Steinheim, Germany) dissolved in 0.63 mL of 2-methyl 2-butanol (Sigma) and was stored at −20 °C. A 1.2% solution, in saline, was prepared freshly before each experiment. All drugs were delivered intraperitoneally at 20 mL/kg except for avertin (25 mL/kg). NPY and [Leu 31, Pro 34]-NPY were obtained from Calbiochem-Novabiochem corporation (La Jolla, CA, USA).
Animals
Establishment of the homozygote Y1 knockout mice (Y1–/–) was previously described (Naveilhan et al. 2001). Heterozygote mice (Y1+/–) with a 129SV × Balb/c hybrid were backcrossed with Balb/c mice. The resulting F1 Y1+/– mice were bred to F2 to generate wild-type (Y1+/+) and Y1–/– mice which were used in the experiments. In the experiments in Figs 2a–c and 3a, Balb/c mice were used (Charles River, Uppsala, Sweden). Mice were housed in a 12 h light : 12 h dark environment (night, 1800–0600 h) and all experiments in this study were performed in the morning (between 0900 and 1100 h).

Pharmacological influence of NPY on LORR induced by anaesthetics. Experiments were performed on Balb/c mice (a–c) and on Y1+/+ and Y1–/– mice (d–f). Mice were anaesthetized with 25 mL/kg of 1.2% (a and d) avertin, (b and e) 60 mg/kg of pentobarbital and (c and f) 150 mg/kg of ketalar. Ten minutes after the beginning of the LORR, mice were injected i. c. v. with CSF, 1 nmol NPY and 2 nmol [Leu 31, Pro 34]-NPY (Leu, Pro) as indicated. CSF, n = 6–15 per group; NPY, n = 4–6 per group and [Leu 31, Pro 34]-NPY, n = 4–6 per group. Values are expressed as percentage of the CSF LORR ± SEM. **P < 0.01; ***P < 0.001; unpaired t-test.
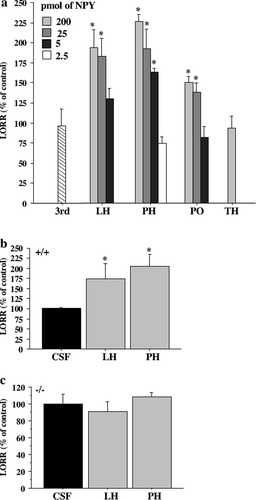
(a) Effect of bilateral intrahypothalamic injection of NPY on LORR induced by pentobarbital (60 mg/kg) in Balb/c mice. Different doses of NPY were injected 10 min after the beginning of LORR induced by pentobarbital in the preoptic (PO), lateral hypothalamic (LH) and posterior hypothalamic (PH) area as well in the third ventricle (3rd) and thalamic area (TH). CSF injected in the different structures did not significantly affect the LORR. These data were pooled and considered as control (n = 14). Three to six mice were used per dose and structure. Doses used are in the upper left corner of the graph and represent the dose used per side (unilateral dose) except for the third ventricle where a single dose of 10 pmol was used (striped bar). (b and c) Effect of NPY (100 pmol, grey bars) or CSF (black bars) injection into the lateral and posterior hypothalamic area of (b) Y1+/+ and (c) Y1–/– mice. Values are expressed as percentage of the CSF injection ± SEM. P < 0.05, unpaired t-test.
NPY injection
These experiments were approved by the local ethics committee, ‘Stockholms Norra Försöksdjarsetiska Nämnd’. To study the effect of NPY on anaesthesia, female adult mice (3–4-month-old) were anaesthetized (i.p.) with either avertin (25 mL/kg of 1.2% avertin solution), pentobarbital (60 mg/kg) or ketamin (150 mg/kg) and placed in a stereotaxic apparatus (Stoelning instruments) with the incisor bar at the level of the interaural line. NPY (0.5 nmol/μL) was dissolved in artificial cerebrospinal fluid (CSF; in mM: NaCl, 124; NaHCO3, 24; KCl, 2.4; KH2PO4, 0.5; CaCl2, 1.4; MgCl2, 2; Na2SO4, 0.5; Glucose, 6; pH 7.4) and the Y1 receptor agonist, [Leu 31, Pro 34]-NPY, in CSF + 0.5% of acetic acid. A 27-gauge cannula was implanted into the left lateral ventricle (AP, +0.0; L, −1.7 from bregma and −2.7 from the dura) and NPY, [Leu 31, Pro 34]-NPY and vehicle (in a total of 2μL at 1.0μL/min) were injected 10 min after the beginning of the loss of the righting reflex (LORR). The needle was left in place for a further 3 min before being slowly withdrawn. The loss of the righting reflex was measured as the amount of time that the animal spent on its back, unable to position itself in an upright position. For the bilateral thalamic and hypothalamic injection, NPY and vehicle were injected in a volume of 0.05μL. Coordinates were determined from the atlas of the mouse brain (Franklin & Paxinos, 1997) and verified using ink injections. NPY was injected at the following coordinates: preoptic region (AP +0.7, L +0.85, −0.85 from bregma and −4.7 from the dura); lateral hypothalamus area (AP −0.3, L +1.2, −1.2 from bregma and −4.8 from dura); posterior hypothalamus (AP −1.8 and L +0.2, −0.2 from bregma and −4.5 from the dura); 3rd ventricle (AP −1, L −0.0 from bregma and −2.1 from the dura) and thalamus (AP −0.7, L +1.5, −1.5 from bregma and −3.2 from the dura).
In situ hybridization
For in situ hybridization, frozen adult brains were positioned on a metalic block and sectioned on a Leitz cryostat in 14-μm-thick sections. Sections were mounted onto slides pretreated with 2% 3-aminopropyl triethoxysilane. (Sigma, St Louis, MO, USA) and kept frozen until used. Probe design and in situ hybridization were performed as previously described (Naveilhan et al., 1998).
Results
Physiological effects of the Y1 receptor in anaesthesia
In order to define the potential role of the Y1 receptor on induced sedation, mice carrying a null mutation of Y1 gene (Y1–/–) (Naveilhan et al. 2001) were injected with different anaesthetics and the LORR was monitored. We used an anaesthetic commonly employed in the laboratory, avertin and also anaesthetics with well described mechanisms of action; pentobarbital, a GABAA agonist and ketalar, an NMDA antagonist. Our results showed that Y1–/– mice exhibited a decrease of 30.6% and 26.8% in LORR compared to control animals (Y1+/+) when avertin (Y1+/+, 70.2 min; Y1–/–, 48.7 min; P = 0.0014) or pentobarbital (Y1+/+, 94.2 min; Y1–/–, 69 min; P = 0.028) were used, respectively (Fig. 1a and b). However, LORR was not affected in mice anaesthetized with ketalar (Fig. 1c; Y1+/+, 39.5 min; Y1–/–, 40.7 min; P = 0.82). These experiments suggest that avertin acts in a manner similar to pentobarbital. Thus, the Y1 receptor appears to play a significant depressant or sedative-facilitating action and in its absence, the mice recover faster from anaesthesia elicited by a GABA agonist and avertin but not when an NMDA antagonist was used.
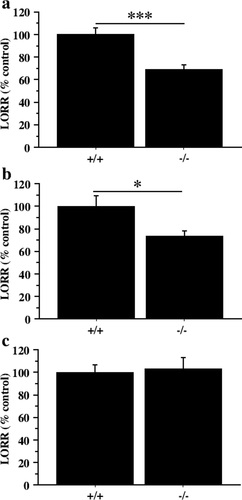
Effect of Y1 mutation on LORR induced by anaesthetics. Y1+/+ (+/+) and Y1–/– (–/–) mice were injected with 25 mL/kg of (a) 1.2% avertin, (b) 60 mg/kg of pentobarbital and (c) 150 mg/kg of ketalar and their respective LORRs were timed. Values are expressed as percentage of Y1+/+ LORR ± SEM. *P < 0.05; ***P < 0.001; unpaired t-test. n = 6 per genotype and condition.
Pharmacological effects of NPY on sedation
We next examined the effects of NPY injections on induced sedation. Balb/c mice, anaesthetized with avertin, pentobarbital or ketalar, received an intracerebroventricular (i.c.v.) injection of 1 nmol NPY and LORR was monitored. All three anaesthetics exhibited an increased LORR after NPY injection compared to vehicle-injected animal (Fig. 2a–c). The effect of NPY was much more potent during avertin (Fig. 2a) and ketalar (Fig. 2c) compared to pentobarbital-induced (Fig. 2b) anaesthesia. The LORR increased to 422%, 170% and 501% following avertin, pentobarbital and ketalar treatment, respectively. However, i.c.v. injection of NPY in awake cannulae implanted mice did not produce any anaesthetic effect (data not shown). This result shows that NPY potentiates the effect of the anaesthetics used in this experiment. Similar results were obtained with a Y1/Y5 receptor agonist, [Leu 31Pro 34]-NPY (2 nmol) during pentobarbital- and avertin-induced anaesthesia (Fig. 2a and b), indicating that the effect of NPY is mediated through interactions with the Y1 or Y5 receptor. In contrast, [Leu 31Pro 34]-NPY did not elicit the full effects of NPY during ketal-induced anaesthesia (Fig. 2c), suggesting the involvement of other NPY receptors when sedation is induced by blocking NMDA receptor activity.
In order to demonstrate that the pharmacological action of NPY is mediated by Y1 receptor, Y1+/+ and –/– animals were injected with 1 nmol of NPY. In Y1+/+ mice, injection of 1 nmol of NPY during avertin, pentobarbital and ketalar anaesthesia increased LORR. However, injection of NPY in Y1–/– mice did not increase the LORR for any of the anaesthetics when compared to the Y1+/+ control animal (Fig. 2d–f). If the results are compared to the CSF-treated Y1–/– animals, a small but statistically significant residual NPY potentiation was seen during avertin anaesthesia (Fig. 2d). From these results we conclude that Y1 is a major receptor mediating NPY effects on induced sleep.
Hypothalamic administration of NPY and anaesthetic-induced sedation
NPY is abundantly expressed in the hypothalamus, a brain region that has been implicated in sleep. Thus, we next examined if hypothalamic nuclei are involved in the NPY-dependent depressant action. To investigate a potential role of NPY in pentobarbital-induced sleep in this region, Balb/c mice were injected with NPY in the lateral (LH), posterior (PH) and preoptic hypothalamic (PO) area as well as in the thalamus (TH) (Fig. 3a). The dose–response relationship following bilateral injections of 2.5–200 pmol of NPY in a volume of 0.05μL were performed 10 min after pentobarbital induced LORR. Even at the highest dose, we did not see any effect on LORR when NPY was injected in the thalamus (Fig. 3a). However, in all hypothalamic regions tested, NPY induced a marked increase in LORR compared to controls. NPY increased-LORR was lower in the PO compared to the PH and LH regions. 25–200 pmol of NPY injected into the LH and PH elicited the maximal increase of LORR. However, at a dose of 5 pmol per hemisphere, NPY was able to increase the pentobarbital-induced LORR only in the PH, showing a maximal responsiveness in this area. The effect was not due to a leakage of NPY in the third ventricle, since administering 10 pmol of NPY into the ventricle did not affect LORR (Fig. 3a striped bar). To define if the Y1 receptor was responsible of this effect, Y1+/+ and Y1–/– mice were injected with 100 pmol of NPY bilaterally into the LH and PH (Fig. 3b and c). The increased LORR in Y1+/+ mice was similar as in previous experiments (Fig. 3b). The NPY potentiation of pentobarbital-induced sleep was completely abolished in Y1–/– mice (Fig. 3c), showing that the Y1 receptor mediates the effect of NPY on pentobarbital-induced LORR. These results show that NPY potentiation of sedation occurrs in the posterior-lateral hypothalamus and is mediated through Y1 receptors in this region.
Localization of Y1 receptor expression
In order to localize the cells expressing Y1 in the lateral and posterior hypothalamus we used in situ hybridization to detect Y1 mRNA expression. We did not detect any signal in the posterior hypothalamic nucleus. However, Y1 mRNA was seen in the medial zone of the mammillary region. The signal was present in the dorsal premammillary and medial mammillary nuclei (Fig. 4).

Analysis of Y1 expression in the posterior hypothalamic area. Brains of wild-type mice were dissected and 14-μm-thick sections were submitted to in situ hydridization using radiolabelled-Y1 oligonucleotides. Dark (a) and bright (b) field micrograph of the mammillary region show expression in the dorsal premammillary nucleus (PMD) and medial mammillary nucleus (MMn). 1 cm, 100 μm.
Discussion
We found that NPY decreased wakefulness in avertin and pentobarbital-induced sedation and analysis of the effects of receptor-specific ligands and Y1–/– mice revealed that this effect of NPY is mediated by interactions with the Y1 receptor. The role of NPY in sleep was found to be intimately linked to the GABAA receptor since the duration of pentobarbital-induced sedation was blunted in the absence of Y1 receptors. In comparison to avertin and pentobarbital, no clear physiological effect of Y1 receptors was found during ketalar-induced sleep.
The Y1 receptor may not be physiologically involved in the NMDA mechanism of sedation since the absence of the Y1 receptor in mice did not influence ketalar-induced sleep. In contrast, injection of either NPY or [Leu 31, Pro 34]-NPY greatly increased ketalar-induced LORR in wild-type mice, but not in Y1–/– mice, showing a pharmacological influence of NPY through the Y1 receptor. However, [Leu 31, Pro 34]-NPY was not as effective as NPY, suggesting the involvement of NPY receptors other than those of the Y1 subtype. Thus, it is conceivable that the participation of another NPY receptor subtype could compensate for the loss of Y1 under physiological situations.
GABA is the major inhibitory neurotransmitter in the mammalian central nervous system and plays a major role in the transition between wake and sleep stages by reducing sleep latency. It is believed to be involved in the induction and the maintenance of NREM sleep through interactions with GABAA receptor subtypes (Lancel, 1999). Y1 receptor deletion greatly decreases the pentobarbital-induced LORR, showing a requirement for a functional NPY system in the GABA agonist-induced depressant action.
NPY exerts powerful anxiolytic actions, opposing the anxiogenic effects of corticotropin releasing hormone (CRH). Administration of CRH and transgenic mice overexpressing CRH gives exaggerated anxiety-related behaviour, while suppressing CRH, by antisense technologies or by blocking CRH-receptor synthesis, produces anxiolytic effects (Skutella et al., 1994; Stenzel-Poore et al., 1994; Liebsch et al., 1995). NPY has a marked anxiolytic profile and when injected into the brain it produces a reduction of anxiety-related behaviour in several models (Heilig et al., 1989, 1992). This effect is specific to NPY peptide fragments that are Y1 receptor agonists. Consistently, C-terminal fragments, which do not interact with the Y1 receptor, do not produce any anxiolytic effect (Heilig et al., 1989) and Y1 receptor antisense sequences cause anxiety-related behaviour (Wahlestedt et al., 1993). Because the opposing actions of CRH and NPY have also been observed in sleep regulation (Yamada et al., 1996; Ehlers et al., 1997b), a common mechanism between sleep and states of depression and anxiety has been proposed. We have microinjected NPY and find that its effect on sedation is localized to the posterior hypothalamus area. Local microinjections of NPY have centred its anxiolytic action to the central nucleus of the amygdala (Heilig et al., 1993). Thus, NPY actions on sedation and anxiety might involve different brain circuits.
Several results have demonstrated that hypothalamic nuclei are involved in the regulation of sleep. Previous findings have shown that the activity of neurons in the posterior hypothalamic area contribute to wakefulness and inactivation of neurons in this area induces sleep (Von Economo, 1918; Lin et al., 1989; Sallanon et al., 1989). Most neurons of the posterior hypothalamus exhibit highest activity and rates of firing during active waking and REM sleep and lowest during slow-wave sleep (SWS) (Sallanon et al., 1989; Szymusiak et al., 1989). The dynamics of activity in the posterior hypothalamus across wake–sleep cycles are correlated to a selective increase in GABA release in the posterior hypothalamus during SWS, while glutamate and glycine levels are unchanged (Nitz & Siegel, 1996). Our results showed that intrahypothalamic injections of NPY were able to increase the sedative effect of a GABA agonist, and in dose–response experiments, the largest response was achieved in the posterior hypothalamic area. Further investigations will be necessary to clarify the role of Y1 receptor subtype in natural sleep.
Acknowledgements
We thank Lotta Johansson for secretarial assistance. This research was supported by the Molecular Biology Department, AstraZeneca (Molndal), Swedish Medical Research Council and Göran Gustafssons Foundation. J.M.C. was supported by EMBO and Human Frontiers Program fellowships.
Abbreviations
-
- CRH
-
- corticotropin releasing hormone
-
- GABA
-
- γ-aminobutyric acid
-
- LH
-
- lateral hypothalamic area
-
- LORR
-
- loss of righting reflex
-
- NMDA
-
- N-methyl-d-aspartic acid
-
- NPY
-
- neuropeptide Y
-
- PH
-
- posterior hypothalamic area
-
- PO
-
- preoptic hypothalamic area
-
- REM
-
- Rapid eye movement.




