Fibroblast growth factor-2 is selectively modulated in the rat brain by E-5842, a preferential sigma-1 receptor ligand and putative atypical antipsychotic
Abstract
Fibroblast growth factor-2 (FGF-2) is a member of a large family of trophic factors whose expression is regulated under several conditions in different areas of the brain. The goal of our experiments was to determine whether the administration of 4-(4-fluorophenyl)-1,2,3,6-tetrahydro-1-[4-(1,2,4-triazol-1-il)butyl] pyridine citrate (E-5842), a sigma-1 receptor ligand and putative atypical antipsychotic, could regulate the expression of FGF-2. After chronic treatment with E-5842 (21 days, and the animals killed 24 h after the last administration), an up-regulation was observed of the expression of FGF-2 mRNA in the prefrontal cortex and the striatum, and a down-regulation of the expression of FGF-2 mRNA in the hypothalamus of the rat brain. Acute treatment with E-5842 (one single administration and animals killed 6 h later) up-regulated FGF-2 expression in the prefrontal cortex, the striatum, the hypothalamus and the hippocampus in a dose-dependent manner. The acute up-regulation was transient and disappeared 24 h after E-5842 administration. The induction of FGF-2 in the striatum after repeated administration has been described for clozapine, but our data concerning regulation in the prefrontal cortex suggest that this effect is unique to E-5852 among other antipsychotics. Given the neuroprotective activity of FGF-2, the data presented here might be relevant to the deficit in cognition and other symptoms that appear in schizophrenia.
Introduction
Schizophrenia is a complex psychiatric disorder characterized by the manifestation of the so-called positive and negative symptoms. A plethora of different antipsychotic compounds exist, some of them known as atypical antipsychotics; these display some advantages over the classic or typical antipsychotics (Arndt & Skarsfeldt, 1998). The atypical compounds show different degrees of efficacy on positive and negative symptoms (Meltzer, 1996), but the mechanism of action of these molecules remains elusive. It is difficult to assign the therapeutic activity of different compounds to the interaction with a given receptor, and molecules with different receptor binding affinity show similar therapeutic activity. 4-(4-fluorophenyl)-1,2,3,6-tetrahydro-1-[4-(1,2,4-triazol-1-il)butyl] pyridine citrate (E-5842) is a new putative atypical antipsychotic with a preferential binding affinity for the sigma-1 receptor (Ki = 4 nm). E-5842 displays moderate to low affinity for other central nervous system receptors, including the dopamine D2, D3 and D4 receptors, the serotonin 5-HT2 and 5-HT1A receptors, the acetylcholine muscarinic receptors or different subtypes of the adrenergic receptors (Guitart et al., 1998). On the other hand, E-5842 is active in different animal models of antipsychotic activity (Guitart et al., 1998), and behaves as an atypical compound in biochemical and electrophysiological studies (Guitart & Farré, 1998; Sánchez-Arroyos & Guitart, 1999). The majority of preclinical tests considered to be models of negative and positive symptoms of schizophrenia are based on the acute activity of the candidate compounds. In this case, it seems clear that the chronic effect of psychotropic drugs has to be considered, and may help to explain their mechanism of action. In fact, changes in protein synthesis as well as in gene expression following repeated treatment with psychotropic drugs should be considered. In this way, changes due to chronic administration of antipsychotics on the expression of immediate early genes, neurotransmitter receptors and other proteins have been recently described (Fitzgerald et al., 1995; Healy & Meador-Woodruff, 1997; Atkins et al., 1999; Riva et al., 1999). We have recently described changes in the mRNA expression for the sigma-1 receptor and several ionotropic glutamate receptor subunits in specific areas of the brain by chronic treatment with E-5842 (Zamanillo et al., 2000; Guitart et al., 2000a).
Fibroblast growth factor-2 (FGF-2) is a member of a large family of trophic factors (Miyamoto et al., 1993; Bikfalvi et al., 1997) whose expression has been shown to be regulated by the action of neurotransmitters (Follesa & Mocchetti, 1993) or learning (Gomez-Pinilla et al., 1998). It has been recently described by Riva et al. (1999) that after chronic treatment with clozapine, levels of FGF-2 mRNA and protein were increased in the rat striatum. Our study was focused on the effect of E-5842 on FGF-2 expression in different brain areas. Our work clearly shows regulation in a region-specific manner, and the results may provide new insights regarding the mechanism of action of E-5842 in the sense of its capacity to regulate neurotrophic molecules in the brain. Some of these data have been reported in abstract form (Guitart et al., 2000b).
Materials and methods
Drugs
Clozapine was obtained from Sigma RBI (St. Louis, MO, USA). E-5842 was synthesized de novo by the Chemistry Department at Esteve S.A. (Barcelona, Spain) (Guitart et al., 1999).
Experimental groups
Male Sprague-Dawley rats (150–200 g) purchased from Iffa Credo (L'Arbresle, France) were used throughout all the experiments. The animals were maintained on a 12-h light/dark cycle with free access to food and water. Saline or E-5842 (20 mg/kg) was intraperitoneally administered to rats once (acute treatment), or over 21 days (chronic treatment). For the dose–response experiments, E-5842 was acutely administered at 5 and 30 mg/kg. In some experiments, clozapine was acutely administered (15 mg/kg, i.p.). Rats were killed by decapitation 6 or 24 h after the single (acute) injection, and 24 h after the last (chronic) injection. The prefrontal cortex, striatum, hippocampus, hypothalamus, pons and cerebellum were rapidly dissected from the brain, frozen on dry ice and stored at −80 °C for further analysis. The prefrontal cortex was dissected out from coronal sections of the brain, approximately in the areas located between 3.7 and 2.2 mm anterior to bregma (sections based on the atlas of Paxinos & Watson, 1986). In some experiments, the heart, lung, liver, spleen and kidney of naive animals were also obtained, frozen and stored as described bellow. All the procedures involving animals and their care were conducted in strict conformity with the European Community Guide for the Care and Use of Laboratory Animals (EEC Council Directive 86/609, OJ L 358,1, Dec. 12, 1987).
RNA isolation
Total RNA was isolated from the different brain areas and the extracerebral tissues. In the case of hypothalamus, tissue from five animals was pooled. Tissues were homogenized directly in a guanidine thyocyanate solution (Trizol, Gibco RBL, Uxbridge, UK) and total RNA isolated by phenol–chloroform extraction (Chomczynski & Sacchi, 1987). Quantification was carried out by spectrophotometry.
Reverse transcription and polymerase chain reaction
Five micrograms of total RNA were reverse transcribed in a total volume of 50 µL of (in mm) Tris buffer (pH 8.3), 50; KCl, 50; MgCl2, 4; deoxynucleoside triphosphates, 0.4; DTT, 10; with 0.5 µg oligo (dT)18 primer and 500 units of M-MLV reverse transcriptase. The reaction was carried out at 37 °C for 1 h. FGF-2 and β-actin fragments were obtained by polymerase chain reaction (PCR) amplification of the reverse transcribed material using 10 pmol of each specific primer. 5′-CTG GCT TCT AAG TGT GTT AC−3′ and 5′-TCA GCT CTT AGC AGA CAT TG-3′ were used as sense and antisense oligos, respectively, to obtain a specific FGF-2 195-bp PCR fragment from cDNA. 5′-CCA GAT CAT GTT TGA GAC CT-3′ and 5′-TAG AGG TCT TTA CGG ATG TCA-3′ were used as sense and antisense oligos, respectively, to obtain a specific β-actin 522-bp fragment from cDNA.
A 43 mer oligodeoxynucleotide (5′-GGA GTA TTT CCG TGA CCG GTA AGT GTT GTA GTT ATT GGA CTC C-3′) was used as FGF-2 probe to ascertain the identity of PCR amplifications. FGF-2 PCR samples were size-fractionated by electrophoresis in a 1.5% agarose gel and then blotted overnight in 10 × SSC. Filters were uv-cross-linked and prehybridized for 30 min in 10 mL of Quichyb solution (Stratagene, La Jolla, CA, USA). Hybridizations were performed for one hour at 68 °C in the same solution with 1 mg denatured salmon testes DNA and the 43 mer oligodeoxynucleotide (radiolabelled at the 5′ termini using T4 polynucleotide kinase and [γ32P] ATP). Filters were washed to a final stringency of 0.1 × SSC, 0.1% sodium dodecyl sulphate at 60 °C. Autoradiograms were obtained after overnight exposure at −80 °C using Hyperfilm β-max films (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Northern blot analysis
The specific FGF-2 195-bp and β-actin 522 bp PCR fragments were used as probes to detect FGF-2 and β-actin mRNA bands, respectively. Ten microgrammes of each RNA sample were size-fractionated by 1% agarose/formaldehyde gel electrophoresis. Before transfer to nylon membranes the gel was stained with ethidium bromide and destained. The quality of the RNA preparation and the positions of ribosomal bands were established at this step. Gels were blotted overnight in 10 × SSC. Filters were uv-cross-linked and prehybridized for 30 min in 10 mL of Quichyb solution (Stratagene, La Jolla, CA, USA). Hybridizations were performed for one hour at 68 °C in the same solution with 1 mg denatured salmon testes DNA and 50 ng denatured DNA probe (radiolabelled by the random priming method). Filters were washed to a final stringency of 0.1 × SSC, 0.1% sodium dodecyl sulphate at 60 °C, and then exposed for several days at −80 °C to Hyperfilm β-max films. Quantification of mRNA was carried by densitometric analysis of the autoradiograms. Data are expressed as mean ± SEM and were analysed using a Student's t-test. The intensity of FGF-2 mRNA bands was normalized relative to that of the β-actin.
Western blot analysis
Rats were acutely or chronically treated with E-5842 as described above, and then the striatum, hippocampus and prefrontal cortex were excised from the brain. Tissues were immediately homogenized in 1% sodium dodecyl sulphate and after protein content determination (Lowry et al., 1951), aliquots containing 30–40 µg of protein were subjected to sodium dodecyl sulphate/polyacrylamide gel electrophoresis. The proteins were then transferred to nitrocellulose and FGF-2 immunolabelled with a monoclonal FGF-2 antibody (1 : 200) (mouse-anti-FGF-2 antibody was obtained from Transduction Laboratories, Lexington, KY, USA). Membranes were then incubated with antimouse IgG conjugated to peroxidase (1 : 1000) (goat-antimouse-IgG conjugated to peroxidase was obtained from Pierce, Rockford, IL, USA).
Immunocomplexes were visualized by using the Supersignal Chemiluminescent substrate (Pierce) according to the manufacture's instructions. Relative levels of proteins were analysed using a Bio-Rad Fluor-S MultiImager. In these experiments, each gel contained saline-treated samples and E-5842-treated samples running under the same conditions. Statistical evaluation of band density values was performed using one-way analysis of variance (anova) followed by Dunnett's t-test. Given that the intensity of the bands varied across experiments, results are expressed as mean percentage of control (saline-treated rats) values ± SEM.
Results
RT-PCR detection of FGF-2 in rat brain
A 195-bp DNA fragment (nucleotides 803–997 of the FGF-2 rat cDNA), encoding amino acid sequences from the C-terminal region of the FGF-2, was obtained by RT-PCR using specific FGF-2 primers and cDNAs from different brain areas (prefrontal cortex, striatum, hippocampus and cerebellum). The 195 DNA fragment from all the brain areas was studied (although with different amplification levels). Figure 1B shows that, in saline-treated animals, the highest levels were found in the hippocampus, whilst similar levels were seen in the prefrontal cortex and the striatum, and relatively low levels in the cerebellum. Moreover, the amplification levels were higher in chronically E-5842-treated rats than in saline treated-rats for in the above referred areas, except for the hippocampus (Fig. 1B). A parallel PCR was performed as a control using the same cDNA samples and specific β-actin primers. Similar levels of a β-actin 522 bp PCR fragment in all the areas from both E-5842-treated and saline-treated rats were observed (Fig. 1A). To insure the identity of the PCR amplifications, an internal 43-mer oligodeoxynucleotide (nucleotides 850–892 of the FGF-2 rat cDNA) was used as probe in Southern blot hybridization of the FGF-2 PCR products as shown in Fig. 1C.
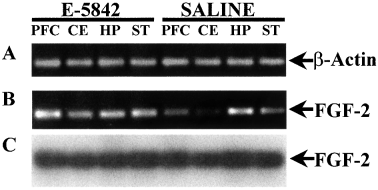
RT-PCR detection of mRNAs for FGF-2. Representative example of ethidium bromide-stained agarose gel of RT-PCR products of (A)β-actin and (B) FGF-2 from saline- and E-5842-treated rats in different brain areas. (C) Southern blot hybridization of the gel B using an internal oligodeoxynucleotide for FGF-2 as a probe. PFC, prefrontal cortex; CE, cerebellum; HP, hippocampus; ST, striatum.
Analysis of the distribution of FGF-2 in rat
The specific FGF-2 195-bp DNA fragment obtained by RT-PCR from total brain was used as a probe to study the distribution of the FGF-2 mRNA in several brain areas and extra-cerebral tissues by Northern blot analysis using high stringency conditions. Our study was focused on prefrontal cortex, striatum, hippocampus, hypothalamus, pons, cerebellum, heart, lung, liver, spleen and kidney. An ≈ 6-Kb band was detected in all cerebral areas studied, with higher expression levels in the hippocampus and hypothalamus, and very low levels in the cerebellum (Fig. 2A). No expression was detected in any of the extra-cerebral tissues studied. The same blots used in this assay were then reprobed for β-actin (the specific β-actin 522-bp PCR fragment obtained previously was used as probe) to ensure each lane had similar levels (Fig. 2B). These results are in agreement with the data reported previously (Shimasaki et al., 1988). Based on those data, we selected prefrontal cortex, striatum, hippocampus and hypothalamus for further studies.
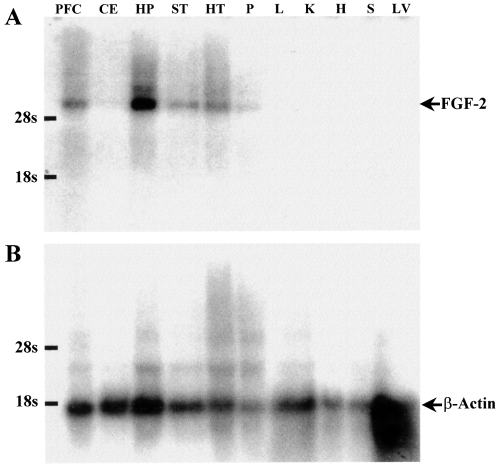
Regional distribution of FGF-2 RNA in different brain areas and other rat tissues. (A) Northern blot analysis of rat tissue RNA samples using a 195-bp PCR FGF-2 fragment as a probe. (B) The same blots were reprobed for β-actin to ensure each lane had similar levels of RNA. PFC, prefrontal cortex; CE, cerebellum; HP, hippocampus; ST, striatum; HT, hypothalamus; P, pons; L, lung; K, kidney; H, heart; S, spleen; LV, liver.
Effect of chronic treatment of E-5842 on FGF-2 mRNA levels
In order to assert the existence of E-5842-dependent changes in FGF-2 mRNA levels in different brain areas, and perform an analysis of such changes, the effects of a prolonged treatment (21 days) with E-5842 (20 mg/kg i.p.) on the expression of the FGF-2 mRNA was studied by means of Northern blot hybridization. As shown in Fig. 3, repeated treatment with E-5842 produced a significant elevation of FGF-2 mRNA levels in prefrontal cortex and striatum, whilst levels of FGF-2 mRNA in the hypothalamus were significantly decreased. The repeated administration of E-5842 had no effect on FGF-2 mRNA apparent levels in the hippocampus.
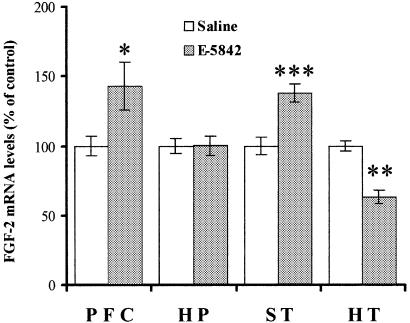
FGF-2 mRNA levels in different rat brain areas after chronic exposure to E-5842 (20 mg/kg i.p., daily for 21 days). The results, expressed as percentage of FGF-2 mRNA levels of saline injected rats, represent the mean ± SEM of (n) independent determinations. PFC, prefrontal cortex (n = 16); HP, hippocampus (n = 13); ST, striatum (n = 22); HT, hypothalamus (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 vs. saline-treated rats (anova with Dunnett's t-test).
Effect of acute treatment with E-5842 and clozapine on FGF-2 mRNA levels
The effect of an acute injection of E-5842 on FGF-2 mRNA levels was also studied. After a single injection of E-5842 (20 mg/kg i.p.), increased levels of FGF-2 mRNA were observed in three out of the four brain areas studied (prefrontal cortex, hippocampus, striatum) as shown in Fig. 4. For these experiments, the animals were killed 6 h after the single injection, a time point that has been reported by Riva et al. (1999), using clozapine, to show regulation of FGF-2 mRNA. In some experiments, the effect of a single administration of clozapine (15 mg/kg, i.p.) was also studied. Levels of FGF-2 mRNA after an acute administration of clozapine were regulated in a similar way (Fig. 4), as has been described previously (Riva et al., 1999), with increased levels of FGF-2 mRNA in the striatum. On the other hand, no variation in the FGF-2 mRNA levels was detected in any of the studied brain areas when the animals were killed 24 h after a single injection of E-5842 (data not shown).
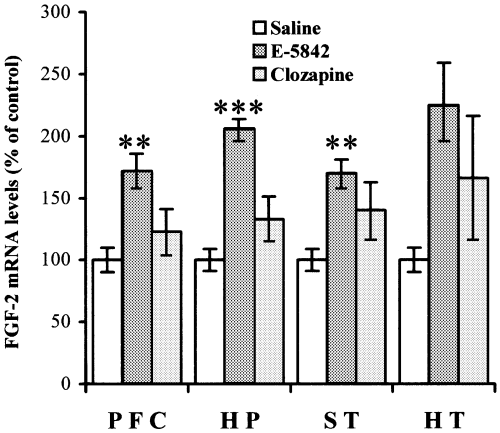
FGF-2 mRNA levels in different rat brain areas 6 h after a single injection of E-5842 (20 mg/kg i.p.) or clozapine (15 mg/kg, i.p.). The results, expressed as percentage of FGF-2 mRNA levels of saline-injected rats, represent the mean ± SEM of independent determinations (n for the E-5842 6 h condition/n for the clozapine 6 h condition). PFC, prefrontal cortex (n = 7/7); HP, hippocampus (n = 8/7); ST, striatum (n = 7/4); HT, hypothalamus (n = 3/2). **P < 0.01, ***P < 0.001 vs. saline treated rats (anova with Dunnett's t-test).
The dose–response effect of acute treatment with E-5842 on FGF-2 mRNA expression in prefrontal cortex, hippocampus and striatum was also studied. As shown in Fig. 5A, a single injection of E-5842 (5 mg/kg, i.p.) produced a moderate increment on the mRNA FGF-2 expression in the three brain areas referred to above, whereas a dose of 30 mg/kg produced the regulation of FGF-2 mRNA up to levels similar to those seen with a 20-mg/kg administration. Figure 5B shows a typical example of Northern blots used for the analysis of this experiment.
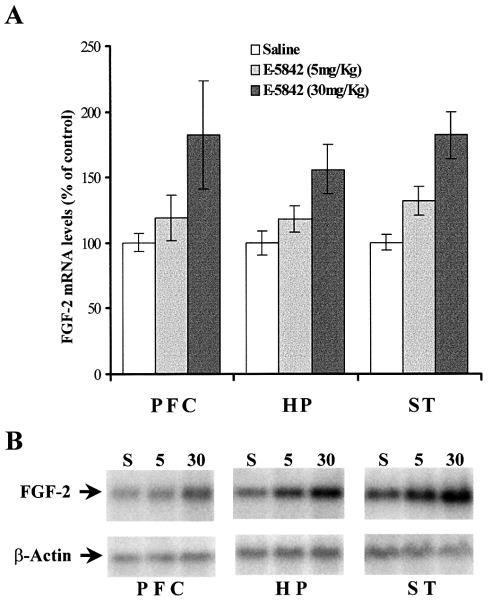
Dose–response analysis of FGF-2 mRNA levels in different rat brain areas after a single injection of E-5842 (5 and 30 mg/kg i.p.). (A) The results, expressed as percentage of saline injected rats, represent the mean ± SEM of six independent determinations. (B) Example of typical Northern blots used for the analysis of these experiments. PFC, prefrontal cortex; HP, hippocampus; ST, striatum.
Effect of chronic treatment with E-5842 on FGF-2 protein levels
The effect observed on the FGF-2 mRNA levels in prefrontal cortex and striatum after chronic and acute treatments of E-5842 was partially paralleled by an elevation of FGF-2 protein in such areas. As shown in Fig. 6, Western blot analysis demonstrated that all FGF-2 isoforms (18, 22 and 24 KDa) (Florkiewicz et al., 1991) were similarly up-regulated in the prefrontal cortex in response to chronic administration of E-5852 (20 mg/kg, i.p., daily for 21 days). A clear tendency to show elevated levels of immunoreactivity was observed in almost all the experiments involving the striatum, but such increase did not reach statistical significance. In contrast, the acute administration of E-5842 (with the animals killed 6 h after E-5842 administration) did not change the immunoreactivity levels of any of the FGF-2 isoforms in any of the brain regions studied as compared to saline treated animals (data not shown).
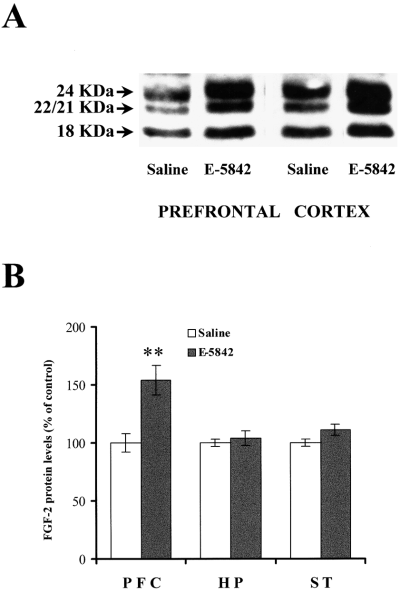
Immunodetection of FGF-2 isoforms by Western blot analysis. (A). Typical autoradiogram showing the effect of a chronic treatment with E-5842 (20 mg/kg, i.p., daily for 21 days) on FGF-2 isoform levels in different brain areas. (B) Graphic representation of FGF-2 immunoreactivity regulation by E-5842. PFC, prefrontal cortex; HP, hippocampus; ST, striatum. Each bar represents the mean ± SEM of data from 12–20 animals from two or three different experiments. **P < 0.001 vs. saline treated rats (anova with Dunnett's t-test).
Discussion
The data reported in our study show that repeated administration of the putative atypical antipsychotic and sigma-1 receptor ligand E-5842 is able to modulate, in a region-specific way, the levels of FGF-2 mRNA and protein. Changes in mRNA levels are also detected by Northern blotting 6 h after a single administration of E-5842, and disappear after 24 h, the time point at which animals from the chronic studies were killed after the last administration.
FGF-2 is a member of the fibroblast growth factors family (Basilico & Moscatelli, 1992; Bikfalvi et al., 1997). FGF-2 is known to be synthesized in the central nervous system, where it plays an important role in neuronal maintenance and repair (Baird, 1994). The physiological importance of FGF-2 in neuronal trophism would suggest that a pharmacological intervention in the sense of increasing the biosynthesis of different neurotrophic factors may have therapeutic significance in the treatment of neuronal diseases. Based on these considerations, the effect of E-5842 promoting the synthesis of FGF-2 could have a relevance regarding its therapeutic potential.
Among the different therapeutic approaches for treating schizophrenia, clozapine is an antipsychotic with characteristics not found in other antipsychotic molecules. It has been described that clozapine (the prototype of atypical antipsychotics) is able to induce FGF-2 expression in the striatum after a chronic treatment, a characteristic not shared by other typical or atypical compounds (Riva et al., 1999). Our results indicate that E-5842 is also able to increase the expression of FGF-2 in the rat striatum, despite its different receptor binding profile as compared to clozapine. On the other hand, and in contrast to classical antipsychotics, E-5842 and clozapine have a clear effect inducing the acute expression of Fos immunoreactivity in the prefrontal cortex while they fail to induce such expression in the striatum (Robertson & Fibiger, 1992; Robertson et al., 1994; Guitart & Farré, 1998). These data, together with the fact that E-5842 seems to have little liability to induce extrapyramidal symptoms, makes E-5842 a good candidate for an atypical compound. Moreover, we show in our work that, after chronic treatment, E-5842 is able to induce the expression of FGF-2 mRNA and protein in some areas of the brain other than the striatum. This up-regulation of FGF-2 mRNA and protein in the prefrontal cortex after chronic treatment is remarkable. To our knowledge this is a characteristic of E-5842 not shared by any other antipsychotic either commercial or in clinical trials. According to recent published reports (Spivak et al., 1997; Karp et al., 1999), clozapine seems to ameliorate tardive dyskinesia and other movement disorders. It has been hypothetized that the induction of FGF-2 in the striatum by clozapine could be a mechanism through which this antipsychotic might ameliorate the movement disorders (Riva et al., 1999). If this is the case, E-5842 behaves in a very similar way inducing FGF-2 expression, thus suggesting that such a beneficial clinical effect could also be expected. Furthermore, it is known that FGF-2 is neuroprotective in experimental parkinsonism in mice (Otto & Unsicker, 1990), and that it stimulates survival and differentiation in dopaminergic neurons (Krieglstein et al., 1998).
Our study also shows that E-5842 is able to induce, after a single administration, FGF-2 mRNA expression in some of the brain areas analysed. In our hands, and using Northern blotting hybridization, the level of induction of FGF-2 mRNA is higher for E-5842 than for clozapine. Using another experimental approach, the RNAase protection assay, it has been previously shown (Riva et al., 1999) that clozapine and the typical antipsychotic chlorpromazine increase striatal FGF-2 mRNA expression, although the effect of chlorpromazine is lost after repeated treatment. The acute effect observed with E-5842 persists in the prefrontal cortex and the striatum, which might suggest a characteristic mechanism of action.
One of the major findings of our work is the up-regulation of FGF-2 mRNA and protein levels in the prefrontal cortex after chronic E-5842 treatment. To our knowledge, variations in FGF-2 expression levels in the prefrontal cortex due to antipsychotic treatment has never been described. Cognitive disturbances are commonly observed in patients with schizophrenia, a brain area that has been repeatedly implicated in the pathophysiology of schizophrenia (Weinberger & Berman, 1988; Goldman-Rakic & Selemon, 1997; Volz et al., 1997; Hazlett et al., 2000). Moreover, a number of clinical studies indicate that abnormalities such as loss of neurons and decrease in weight and volume of the cortex are observed in several cortical areas (Roberts, 1990; Schroeder et al., 1996). In the same way that an induced increase in FGF-2 expression levels in the striatum might be associated to the amelioration of extrapyradimal symptoms (Riva et al., 1999), it could be suggested that the up-regulation of FGF-2 mRNA and protein in the prefrontal cortex might be associated to an amelioration of cognition deficits. In has been proposed that the increase in c-fos expression in the prefrontal cortex by atypical antipsychotics could be related to a possible effect on negative symptoms of schizophrenia (Robertson et al., 1994) although this assumption has to be clearly determined. E-5842 shares with clozapine and other atypical compounds this characteristic (Guitart & Farré, 1998). In a similar way, it could be suggested that regulation of FGF-2 in the prefrontal cortex would represent another marker of putative activity on the negative symptoms of schizophrenia. If the proposed hypothesis of a link between hypofrontality and deficit cognition is correct, then it could be a relationship between up-regulation of FGF-2 expression and improvement of some negative symptoms although, again, this assumption should be clearly demonstrated. E-5842 and other putative antipsychotics could be supporting the survival of selected neurons not only in the striatum but also in the prefrontal cortex.
The role of the sigma receptors is controversial, and it has been hypothesized that the activation of this receptor triggers its translocation to the plasma membrane and hence regulates several components implicated in signal transduction (Morin-Surun et al., 1999). Sigma receptor ligands show neuroprotective actions on dopaminergic neurons and may attenuate N-methyl-d-aspartate cytotoxicity (Gronier & Debonnel, 1999; Shimazu et al., 2000). It would be interesting to determine the exact relationship between the E-5842 effect on FGF-2 expression, and whether the neuroprotection reported could be mediated by FGF-2. In summary, the present results, together with results published by others, point to the importance of FGF-2 expression after the administration of clinically proven antipsychotics and putative new ones such as E-5842. FGF-2 expression could be considered a good indicator of anatomical sites activated by these compounds, and in close relationship with the amelioration of some of the symptoms of schizophrenia.
Abbreviations
-
- E-5842
-
- 4-(4-fluorophenyl)-1,2,3,6-tetrahydro-1-[4-(1,2,4-triazol-1-il)butyl] pyridine citrate
-
- FGF-2
-
- fibroblast growth factor-2
-
- PCR
-
- polymerase chain reaction.




