Defining the role of Drosophila lateral neurons in the control of circadian rhythms in motor activity and eclosion by targeted genetic ablation and PERIOD protein overexpression
Abstract
The ventral lateral neurons (LNvs) of the Drosophila brain that express the period (per) and pigment dispersing factor (pdf) genes play a major role in the control of circadian activity rhythms. A new P-gal4 enhancer trap line is described that is mostly expressed in the LNvs This P-gal4 line was used to ablate the LNvs by using the pro-apoptosis gene bax, to stop PER protein oscillations by overexpressing per and to block synaptic transmission with the tetanus toxin light chain (TeTxLC). Genetic ablation of these clock cells leads to the loss of robust 24-h activity rhythms and reveals a phase advance in light–dark conditions as well as a weak short-period rhythm in constant darkness. This behavioural phenotype is similar to that described for disconnected1 (disco1) mutants, in which we show that the majority of the individuals have a reduced number of dorsally projecting lateral neurons which, however, fail to express PER. In both LNv-ablated and disco1 flies, PER cycles in the so-called dorsal neurons (DNs) of the superior protocerebrum, suggesting that the weak short-period rhythm could stem from these PDF-negative cells. The overexpression of per in LNs suppresses PER protein oscillations and leads to the disruption of both activity and eclosion rhythms, indicating that PER cycling in these cells is required for both of these rhythmic behaviours. Interestingly, flies overexpressing PER in the LNs do not show any weak short-period rhythms, although PER cycles in at least a fraction of the DNs, suggesting a dominant role of the LNs on the behavioural rhythms. Expression of TeTxLC in the LNvs does not impair activity rhythms, which indicates that the PDF-expressing neurons do not use synaptobrevin-dependent transmission to control these rhythms.
Introduction
Circadian clocks control internal temporal organization of living organisms and allow them to anticipate the daily changes in environmental conditions. The biological importance of circadian clocks is illustrated by the large number of physiological and behavioural processes that display circadian rhythms (Moore-Ede et al., 1982; Saunders, 1982). Molecular genetics studies have shown that circadian clocks require a set of genes involved in a transcriptional feedback loop, and that most of them are remarkably conserved between fruit flies and mammals (reviewed in Dunlap, 1999; King & Takahashi, 2000; Scully & Kay, 2000). In both cases, a brain-located circadian pacemaker is responsible for the control of activity rhythms. In the mammalian brain, this circadian function is carried out by the suprachiasmatic nucleus (SCN) of the hypothalamus (reviewed in Klein et al., 1991). In insects, it has been proposed that the activity pacemaker stems from a limited number of neurons, in the accessory medulla of the optic lobe, that synthesize the pigment dispersing factor (PDF) neuropeptide (reviewed in Helfrich-Förster et al., 1998).
The expression of the clock genes within the brain provides a framework for understanding the anatomical and functional organization of the pacemaker that controls activity rhythms. The expression of period (per), the first discovered clock gene, has been extensively studied in the Drosophila brain. The PER protein is detected in scattered glial cells and at least four groups of neurons (Siwicki et al., 1988; Zerr et al., 1990; Ewer et al., 1992; Frisch et al., 1994; Kaneko et al., 1997; Helfrich-Förster et al., 1998; Kaneko, 1998). In the lateral brain, the dorsal lateral (LNds) and ventral lateral neurons (LNvs) show circadian fluctuations of PER expression with high levels of the protein at the end of the night (Zerr et al., 1990; Frisch et al., 1994; Kaneko et al., 1997). The PDF-expressing LNvs, located in the accessory medulla, can be subdivided into four small cells (s-LNvs) already present in the larval brain and four to six large cells (l-LNvs) detected from mid-metamorphosis (Helfrich-Förster, 1997). The l-LNvs have large arborizations at the periphery of the medulla and send projections to the contralateral LNvs through the posterior optic tract (POT) (Helfrich-Förster & Homberg, 1993; Nässel et al., 1993; Helfrich-Förster, 1995), whereas the s-LNvs project dorsally to the superior protocerebrum (Helfrich-Förster & Homberg, 1993; Nässel et al., 1993; Helfrich-Förster, 1995) where PDF accumulation within their axons terminals is rhythmically regulated (Helfrich-Förster et al. 2000; Park et al. 2000). PER is also expressed in the dorsal neurons (DNs) of the superior protocerebrum (Siwicki et al., 1988; Ewer et al., 1992) where protein oscillations have been shown to occur in light–dark (LD) conditions (Frisch et al., 1994; Kaneko et al., 1997).
The respective contribution of the different PER-expressing brain cells to the generation of activity rhythms has been addressed in several studies. Mosaic analysis has suggested that glial PER was able to generate weak long-period activity rhythms (Ewer et al., 1992). In the largely arrhythmic disconnected mutant (Dushay et al., 1989), a comparison of locomotor behaviour and PDF expression in the brain has shown that the existence of strong activity rhythms in a very small number (1–2%) of individuals was correlated with the detection of PDF-stained LNvs that project dorsally (Helfrich-Förster, 1998). The use of per transgenes to restore rhythmicity in per01 arrhythmic mutants has underlined a correlation between PER expression in the LNs and robust activity rhythms (Frisch et al., 1994). In another study, per expression in unidentified glass-expressing cells of the central brain was sufficient to generate long-period activity rhythms in per01 flies (Vosshall & Young, 1995).
This paper analyses the role of the PER- and PDF-expressing LNvs in the generation of activity and eclosion circadian rhythms by using a new P-gal4 enhancer-trap line (gal1118), which is mostly expressed in the LNvs, to kill these cells or to target PER overexpression. We show that both treatments disrupt circadian rhythmicity, although to a different degree. We also show that autonomous PER cycling occurs in other central brain neurons and discuss the possibility that these cells contribute to the behavioural rhythms. While this manuscript was in preparation, genetic ablation as well as PER overexpression experiments were reported using other P-gal4 drivers and lead to similar defects of the activity rhythms (Renn et al., 1999; Kaneko et al. 2000). These results are discussed in relationship to the present study.
Materials and methods
Fly strains
Drosophila cultures were maintained on a 12-h light–dark cycle on corn meal–yeast–agar medium at 25 °C and 50% relative humidity. All the fly lines carried the white mutation on the X chromosome. The alleles of the X-linked genes disconnected and period were disco1 and per01 (associated with the forked marker for the former). To make the upstream activating sequence (UAS)-per constructs, a per cDNA of 3.9 kb beginning at the SpeI site (46 bp upstream from the ATG) and ending at position 4230 in the mRNA (134 bp upstream of the HindIII site in the 3′ untranslated region (UTR) was cloned into the pUAST vector by XhoI and XbaI sites. Two independent lines were obtained by microinjection and used to generate five additional lines by P-element mobilization, including the third chromosomal lines UAS-per16 and UAS-per19. The UAS-bax construct used to ablate cells by activating cell death is described elsewhere (Gaumer et al., 2000). Briefly, an EcoRI-XbaI fragment of murine bax cDNA containing the full-length coding region was ligated into pUAST, the resulting plasmid was microinjected and several independent insertions were obtained, comprising the UAS-bax1 insertion on the third chromosome that was used in this study. The UAS-TeTxLC (TeTxLC, tetanus toxin light chain) construct allows GAL4-induced synthesis of the tetanus toxin light chain which blocks synaptobrevin-dependent neurotransmitter release (Sweeney et al., 1995). Two lines of the active toxin (tntG, tntH) that are strongly activated by gal4 drivers and a line bearing an insertion coding for a mutant inactive protein (tntV1-A) were used (all second chromosomal lines), and have been described previously (Sweeney et al., 1995). A UAS-lacZ reporter was used in our initial disco1 studies, but was then replaced by a UAS-gfp (GFP-A65T) for better sensitivity (see text). The two lines carry insertions on the second chromosome and were provided by the Bloomington stock centre.
Behavioural analyses
Behavioural analyses were carried out in LD 12 : 12 h conditions and in constant darkness (DD) at 20 °C. Temperature was controlled via a Temperatel processor (SLEL, France), reducing fluctuations around the set point to < 0.3 °C, in both light-on and light-off conditions. Light was provided by cool white fluorescent lamps. The locomotor activity of young males (1–7 days) was followed with Drosophila activity monitors (Trikinetics Inc., Waltham, USA). For LD 12 : 12 analysis, flies were left in LD for 1–2 days before starting to collect data. For DD analysis, flies were first entrained in LD 12 : 12 for 2–4 days and recorded in DD for 6–8 days. LD and DD average activity plots were generated according to Hamblen-Coyle et al. (1992) and Wheeler et al. (1993). Circadian rhythmicity in DD was tested by χ2 periodogram analysis as described in Hamblen et al. (1986). Only flies with a period of between 15 and 32 h (a range that includes all known rhythmic clock mutants with altered periods) and a power (height of the periodogram peak above the 5% significance threshold) > 20 (with filter on) were considered circadian rhythmic. To assess the rhythmicity of eclosion, ≈ 15 males and 15 females were introduced into 150-mL vials, with a separable bottom part containing standard medium. Eggs were laid for 10–11 days at 20 °C in LD 12 : 12 conditions. When the top part of the vials was covered with pupae, the adults were discarded and the food-containing bottom of the vials was replaced with a clean empty one. Lights were switched off when the first dark pupae appeared. Newly eclosed flies were emptied from the vials every 4 h, under red safelight illumination, for 3–4 days. For each time point, the number of eclosed flies was calculated with the formula n= sample weight/average fly weight (average fly weight = 1 mg), because pilot experiments had shown that the eclosion curves obtained by counting and by weighing the flies were indistinguishable.
Immunocytochemistry and GFP fluorescence
All experiments were performed on whole-mounted adult or larval brains. CNSs from third instar larvae and adults were dissected into phosphate-buffered saline (PBS), fixed in 4% formaldehyde in PBS for 2 h at room temperature (RT) and washed for 6 × 10 min in OX-PBS (PBS and Thimerosal) with 0.3% Triton X-100 (OX-PBS-T). Tissues were pretreated with OX-PBS with 1% Triton X-100 for 10 min at RT and blocked in OX-PBS-T containing 10% normal goat serum (NGS; Sigma #G9023) for 1 h at RT. Then they were incubated at 4 °C with rabbit antifull-length PER serum (Stanewsky et al., 1997) that had been adsorbed against per01 adult acetone powder, at 1 : 15 000 in OX-PBS-T containing 5% NGS (OX-PBS-T-N) for at least 72 h, rabbit anticrab β-PDH serum (Dircksen et al., 1987) at 1 : 5000 in OX-PBS-T-N for 48 h, or anti-TeTxLC monoclonal antibody (J. Thierer & H. Niemann, unpublished data) at 1 : 2000 in OX-PBS-T-N for 48 h. After washing for 6 × 10 min in OX-PBS-T, samples were labelled with Texas red-conjugated goat antirabbit IgG antibody (Cappel #55675; Costa Mesa, USA) at 1 : 1000 in OX-PBS-T-N for 3 h at RT or overnight at 4 °C. They were washed for 6 × 10 min in OX-PBS, then mounted in Mowiol. To detect GFP expression, CNSs were dissected into saline buffer, fixed, washed and mounted. Preparations were examined immediately using an epifluorescence microscope (Zeiss Axioplan2) and a cooled digital camera (Diagnostic Instruments SPOT2, USA). Fluorescence intensity was quantified from digital images using the following formula: I = 100 × (S − B)/B, where S is the average fluorescence intensity (which was never saturating on a 0–255 scale) of the labelled cells and B is the average intensity in regions adjacent to the cells. It thus represents the percentage fluorescence above background.
Immunoblotting
Protein extracts were made from ≈ 20 heads for each time-point. Frozen heads were homogenized in 100 µL ice-cold extraction buffer in mm: Tris pH 7.5, 50; NaCl, 100; NaF, 50; EDTA, 5; [4-(2-aminoethyl)-benzenesulfonylfluoride (AEBSF), 1; plus Triton X-100, 1%; aprotinine, 1%; chymostatin, pepstatin, leupeptin and antipapain, 1 µg/mL each]. After centrifugation, supernatant fractions containing 50 µg of proteins were mixed with SDS sample buffer, boiled, and analysed by protein immunoblot as previously described (Edery et al., 1994). The following modifications were made: rabbit anti-PER antibody was adsorbed against per01 adult head acetone powder and incubated with a per01 head immunoblot before being used at a 1 : 10 000 dilution. Anti-PER was detected with an antirabbit IgG horseradish peroxydase-conjugated antibody (Amersham) diluted 1 : 3000.
Results
The gal1118 enhancer trap line is mostly expressed in the PDF-expressing lateral neurons of the central brain
The gal1118 line was generated in the course of a P-gal4 enhancer-trap screen aimed at isolating genes expressed in the central brain (Boquet et al., 1999). The gal1118 insertion is located in cytological position 82D-E on the third chromosome and does not correspond to any known gene involved in circadian rhythms. Flies carrying one or two copies of the gal1118 insertion display a 1-h lengthening of their circadian period (Table 1). The period change is lost by excision of the P element (A. Lamouroux & F. Rouyer, unpublished results), indicating that it is a consequence of the insertion, although it is not known whether it is due to the disruption of the genomic sequence or to the production of large amounts of GAL4 protein that may alter the functioning of the gal1118-expressing cells.
| Genotype | Number of flies | Number of circadian rhythmic flies (%) | Mean period(h) | Meanpower |
|---|---|---|---|---|
| w | 102 | 97 (95) | 23.8 ± 0.1 | 106 ± 3 |
| w;;gal1118/+a | 54 | 51 (95) | 24.8 ± 0.1b | 101 ± 4 |
| w;;gal1118 a | 127 | 118 (93) | 25.0 ± 0.1b | 111 ± 5 |
| w disco 1 f | 26 | 6 (23) | 19.2 ± 0.5 | 34 ± 6 |
| w disco 1 f;;gal1118/+a | 66 | 23 (35) | 21.1 ± 0.8c | 38 ± 5 |
| w disco 1 f;;gal1118 a | 35 | 11 (31) | 22.5 ± 0.7c | 36 ± 6 |
| w;;gal1118/UAS-baxa | 122 | 21 (17) | 22.5 ± 0.4 | 33 ± 2 |
| w;;UAS-bax/TM3 | 43 | 40 (93) | 23.7 ± 0.1 | 112 ± 6 |
| w;;gal1118 UAS-per16/+ | 39 | 33 (84) | 24.3 ± 0.1 | 87 ± 8 |
| w;;gal1118 UAS-per16 | 53 | 5 (9) | 25.2 ± 1.0 | 33 ± 3 |
| w;;UAS-per16 | 61 | 52 (85) | 24.3 ± 0.1 | 113 ± 6 |
| w;;gal1118 UAS-per19/+ | 17 | 9 (53) | 25.5 ± 0.3 | 41 ± 7 |
| w;;gal1118 UAS-per19 | 18 | 3 (17) | 26.0 ± 6.0 | 25 ± 3 |
| w;UAS-per19 | 31 | 31 (100) | 24.3 ± 0.1 | 138 ± 14 |
| w;UAS-tntG/+;gal1118/+ | 31 | 26 (84) | 24.6 ± 0.1 | 73 ± 6 |
| w;UAS-tntG; gal1118 | 73 | 68 (93) | 24.7 ± 0.1d | 92 ± 4 |
| w;UAS-tntG | 8 | 8 (100) | 23.5 ± 0.0 | 136 ± 5 |
| w;UAS-tntH/+;gal1118/+ | 32 | 30 (94) | 24.8 ± 0.1 | 82 ± 6 |
| w;UAS-tntH; gal1118 | 29 | 30 (87) | 24.6 ± 0.2d | 80 ± 6 |
| w;UAS-tntH | 10 | 10 (100) | 23.6 ± 0.1 | 88 ± 14 |
| w;UAS-tntV1-A; gal1118 | 10 | 10 (100) | 24.5 ± 0.1 | 123 ± 11 |
- The analysis was carried out over 6–8 days in DD, following a 2–4-day entrainment period in LD 12 : 12 h. The tested genotypes are listed on the left. aSome of the individuals in these genotypes carry one or two copies of the UAS-gfp or UAS-lacZ reporters, which did not significantly affect behaviour (not shown). Criteria used to define circadian rhythmic flies are described inMaterials and methods. The mean values of individual flies periods and associated powers are given ± SEM. bw;;gal1118 and w;;gal1118/+ flies show a slight but significant (P < 0.01, unilateral Student's test) period increase compared to wild-type (w) but are not significantly different from each other (P > 0.1). cSimilar effects held in a disco 1 background, with one or two copies of gal1118 significantly increasing the period (P < 0.01), whereas the increase seen between one copy and two copies of gal1118 was not significant (P > 0.1). dF-test suggests that the variances of the period distributions for these two genotypes are significantly different (P < 0.02).
gal1118 expression was studied in the adult brain. Using UAS-gfp or UAS-lacZ as reporters, no difference could be detected between expression at Z9 and ZT22 (ZT, Zeitgeber time) in a wild-type background nor between wild-type and per01 or tim01 mutants (data not shown). In w;UAS-gfp/+;gal1118/+ flies, the gal1118 expression pattern closely resembles the PDF one (Helfrich-Förster & Homberg, 1993; Helfrich-Förster, 1997), namely two small sets of cell bodies in the lateral brain with projections into the optic lobe medulla, toward the superior protocerebrum and through the posterior optic tract (Fig. 1A and B). However, gal1118 is not expressed in the few PDF-expressing cells of the tritocerebrum (PDF-Tri in Fig. 1B) nor in the PDF neurons of the abdominal ganglion (PDF-Ab, not shown). gal1118 cells are observed at the surface of the medulla in young adults (gme for gal1118 medulla, Fig. 1A), but the signal strongly decreases in the ageing fly (not shown).
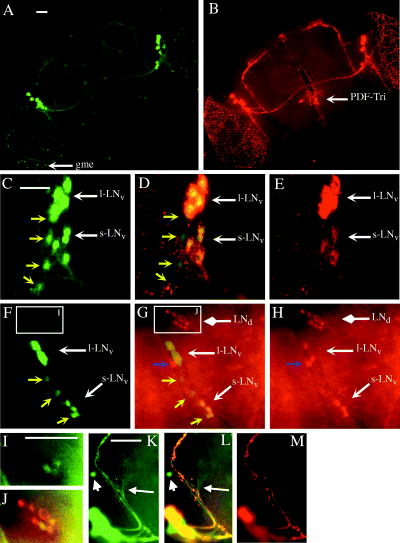
Expression of gal1118, PDF and PER expression in the brain of heterozygous w;UAS-gfp/+;gal1118/+ adult flies. (A) GFP fluorescence and (B) PDF immunocytochemistry (Texas red-coupled antibody) double labelling. gme, gal1118-expressing cells in the medulla; PDF-Tri, PDF-expressing cells of the tritocerebrum. (C–E) GFP fluorescence (C and D) and PDF immunocytochemistry (D and E) double labelling in the region of the lateral neurons. s–LNv, PDF-expressing small cell body ventral lateral neurons (four cells); l-LNv, PDF-expressing large cell body ventral lateral neurons (four cells). Yellow arrows point to four gal1118-positive PDF-negative s-LNv-like cells. (F–H) GFP fluorescence (F and G) and PER immunocytochemistry (Texas red-coupled antibody) (G and H) double labelling in the lateral neuron region at ZT22. LNd, PER-expressing dorsal lateral neurons (6–7 cells). Yellow arrows point to three gal1118-positive PER-negative s-LNv-like cells whereas three s-LNv and two L-LNv are labelled with the two markers. The blue arrow points to a single PER-positive gal1118-negative cell. No PER-positive cells were detected in per01 brains (not shown). (I and J) Close-up of the LNd region (from F and G, respectively). The green (GFP) signal was enhanced, allowing to revelation of weak gal1118 expression in three of the LNds. According to GFP signal quantification (not shown), gal1118 expression is at least 10 times lower in the LNds than the LNvs. (K–M) Close-up of the LNv dorsal projections (same brain as in A and B). The white arrow points to the gal1118-positive PDF-negative projection and arborization that comes from the gal1118-positive PDF-negative s-LNv-like cells (not shown). The short arrow points to a dorsolateral PDF-negative gal1118 cell that is mostly visible in homozygous w;UAS-gfp;gal1118 brains (gd2; see Fig. 2F– H). Scale bars, 50 µm.
In the lateral brain of w;UAS-gfp/+;gal1118/+ flies, gal1118‘w;UAS-gfp;gal118/+’ expression is detected in 3–8 small cells (s-LNv-like in Table 2) and 3–6 large cells (l-LNv-like in Table 2) (Fig. 1A, C and F and Table 2). Although the number of s-LNv-like cells is often difficult to estimate accurately because of the presence of numerous gal1118-labelled neuronal processes in this region, it is larger on average than the number of PDF s-LNvs (5 vs. 3.5, Table 2) whereas the number of l-LNv-like cells is similar to the number of PDF l-LNvs (4 vs. 3.8, Table 2; see also Helfrich-Förster, 1997). To identify these different cells, double-labelling experiments were performed with GFP fluorescence and PDF (Fig. 1C–E) or PER (Fig. 1F–H) immunocytochemistry. These experiments show that gal1118 is strongly expressed in the PDF-positive and PER-positive s- and l-LNvs (Table 2). In agreement with more gal1118- than PDF-expressing s-LNv-like cells, double labelings show that gal1118 is also expressed (although less strongly) in 1–4 s-LNv-like cells that do not express PDF, whereas no cells staining for PDF only could be observed (Fig. 1C–E and Table 2). gal1118 cells that do not express PER were also identified (Fig. 1F–H and Table 2) and because there are no PER-negative PDF-positive cells (Helfrich-Förster, 1995), we assume that the PER-negative gal1118 cells are the same cells as the PDF-negative gal1118 cells. These additional s-LNv-like cells revealed by gal1118 send projections that follow the s-LNvs dorsal projections but stop shortly to arborize without reaching the dorsal brain (Fig. 1K–M), whereas each of the projections going further dorsally is both GFP- and PDF-labelled in w;UAS-gfp/+;gal1118/+ brains (Fig. 1K–M, and data not shown). A single PER-positive LNv-like cell (PER-only cell in Table 2 and Fig. 1F–H), which could be the same cell as the PDF-negative PER-expressing fifth LN described in the larval brain (Kaneko et al., 1997), does not express gal1118. This cell accounts for the excess of PER- vs. PDF-expressing cells in the s-LNv-like group (4.2 vs. 3.5 in Table 2).
| Mean number of cells ± SEM (number of hemispheres used for cell counting) | |||||
|---|---|---|---|---|---|
| Adult | |||||
| Genotype | Labelled cells | Third instar Larva, Larval LN-like cells | s-LNv-like cells | l-LNv-like cells | LNd-like cells |
| w;UAS-gfp/+;gal1118/+ | GFPb | 4.0 ± 0.0 (21) | 5.0 ± 0.3 (33) | 4.0 ± 0.2 (33) | 2.5 ± 0.7 (33) |
| w;;gal1118/+ a | PERc | 4.9 ± 0.1 (15) | 4.2 ± 0.2 (29) | 3.8 ± 0.2 (29) | 5.7 ± 0.3 (29) |
| w;UAS-gfp/+;gal1118/+ | GFP + PERd | 4.0 ± 0.0 (6) | 3.5 ± 0.4 (6) | 3.3 ± 0.4 (6) | 2.3 ± 0.8 (6) |
| GFP onlyd | 0.0 ± 0.0 (6) | 1.2 ± 0.5 (6) | 0.7 ± 0.5 (6) | 0.0 ± 0.0 (6) | |
| PER onlyd | 0.8 ± 0.2 (6) | 0.7 ± 0.2 (6) | 0.0 ± 0.0 (6) | 2.8 ± 0.7 (6) | |
| w;;gal1118/+ a | PDFe | 4.0 ± 0.0 (15) | 3.5 ± 0.2 (21) | 3.8 ± 0.2 (21) | 0.0 ± 0.0 (21) |
| w;UAS-gfp/+;gal1118/+ | GFP + PDFf | 4.0 ± 0.0 (15) | 3.4 ± 0.4 (5) | 3.8 ± 0.2 (5) | ND |
| GFP onlyf | 0.0 ± 0.0 (15) | 3.4 ± 0.6 (5) | 0.0 ± 0.0 (5) | ND | |
| PDF onlyf | 0.0 ± 0.0 (15) | 0.0 ± 0.0 (5) | 0.0 ± 0.0 (5) | ND | |
- The tested genotypes are listed on the left. Labelled cells were detected by gal1118-driven GFP fluorescence and PER as well as PDF immunocytochemistry in single or double (GFP/PER or GFP/PDF) labelling experiments (see Materials and methods). Dissections were carried out in LD 12:12 conditions. For experiments including PER immunocytochemistry, flies were collected at ZT21–22. All other dissections were performed during daytime. aIncludes individuals with one copy of UAS-gfp. bData from both GFP/PER and GFP/PDF double labelings (and GFP single labelings in adults). cData from both PER single- and GFP/PER double-labelings. dData from GFP/PER double labelings. eData from GFP/PDF double labelings (and PDF single labelings in adults). fData from GFP/PDF double labelings. For double labelling experiments, GFP + PER or GFP + PDF means that only cells that were labelled with both markers were counted, whereas GFP only, PER only or PDF only means that cells labelled with only the indicated marker were counted. LN-like, s-LNv-like, l-LNv-like and LNd-like cells means appropriately sized cells that were counted in the area where the PER-expressing larval LNs, or adult s-LNvs, l-LNvs and LNds, respectively, are located. Weakly stained larval gd2L cells (see text) were not counted. ND, not determined.
As shown in Fig. 1G–H and Table 2, other PER-expressing cells of the lateral brain are the 5–8 LNds (Ewer et al., 1992) that do not express PDF (Helfrich-Förster, 1995). These cells appear to be gal1118-negative in most of the w;UAS-gfp/+;gal1118/+ brains as shown in Fig. 1A and F. However, some brains show very weak gal1118 expression in ≈ 50% of the LNds as illustrated in Fig. 1I and J and Table 2 (see also below). No gal1118 expression could be detected outside the brain on sections of adult heads (using UAS-lacZ) or whole white pupae (using UAS-gfp) although weak expression could be easily missed in the whole pupae sections (data not shown).
In order to detect cells weakly expressing gal1118, we also analysed gal1118 cells in flies carrying two copies of the gal1118 and UAS-gfp insertions (w;UAS-gfp;gal1118). In addition to the strong LNv labelling described above, w;UAS-gfp;gal1118 brains reveal the weak gal1118 expression in the PER-expressing LNds (Fig. 2A, C–E). Several other groups of cells weakly expressing gal1118 can be detected in the homozygous w;UAS-gfp;gal1118 flies. Ten to fifteen cells termed gd1 (for gal1118 dorsal 1) are located in the same region as the previously reported PER-expressing DN1 and DN2 neurons, close to the ending of the LNvs dorsal projections (Fig. 2B and F and Kaneko et al., 1997; Kaneko, 1998). Double labelings indicate that some of the cells in the gd1 group are PER-expressing DN1s, whereas two slightly more ventral PER-positive cells are likely to be the PER-expressing DN2s (Fig. 2F–H). A pair of gal1118 cells, termed gd2, are located laterally to the gd1 cells not far from the PER-expressing DN3 cluster (Fig. 2B and F and Kaneko et al., 1997; Kaneko, 1998), but do not express PER (Fig. 2F–H). The two other characterized groups of gal1118 cells are a small group of large-cell-body neurons located at the surface of the central dorsal brain and projecting into the pars intercerebralis (gpi), and ≈ 20 cells located underneath the calyces of the mushroom bodies (gmbc). These two groups of cells were found to display variable levels of gal1118 expression but did not express PER (not shown; see also Kaneko et al., 1997; Kaneko, 1998) nor PDF (see Fig. 1B and Helfrich-Förster & Homberg, 1993; Helfrich-Förster, 1997), as several other cells scattered through the brain (Fig. 2A and B and data not shown).
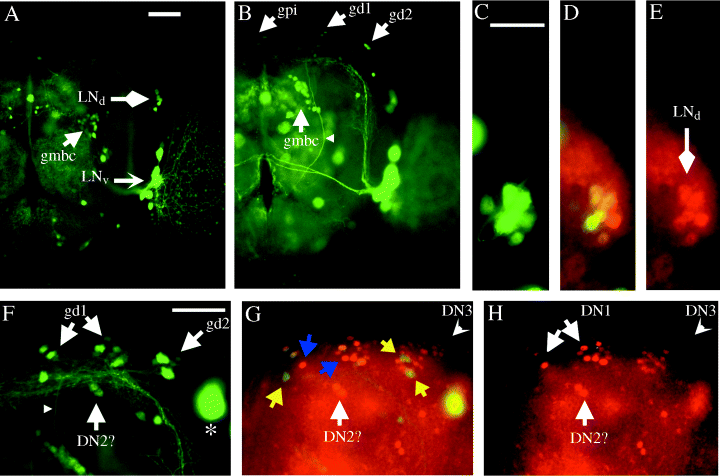
Expression of gal1118 and PER in the brain of homozygous w;UAS-gfp;gal1118 adult flies. GFP fluorescence in (A) an anterior view (focus on the LN cell bodies) and (B) a more posterior view (focus on the LN dorsal projections). LNd, dorsal lateral neurons; LNv, ventral lateral neurons; gpi, gal1118-weakly expressing cells of the pars intercerebralis. The staining in the latter cells can be higher in some brains, as the weak and diffuse signal seen in the region of the antennal lobe neuropile between the LNvs and the oesophagus. gd1 and gd2, gal1118-expressing cells of the dorsal brain. The gd2 cells send a projection that goes down through the central brain (small arrowhead). gmbc, gal1118-expressing cells located near the mushroom bodies calyces. (C–E) GFP fluorescence (C and D) and PER immunocytochemistry (D and E) double labelling in the region of the dorsal lateral neurons at ZT21. (F–H) GFP fluorescence (F and G) and PER immunocytochemistry (G and H) double labelling in the region of the dorsal brain at ZT21. DN1, DN2 and DN3 are PER-expressing dorsal neurons; DN2 identification is not certain as these cells are difficult to differentiate from DN1. The yellow arrows point to gal1118-positive PER-negative cells (one gd1 on the left, two gd2 on the right). The blue arrows point to PER-positive gal1118-negative DN1 cells. The small arrowhead points to the gd2 projection. The asterisk indicates a large ventral lateral neuron that is out of focus. Scale bars, 50 µm.
In the brain of w;UAS-gfp/+;gal1118/+ third instar larvae, gal1118 strongly labelled four dorsally projecting neurons in each brain hemisphere (Fig. 3A and Table 2), that appear similar to the four PDF-expressing neurons (larval LNs) (Fig. 3B, Table 2) previously identified in the larval brain (Helfrich-Förster, 1997). Weak staining was also often observed in two dorsolateral cells termed gd2L (Fig. 3A and C). As in the adult, there was no gal1118 expression in the PDF-Ab neurons (Helfrich-Förster, 1997) at the tip of the ventral ganglion (Fig. 3A and B). Double labelling experiments showed that the cells strongly expressing gal1118 are the PDF larval LNs (Fig. 3C–E) that persist through metamorphosis to become the adult s-LNvs (Helfrich-Förster, 1997). Homozygous w;UAS-gfp;gal1118 larval brains were also analysed to detect cells with weaker gal1118 expression. In addition to the four larval PDF-expressing LNs, four other PDF-negative gal1118 cells were detected in each hemisphere of the homozygous brains (Fig. 3F), that include the two gd2L cells faintly detected in heterozygous brains. A single neuron (gd1L) located dorsally to the LNs axon terminals sends projections into the mid-brain that finally go to the corpora cardiaca of the ring gland (CC) through the interhemispheric region (Fig. 3F, J and K). The two gd2L neurons also project to the CC through the same path (Fig. 3F, J and K) and look very much like the adult gd2 cells (Fig. 2B and F). A fourth gal1118 neuron (named EH; see below) is located close to the interhemispheric region and sends projections toward the dorsal brain and to the CC (Fig. 3F, J and K). Double labelling experiments with anti-eclosion hormone (EH) antibody (Fig. 3I) show that this cell is the previously described EH-synthesizing neuron (McNabb et al., 1997). Interestingly, the dorsal projections of the EH neuron arborize close to the LNs dorsal projection terminals (Fig. 3I and K). Double labelling with anti-PER antibody showed that besides the LNs, none of the larval gal1118 neurons (namely gd1L, gd2L and EH) expressed PER (Fig. 3F–H).
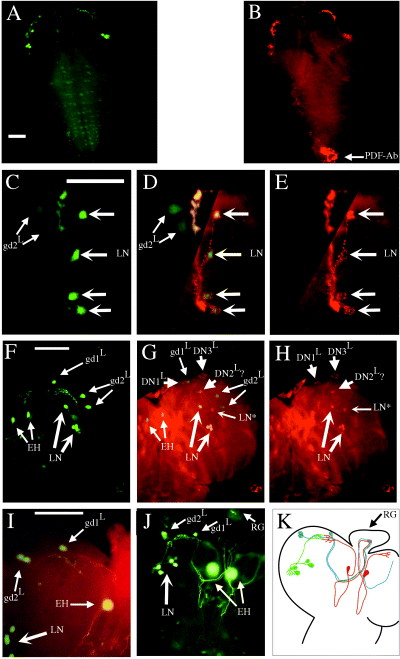
Expression of gal1118, PDF and PER in the third instar larval brain. (A–E) heterozygous w;UAS-gfp/+;gal1118/+ brains. GFP fluorescence (A) and PDF immunocytochemistry (B) double staining. The weak GFP staining in the subesophageal region and in the thoracic ganglion is due to nonspecific expression of the UAS-gfp transgene independently of gal1118 (not shown). (C–E) GFP fluorescence (C and D) and PDF immunocytochemistry (D and E) double labelling in the region of the lateral neurons. (F–K) Homozygous w;UAS-gfp;gal1118 brains. GFP fluorescence (F and G) and PER immunocytochemistry (G and H) double labelling at ZT21. No PER-positive cells were detected in per01 brains (not shown). (I) GFP fluorescence and eclosion hormone immunocytochemistry. (J) GFP fluorescence with focus on the projections of the gal1118 cells going to the ring gland (RG). Many neurites but no cell bodies were detected in the RG when this structure was focused on (not shown). (K) Drawing of gal1118 cells and their projections: larval lateral neurons (green), eclosion hormone synthesizing neurons (red), larval dorsal gal1118 neurons (blue). LN, PDF-, PER- and gal1118-expressing lateral neurons; gd1L and gd2L, larval dorsal gal1118 neurons; LN*, PDF and gal1118-negative PER-expressing lateral neuron; DN1L, DN2L and DN3L, PDF and gal1118-negative PER-expressing larval dorsal neurons. The identification is not certain for DN2L, which have been shown to display an out-of-phase PER cycling (Kaneko et al., 1997). EH, eclosion hormone synthesizing neurons. Scale bars, 50 µm.
Lateral neurons are present in the majority of adult disco1 flies but fail to express PER
Lack of PER and PDF staining in previous studies has shown that PER and PDF do not accumulate in the brains of the vast majority of disco1 flies (Zerr et al., 1990; Helfrich-Förster & Homberg, 1993; Helfrich-Förster, 1998). We used the strong gal1118 expression in the PDF-expressing LNvs to test whether those neurons were still present in the disco1 brain. The study was initiated with a UAS-lacZ reporter gene. No LN-like labelling was detected in any disco1 larval brain (in the presence of one copy each of gal1118 and UAS-lacZ, data not shown), and 62% of the adult brains displayed stained cells (e.g. Figure 4C and D). Using a UAS-gfp reporter resulted in much greater sensitivity in both larvae (Fig. 4A and B) and adults (compare Fig. 4C and D with Fig. 4E and F), and the latter reporter was used for cell characterization and counting.
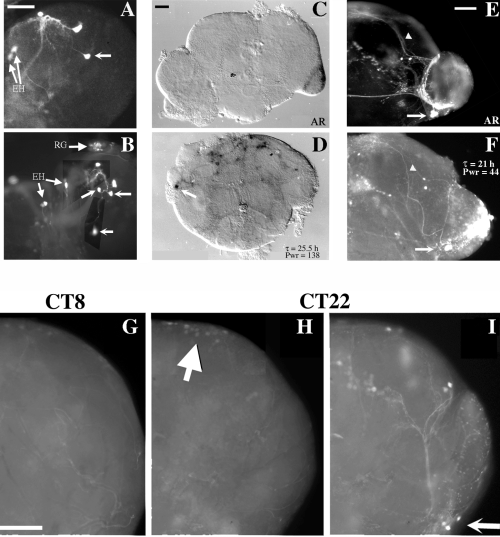
Expression of gal1118 and PER in disco1 brains. (A and B) gal1118 expression in third instar larval brains of w disco1fflies carrying a single copy of gal1118 and one (A) or two (B) copies of UAS-gfp. GFP labelling in gd1L, gd2L and EH neurons of the disco1 brains was usually stronger than in wild-type brains. Depending on the orientation of the larval brain on the coverslip, the three dorsal gal1118 positive cells (one gd1 L and two gd2 L; see Fig. 3) are sometimes located close to the LNs even in a wild-type brain, and the location of labelled cell bodies was even more difficult to ascertain in the disorganized disco1 brain. Because at this stage gal1118 only labels four larval LNs, as well as one EH and three dorsal cells, we conservatively chose to count as putative LNs only cells in excess of three, in addition to the centrally located EH neuron, in a given disco1 larval brain hemisphere. The unlabeled arrows point to putative LN cell bodies. RG, Ring gland. (C–F) gal1118 expression in adult brains of behaviourally tested w disco1f flies carrying a single copy of gal1118 and one copy of UAS-lacZ (C and D) or UAS-gfp (E and F). Brains were dissected at the end of the behavioural test (see Table 3). The circadian phenotype is indicated in the figure (τ, circadian period; Pwr, power; AR, arrhythmic). Arrows point to putative LN cell bodies and arrowheads to the dorsal projections. In the adult brain, only GFP-labelled cells satisfying the following criteria were counted as LNs: (i) a ventrolateral location appropriate for lateral neurons; (ii) the presence of projections reaching the dorsal protocerebrum, higher than the calyces of the mushroom bodies, where no other gal1118 cells project in the wild-type. All the cell bodies of a cell cluster from which a dorsal projection was originating were counted as putative LNs, so the number of dorsally projecting cell bodies could be lower. (G–I) Comparison of PER expression (G and H) and gal1118-driven GFP fluorescence (I) by double labelling in w;UAS-gfp/+;gal1118/+ brains. Flies were entrained for 4 days in LD 12 : 12 and left for 24–48 h in the dark before brain dissection at CT8 (G) or CT22 (H and I). Arrow in I points to the PER-negative putative LNs. Arrow in H points to the dorsal neurons cyclically expressing PER. Scale bars, 20 µm (A, for A and B), 50 µm (C, for C and D; E, for E and F; G, for G–I).
In the larva, 10/13 wdisco1f brains carrying one copy of gal1118 and either one or two copies of UAS-gfp displayed one or more putative LNs (Table 3; see Fig. 4 legend for the identification of putative larval LNs). In contrast, neither PDF (10/10 brains) nor PER (20/20 brains at ZT21) staining was ever detected in the putative LNs of disco1 larval brains (data not shown). In the adult, 83% of the disco1 brains displayed at least one putative LN (Table 3; see Fig. 4 legend for the identification of putative adult LNs). These putative LNs were not labelled with the anti-PDF antiserum, which on the other hand clearly revealed the PDF-Tri neurons (7/7 brains, data not shown), as previously reported in earlier studies (Helfrich-Förster & Homberg, 1993; Helfrich-Förster, 1998).
| Stage | Behaviour | Number of flies(%) | Period(h) | Power | Number of dissected brains | Brains with at least one putative LNa: number (%) | Putative LNs per hemispherea,b: mean ± SEM | Genotype of the dissected brains |
|---|---|---|---|---|---|---|---|---|
| Third | – | – | – | – | 13 | 10 (77) | 0.7 ± 0.2 | wdisco 1 f; UAS-gfp/(+ or UAS-gfp);gal1118/+; |
| instar | ||||||||
| larva | ||||||||
| Adult | Not analysed | – | – | – | 29 | 24 (83) | 1.5 ± 0.2 | wdisco 1 f; UAS-gfp/(+ or UAS-gfp);gal1118/+; |
| Adult | Robust circadian | 2 (2) | 25.0 ± 0.5 | 115 ± 24 | 1 | 1 (100) | 6.0 | |
| Weak circadian | 32 (32) | 21.3 ± 0.6 | 33 ± 2 | 10 | 7 (70) | 1.7 ± 0.4 | wdisco1f; UAS-gfp/+;gal1118/(+ or gal1118). | |
| AR | 67 (66) | – | – | 9 | 7 (78) | 1.3 ± 0.4 | ||
| Total | 101 (100) | – | – | 20 | – | – | ||
- The gal1118-expressing cells were identified by GFP fluorescence. aSeeFig. 4 legend for the identification of putative LNs in larval and adult disco1 brains. bAll brains, including those with no detected cells, were taken into account for the calculation of the putative LNs mean number. Larvae and behaviourally unanalysed adults were dissected during daytime in LD 12 : 12 conditions. wdisco1f flies carrying various combinations of gal1118 and either the UAS-gfp or UAS-lacZ reporter constructs were used in the behavioural assay as described in Table 1. Among the circadianly rhythmic flies (see Materials and methods), only two individuals displayed periods between 24 and 26 h (in the same range as the w;;gal1118/+ or w;;gal1118 controls; see Table 1) and high powers (> 60), and were therefore classified as robust circadian. The brain of one of these two flies (carrying one copy of UAS-lacZ) is shown in Fig. 4D and displayed LN-like cells with dorsal projections. All the other rhythmic flies were classified as weak circadian. AR, arrhythmic. Among the tested flies carrying one or two copies of gal1118and one copy of the UAS-gfp reporter, samples from the three behavioural categories (including the second fly classified as robust circadian) were used for the anatomical study by dissecting the brain at the end of the behavioural assay. The UAS-lacZ reporter yielded similar results, but with lower numbers of expressing individuals and putative LNs (not shown). Mean values are given ± SEM.
Only 34 of 101 behaviourally tested disco1 flies (34%), carrying one or two copies of the gal1118 insertion, were classified as rhythmic, with a mean period 2.5–3.7 h shorter than the corresponding controls (Table 1). Two of these rhythmic flies had a clearly distinct phenotype, with a robust circadian rhythmicity indistinguishable from wild-type, whereas the remaining 32 rhythmic flies had much weaker (compare powers) short-period rhythms (Table 3). In LD 12 : 12, the evening activity peak of the disco1 flies was advanced compared to the controls (Fig. 5A and D), consistent with a fast-running clock. That peak was observed even for the individuals which were subsequently arrhythmic in DD (not shown), as described previously (Dushay et al., 1989; Hardin et al., 1992; Wheeler et al., 1993; Helfrich-Förster, 1998). The distribution of the average activity over a 24-h window shifts from nonrandom during the first 2 days in DD (Fig. 5E) to random during the next two days (Fig. 5F), indicating that the disco1 flies transiently keep a synchronized rhythmic behaviour.
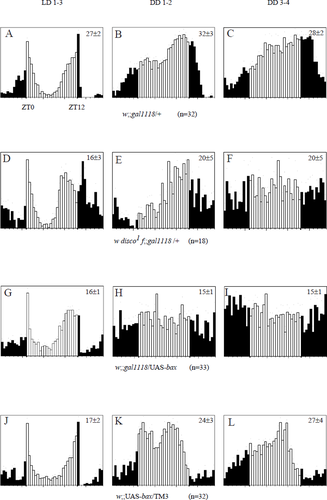
Locomotor activity of w disco1f and w;;gal1118/UAS-bax flies. Histograms represent the distribution of the activity (number of events per 0.5-h bin) through 24 h, averaged for several flies during several days. Activity levels are normalized independently for each set of data, so the ordinates cannot be compared between different graphs. The mean ± SEM daily activity (number of events per bin averaged over 48 bins) is given in the upper right corner of each panel; n, number of flies. The analysis was performed separately in LD 12 : 12 conditions over 3 days (LD 1–3: A, D, G and J), then in constant darkness during the first two days after lights off (DD 1–2: B, E, H and K) and during the next two days (DD 3–4: C, F, I and L). The open bars indicate activity exhibited during the day phase in LD conditions or subjective day in DD, whereas the solid bars indicate activity exhibited during the night phase in LD or subjective night in DD. ZT0 is light on and ZT12 is light off in LD conditions. Dots indicate the SEM of the average activity for each bin. Some of the w disco1f;gal1118/+ and w;;gal1118/UAS-bax individuals used for the analysis also carried a single UAS-lacZ or UAS-gfp insertion.
Samples of the tested disco1 individuals belonging to different behavioural categories were then compared for brain anatomy. We found the same proportion of brains without dorsally projecting gal1118-positive neurons among arrhythmic and weak short-period rhythmic disco1 flies (Table 3 and Fig. 4E and F). In contrast, such dorsally projecting neurons were seen in both of the two flies with robust rhythmicity (see Table 3). One of them was obviously of the connected phenotype (Steller et al., 1987), with close-to-wild-type anatomy (Table 3) but reduced gal1118-driven GFP intensity (not shown). The other one was a UAS-lacZ-bearing fly of the unconnected phenotype, whose contracted gal1118 expression pattern nevertheless included dorsal projections (Fig. 4D).
We wondered whether the short-period residual rhythmicity of the disco1 flies in constant darkness could be related to PER cycling in some central brain neurons. Although gal1118-positive putative LNs are observed in the majority of disco1 brains (see above), no PER was ever detected at circadian time (CT8; 16 brains) or CT22 (18 brains) in those flies as illustrated in Fig. 4G–I. In contrast, PER was detected at CT22 but not at CT8 in one or two groups of dorsal neurons, at locations very similar to those found in the wild-type and with a similar staining difference between the two time points (compare Fig. 4G and H with Fig. 6E and F). This indicates that PER is cycling in a circadian manner in the DNs of disco1 flies.
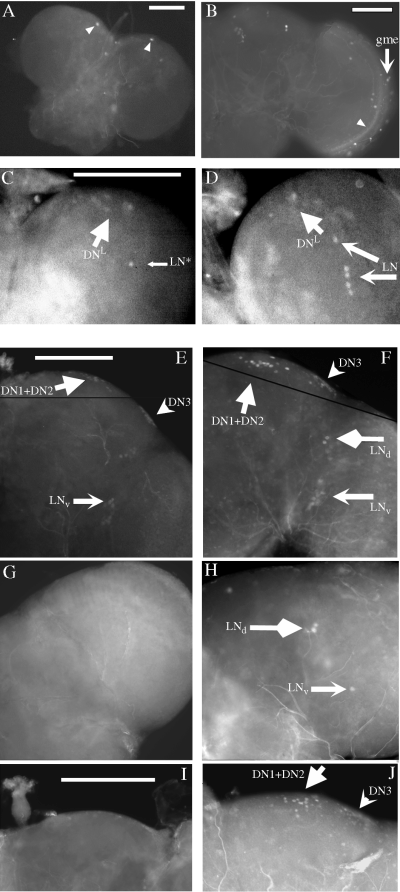
Expression of gal1118 and PER in w;UAS-gfp/+;gal1118/UAS-bax brains. GFP fluorescence in the third instar larval (A) and adult (B) brain of w;UAS-gfp/+;gal1118/UAS-bax flies; gme, gal1118 cells of the medulla. The arrowheads point to GFP-independent brain autofluorescence. PER immunocytochemistry in the larval brain of w;UAS-gfp/+;gal1118/UAS-bax (C) and control w;UAS-gfp/+;gal1118/+ (D) flies at ZT21. DN, dorsal neurons; LN, lateral neurons; LN*, single gal1118-negative PER-expressing LN. (E–J) PER immunocytochemistry in the adult brain of w;UAS-gfp/+;gal1118/+ (E, F) and w;UAS-gfp/+;gal1118/UAS-bax (G–J) flies. Flies were entrained for 3–4 days in LD 12 : 12, and then left for 24–48 h in the dark before brain dissection at CT9 (E,G,I) or CT22 (F,H,J). DN1 + 2 and DN3, dorsal neurons; LNd, dorsal lateral neurons; LNv, ventral lateral neurons. Scale bars, 100 µm.
Genetic ablation of the PDF LNvs strongly alters activity rhythms but does not prevent PER cycling in other PER-expressing neurons
The gal1118 line was used to express the pro-apoptosis gene bax in the lateral neurons. bax is a mammalian gene that also induces apoptosis in Drosophila (Gaumer et al., 2000). gal1118-driven bax expression resulted in 85% lethality, mostly at embryonic stages, and half of the surviving adults had shrivelled wings. The finding of ≈ 70% lethality in flies ablated for the EH neurons (McNabb et al., 1997) suggests that the killing of these cells in the w;;gal1118/UAS-bax flies is likely to explain most of this effect, but other gal1118-expressing neurons may also be involved. Surviving individuals with expanded or shrivelled wings did not show major differences in their viability, at least up to 10–15 days of age, and were used for both the anatomical and behavioural analyses.
To control for the effect of ablation, we analysed the brain of flies carrying a UAS-gfp transgene in addition to the UAS-bax one (w;UAS-gfp/+;gal1118/UAS-bax). No gross brain rearrangement was observed either in larval or adult brains. In third instar larvae, a complete ablation of gal1118 cells was observed (Fig. 6A and Table 4). In agreement with the ablation of the four PDF- and gal1118-expressing LNs, anti-PER antibody staining at CT22 revealed a single PER-expressing cell in the LN region (Fig. 6C and Table 4), whereas five PER-expressing LNs were observed in the wild-type larval brain (Fig. 6D; see also Fig. 3 and Table 2). As expected from the nonoverlapping gal1118- and PER-expression patterns in the larval dorsal brain (Fig. 3F–H), PER-expressing cells were detected in 4/5 brains (see Fig. 6C).
| Third instar larval brain | Adult lateral brain | Adult dorsal brain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Larval LN-like cells | s-LNv-like cells | l-LNv-like cells | LNd-like cells | ||||||||||
| Genotype | Labelling | Time | Cells:n ± SEM | Hemisphere with ≥ 1 cell | Time | Cellsn ± SEM | Hemisphere with ≥ 1 cell | Cellsn ± SEM | Hemisphere with ≥ 1 cell | Cellsn ± SEM | Hemisphere with ≥ 1 cell | DN1+DN2-like cells Hemisphere with ≥ 1 cell | DN3 Hemispheres with ≥ 1 cell |
| w;UAS-gfp/+; | GFP | –a | 0.0 ± 0.0 | 0/20 | –a | 0.0 ± 0.0 | 0/66 | 0.0 ± 0.0 | 1/66 | 0.3 ± 0.1 | 10/66 | 0/66 | NA |
| gal1118/UAS-bax | |||||||||||||
| w;;gal1118/UAS-bax | PER | ZT21 | 0.9 ± 0.1 | 11/12 | CT22 | 0.7 ± 0.1 | 12/17 | 0.0 ± 0.0 | 0/17 | 2.6 ± 0.3 | 15/17 | 17/17 | 17/17 |
| – | – | – | CT9 | 0.0 ± 0.0 | 0/14 | 0.0 ± 0.0 | 0/14 | 0.0 ± 0.0 | 0/14 | 8/14 | 10/14 | ||
- The tested genotypes are listed on the left. For PER staining, larvae were collected at ZT21 in LD 12:12 conditions and adult flies were entrained for 3–4 days in LD 12 : 12 and left for 24–48 h in the dark before brain dissection at CT9 or CT22. aDissections for GFP fluorescence were done during daytime in LD12:12. LN-like, s-LNv-like, l-LNv-like and LNd-like are as in Table 2. DN1 + DN2-like cells are DN1 + DN2 PER neurons for PER labelings and gal1118 cells located in the same dorsal region for GFP fluorescence (gd1 cells, see Fig. 2). When visible, PER staining in the DN1 + DN2 and DN3 neurons was lower at CT9 than at CT22 (Fig. 6I and J and data not shown). NA: not applicable. Mean values are given ± SEM.
In adult brains, we observed the complete disappearance of the gal1118-driven GFP expression in the LNvs region (Fig. 6B and Table 4), whereas gal1118 cells at the periphery of the medulla were observed in 16/33 brains (Fig. 6B). In a minority of the brains, very few GFP-positive cells were observed in the region of the LNds (Table 4). PER expression was then characterized in the brains of flies with and without gal1118 cells. As illustrated in Fig. 6F, control brains contain several groups of neurons expressing PER at CT22: the LNvs and LNds in the lateral region and the DNs in the dorsal protocerebrum (Siwicki et al., 1988; Zerr et al., 1990; Ewer et al., 1992; Frisch et al., 1994). The latter group has been subdivided into three subgroups: the central DN1, two slightly more ventral DN2 cells and the lateral DN3 cluster (Kaneko et al., 1997; Kaneko, 1998). We could not readily distinguish the DN2 cells among the larger DN1 group in many brains, but the more lateral DN3 group could easily be identified and analysed separately from the DN1 + DN2 set. In w;UAS-gfp/+;gal1118/UAS-bax brains, as well, four groups of PER-expressing cells were detected at CT22 (Fig. 6H and J). In agreement with the presence of a single gal1118-negative PER-positive LNv-like cell and with the excess of PER-labelled vs. GFP-labelled LNds in w;UAS-gfp/+;gal1118/+ brains (see Fig. 1 and Table 2), a single PER-expressing LNv-like and two or three PER-expressing LNds were labelled in w;UAS-gfp/+;gal1118/UAS-bax brains (Fig. 6H and Table 4). In the dorsal brain, large groups of central DNs and laterally located DN3 were found (Fig. 6J and Table 4), as expected from the only partial overlap between gal1118 dorsal cells (gd1) and PER-expressing DN1 + DN2 group and from the absence of gal1118 expression in the PER-expressing DN3 (see Fig. 2F–H).
PER temporal expression was analysed in constant darkness by comparing PER staining at CT9 (Fig. 6E, G and I) and CT22 (Fig. 6F, H and J). In control brains, PER cycles in the LNs (both LNvs and LNds) as previously reported (Zerr et al., 1990), but also in the central DNs and in the DN3 group with a phase similar to the one of the LNs (Fig. 6E and F; see also Fig. 9E). In the w;UAS-gfp/+;gal1118/UAS-bax brains, PER levels were found to cycle with a phase similar to the wild-type in all of the nonablated PER-expressing neurons: the LNds and the single LNv-like cell as well as the DN1 + DN2 and DN3 (Fig. 6G–J and Table 4).
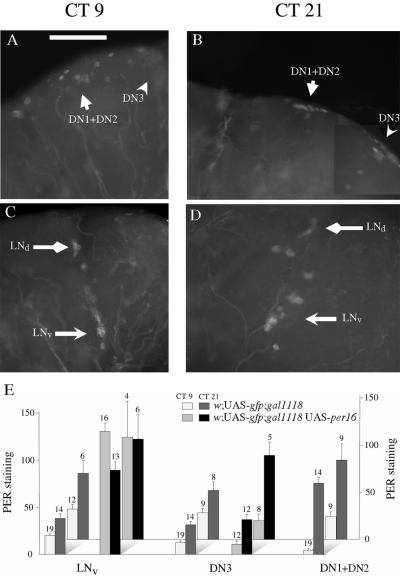
PER expression in w;;gal1118 UAS-per16 adult brains in DD. Flies were entrained during 4 days of LD 12 : 12 and brains were dissected during the first day in DD at CT9 (A and C) or CT21 (B and D). Anti-PER immunostaining was detected in the LNvs and LNds in the lateral brain (C and D), and in the DN3 and a group of neurons comprising the DN1 + DN2 as well as gal1118-expressing cells (gd1) in the dorsal brain (A and B). (E) PER immunoreactivity quantification in the different groups of positive cells of control w;UAS-gfp;gal1118 brains and w;UAS-gfp;gal1118 UAS-per16 brains. The results of two independent experiments are shown separately on the graph. The signal intensity is reported in arbitrary units (see Materials and methods) with a bar representing the associated SEM. The number of brain hemispheres analysed is shown above each histogram. Statistical analysis (one-tailed t-test) indicates that CT21 values are significantly higher (P < 0.03) than CT9 values for LNvs, DN3s and DN1s in control w;UAS-gfp;gal1118 brains and for DN3s (P < 0.01) in w;UAS-gfp;gal1118 UAS-per16 brains. Scale bar, 50 µm.
The locomotor activity rhythm of the w;;gal1118/UAS-baxadult flies was tested in DD and 83% of the flies were found to be arrhythmic (Table 1). Similarly to disco1, the rhythmic flies showed a shortening of the period, with a mean value of 22.5 h (± 0.4 h) and a low power (Table 1). The genetic ablation of the PDF-expressing LNvs with the hid and reaper apoptosis genes of Drosophila has been recently reported and led to similar findings (Renn et al., 1999). In LD 12 : 12 conditions, the w;;gal1118/UAS-bax flies showed a clear anticipation of the light-to-dark transition with a shift of the evening activity peak ≈ 2 h earlier than the controls (compare Fig. 5G with Fig. 5A and J). As for disco1, a clear lights-off anticipation was detected in LD for the majority of the flies, regardless of their behaviour in DD (not shown). Conversely to the disco1 (Fig. 5E) and pdf01 (Renn et al., 1999) flies, no clear rhythm was displayed in average activity plots during the first two days in DD (Fig. 5H, I, and Fig. 5B, C, K and L for the controls).
Gal1118-driven PER overexpression abolishes activity and eclosion rhythms
The above results indicate that the absence of PER (and PDF) expression in the LNs of the disco1 flies or the ablation of the PDF-expressing LNvs in the w;;gal1118/UAS-bax flies lead to an advanced evening peak in LD and a mostly arrhythmic locomotor activity in DD with a weak short-period rhythm for a minority of the individuals. We wondered whether similar effects would be produced by blocking PER oscillations with saturating levels of PER in gal1118 flies carrying a UAS-per cDNA construct.
Two transgenic lines bearing independent UAS-per insertions were generated and tested for their rhythmic behaviour under DD and LD conditions with one or two copies of the gal1118 driver (Table 1). gal1118-driven per expression suppressed activity rhythms in a PER dose-dependent manner, as the number of arrhythmic flies strongly increases between heterozygous w;;gal1118UAS-per/++ and homozygous w;;gal1118 UAS-per. For both UAS-per16 and UAS-per19 insertions, almost complete arrhythmicity was observed in homozygous w;;gal1118UAS-per (Table 1). In heterozygous w;;gal1118 UAS-per/++ individuals, a clear effect is seen with the UAS-per19 insertion whereas almost no effect was observed with the UAS-per16 insertion, which has lower levels of PER in the gal1118 cells (not shown). In contrast to the disco1 and w;;gal1118/UAS-bax flies, no short-period weak rhythm could be detected in the PER-overexpressing lines. However, anticipation of the lights-off transition could be observed in LD 12 : 12 conditions, even for the strongly arrhythmic w;;gal1118UAS-per16 (Fig. 7G) and w;gal1118 UAS-per19 (Fig. 7M) homozygous genotypes, as opposed to the complete absence of such anticipation in the arrhythmic per01 mutant (Fig. 7S), as previously described (Wheeler et al., 1993). The anticipation of lights-off is rather poor with the UAS-per19 insertion but very robust with the lower PER-expressing UAS-per16. Conversely to the disco1 and LNv-ablated flies, no phase advance was observed for the anticipation of lights-off in LD (Fig. 7G and M). In the same line, no weak short-period rhythm could be detected in constant darkness (Table 1), even during the first days in DD (Fig. 7H, I and N, O).
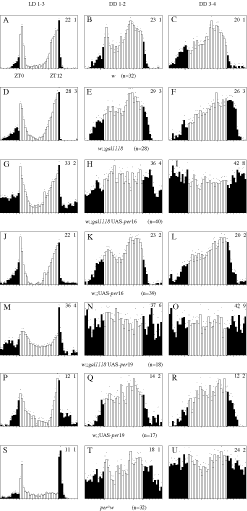
Locomotor activity of w;;gal1118 UAS-perand control flies in LD and DD conditions. Data are represented as described in Fig. 5. The analysis was performed separately in LD 12 : 12 conditions over 3 days (LD 1–3: A, D, G, J, M, P and S), then in constant darkness during the first two days after lights off (DD 1–2: B, E, and H, K, N, Q and T) and during the next two days (DD 3–4: C, F, I, L, O, R and U).
Eclosion rhythms were also analysed for the two lines overexpressing PER under gal1118 control. Arrhythmic eclosion was observed for both heterozygous w;;gal1118 UAS-per19/++ and homozygous w;;gal1118 UAS-per19 (Fig. 8). Homozygous w;;gal1118 UAS-per16 were also arrhythmic whereas heterozygous w;;gal1118 UAS-per16/++ were not (not shown), in agreement with the lower GAL4-dependent PER expression observed with the UAS-per16 insertion. As for activity, the differences between heterozygous and homozygous flies for the UAS-per16 insertion suggest that the suppression of the eclosion rhythm depends upon PER dose.
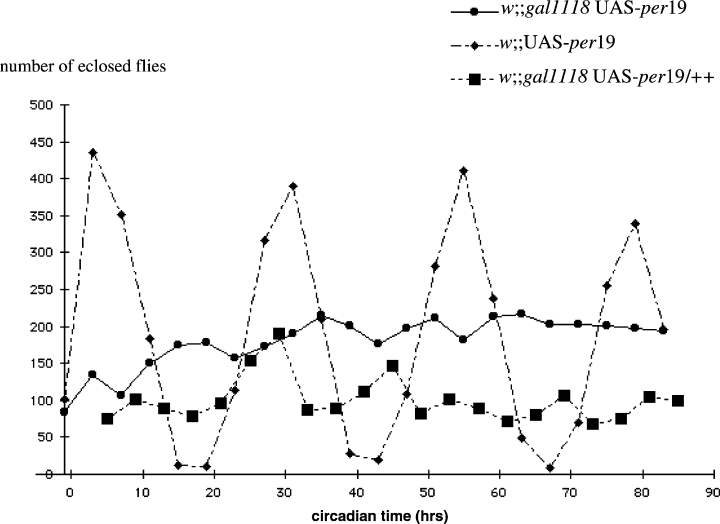
Eclosion behaviour of w;;gal1118UAS-per19 flies in DD. The eclosion vials were seeded with the homozygous stocks for the w;;gal1118 UAS-per19 and w;;UAS-per19 genotypes, and with w;;gal1118 males and w;;UAS-per19 virgin females to obtain the w;gal1118 UAS-per19/+ progeny. Newly eclosed flies were collected every 4 h (see Materials and methods), and their number is plotted as a function of circadian time. CT0 is 36 h after light is turned off for the last time in the LD regime. The lower number of eclosed flies for the heterozygous w;;gal1118 UAS-per19/+ genotype only reflects a lower number of culture vials used for the experiment and not decreased emergence.
PER levels oscillate in the DN3 dorsal neurons and the eye photoreceptors of arrhythmic flies overexpressing PER under gal1118 control
We performed an analysis of PER expression in the brains of the behaviourally arrhythmic w;;gal1118 UAS-per16 flies in DD to verify that PER cycling would be abolished in the gal1118-expressing neurons and to test whether changes of PER expression could be detected in the other PER-expressing cells. In agreement with the behavioural results, high and constant PER expression was detected in the LNvs (Fig. 9C–E), whereas PER levels cycled in the control flies (Fig. 9E). As expected from the gal1118-driven GFP expression pattern in the homozygous w;UAS-gfp;gal1118 genotype (see Fig. 2), lower level noncycling PER expression seems to occur in the LNds (Fig. 9C and D), and in dorsal cells (gd1) overlapping with the DN1 + DN2 PER-expressing cells (Fig. 9A and B). The proximity and therefore frequent overlap between the strongly PER-expressing l-LNvs and the weakly expressing LNds prevented us from unambiguously quantifying PER in the LNds. Although strong PER oscillations were observed in the DN1 + DN2 group of the control brains (Fig. 9E), the overlap between the DN1 + DN2 group and the gd1 cells also prevented us from unambiguously quantifying PER in this region. However, PER expression could be analysed in the DN3 and showed strong oscillations in both the w;;gal1118 UAS-per16 brains and the controls (Fig. 9A, B and E). In contrast, larval DN3 do not show PER cycling (Kaneko et al., 1997), suggesting that larval and adult DN3s are different cells or change their PER expression during metamorphosis.
PER oscillations have been shown to occur in the eyes of the arrhythmic disco1 mutant (Zerr et al., 1990; Hardin et al., 1992) and in the photoreceptors of per01 flies carrying a per construct that constitutively express per mRNA in these cells only, indicating that the eye photoreceptors contain an autonomous oscillator (Cheng & Hardin, 1998). Furthermore, the constitutive overexpression of per in the eye photoreceptors has no effects on the rhythmic behaviour and PER oscillations in a per+ brain (Zeng et al., 1994). To test further the independence of the eye clock with respect to the brain, we have analysed the expression of PER that emanates mainly from the eye photoreceptors in the arrhythmic w;;gal1118UAS-per16 flies, which have strong noncycling PER expression in the LNvs (see above). Anti-PER antibody staining on head sections showed that the eye photoreceptors represent the major contribution to head PER expression in these flies, as is the case for the wild-type flies (data not shown), allowing us to use head protein extracts to analyse PER expression from the eye. Western blots of heads were made from w;;gal1118 UAS-per16 and control w;;UAS-per16 flies in DD conditions and showed that PER cycled with similar overall levels and a peak around CT18 in both genotypes (Fig. 10).
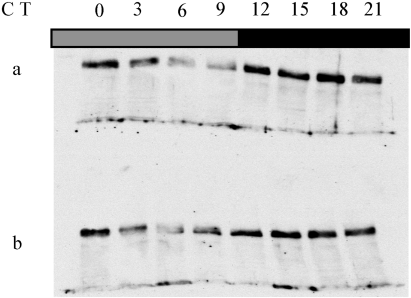
PER expression in the heads of (a) w;;gal1118 UAS-per16 and (b) control w;;UAS-per16 genotypes. Flies were entrained for 3 days in LD 12 : 12 and collected every 3 h during the first day in DD. Total head extracts were analysed by anti-PER immunoblotting.
Expression of the tetanus toxin light chain in the LNvs does not alter rhythmic behaviour
Knowing that the LNs are involved in both activity and eclosion rhythms, we wondered whether gal1118-driven expression of the tetanus toxin light chain, which should block the synaptic output from the LNs, might alter rhythmicity. Flies bearing gal1118 and two different UAS-tnt insertions known to give strong GAL4-dependent expression (Sweeney et al., 1995) were generated. They were verified for the presence of the tetanus toxin light chain in the gal1118 neurons by immunohistochemistry using anti-TeTxLC antibody (not shown). Activity rhythms of flies expressing the TeTxLC under gal1118 control were tested in both LD and DD conditions. In either conditions, flies carrying one copy of gal1118and one copy of UAS-tntG or UAS-tntH appear to be unaffected compared to w;;gal1118/+controls (Table 1 and Fig. 11). In flies carrying two copies of gal1118 and two copies of either one of the two UAS-tnt insertions, no strong effect could be seen when comparing them with w;;gal1118 and w;UAS-tnt, or with a w;UAS-tntV1-A;gal1118 line expressing an inactive form of the toxin (Sweeney et al., 1995) (Table 1 and Fig. 11). A slight increase in the variability of the individual circadian periods of the w;UAS-tntH;gal1118 line is suggested by the higher SEM value of tau (0.2 vs. 0.1 for the w;UAS-tntG;gal1118 line and controls; see Table 1), and may reflect a looser control of the period in this genotype. Indeed, a weakening of the rhythmicity occurs at the end of the 6-day period of DD analysis when the activity is plotted by fractions of 2 days (compare days 1–4, Fig. 11N, with days 5–6, Fig. 11P). This long-term effect has been seen only with the UAS-tntH insertion, in the presence of two copies of gal1118 and UAS-tntH. No similar weakening of the rhythm was found for the homozygous w;UAS-tntG;gal1118 line during a 15-day experiment (not shown).
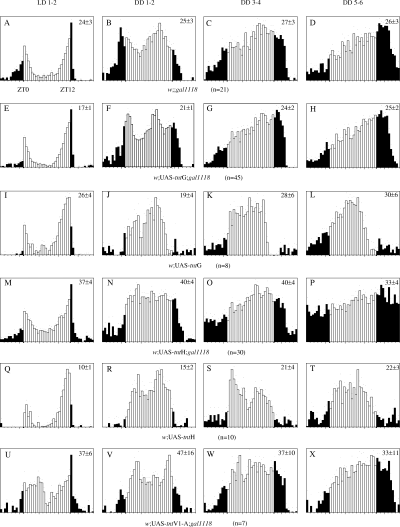
Locomotor activity of TeTxLC-expressing flies in LD and DD conditions. Data are represented as described in Fig. 5. The analysis was carried out separately in LD 12 : 12 conditions over 2 days (LD 1–3: A, E, I, M, Q and U), then in constant darkness during the first 2 days after lights off (DD 1–2: B, F, J, N, R and V), during the 3rd and 4th days (DD 3–4: C, G, K, O, S and W) and during the 5th and 6th days (DD 5–6: D, H, L, P, T and X).
Discussion
In this study, we have used a P-gal4 insertion line that is strongly expressed in the PDF-expressing LNvs of the Drosophila brain to analyse the role of these neurons in the generation of the locomotor activity and eclosion rhythms. The results indicate that the PDF LNvs are required for the generation of a robust 24-h rhythmicity, although their absence or alteration reveals weak short-period rhythms. These data are discussed with respect to the complete arrhythmicity that is induced by the overexpression of PER in the LNs and to the nature of the other pacemaker cells that could participate in the control of the behavioural rhythms.
The disco1 mutants show extensive cell degeneration and neuronal differentiation defects in the developing optic lobe (Steller et al., 1987; Campos et al., 1995). We have analysed the anatomical defects of this mostly arrhythmic mutant using gal1118-driven GFP expression. Our data show that at least one gal1118-expressing cell with dorsal projections can be detected in the lateral brain in a majority of the disco1 brains (83%; see Table 3). The results also indicate that the PER protein is absent from these cells, in agreement with previous data reporting PER expression (and cycling) in disco1 glia and photoreceptors but not in LNs (Zerr et al., 1990). Because they do not express detectable levels of PER or PDF, anatomical criteria were used to define gal1118 cells as LNs in the larval and adult disco1 brains (see legend of Fig. 4). The detection of PDF- and PER-negative LNs in disco1 mutants suggests that the disco gene may somehow control the expression of clock genes in the LNs, in addition to or rather than their presence.
Our findings should be compared with previous work using PDF as a marker (Helfrich-Förster & Homberg, 1993; Helfrich-Förster, 1997; Helfrich-Förster, 1998), which concluded that the LNvs are completely missing in 98% of the adult and 100% of the larvae in the disco1 mutant (Helfrich-Förster, 1998). Why was PDF not detected in LNvs in so many of the adult disco1 brains, if such cells are present in most of them? One possibility is that the gal1118-driven GFP expression is more sensitive in detecting the LNvs. More probably, the disco1 mutation induces the complete loss of pdf expression whereas the gal1118 locus is less affected. The small number of gal1118-positive LNvs in disco1 brains could be due to a reduced expression of this marker and/or to a reduced number of neurons. Whereas the two (out of 101) individuals with wild-type-like rhythms had putative LNs, we found no correlation between the presence of gal1118-expressing LNvs (or their average number) and the weak short-period rhythmicity displayed by one third of the disco1 flies (see Table 3). Such residual rhythmicity thus appears to be independent of the presence of PER- and PDF-expressing LNvs.
A similar behaviour was displayed by flies ablated for the gal1118-expressing cells, with 83% of the viable individuals showing a loss of locomotor activity rhythms in DD and 17% of them displaying weak short-period rhythms (see also Renn et al., 1999). As predicted from the gal1118 expression pattern in w;UAS-gfp/+;gal1118/+ flies, the PER- and PDF-expressing LNvs are absent from the brain of virtually all the w;;gal1118/UAS-bax flies (see Table 4), whereas the other PER-expressing neurons (the single PDF-negative LNv, the LNds and the DNs) could be detected, although in a reduced number for the LNds. Other gal1118 cells labelled in the w;UAS-gfp/+;gal1118/+ brains are also missing (as gal1118-negative cells could be altered in the disco1 brains), but their lack of PER and PDF expression strongly suggests that their ablation is not the cause of the arrhythmicity of the w;;gal1118/UAS-bax flies. We therefore believe that the ablation of PDF- and PER-expressing LNvs leads to the mostly arrhythmic phenotype and unmasks a weak short-period rhythm in a minority of the individuals.
The LD behaviour of the disco1 (Dushay et al., 1989; Hardin et al., 1992) and w;;gal1118/UAS-bax flies indicates that anticipation of the light-to-dark transition does not require the LNvs. The weak rhythmic behaviour that is displayed in DD by 32% and 17% of the disco1 and w;;gal1118/UAS-bax flies, respectively, has a short period that is compatible with the phase-advanced LD rhythm and could be generated by a persistence of the latter as a weak fast-running oscillator in DD. This short-period rhythm is not detected in per01 mutants, indicating that it requires PER to be expressed. As PDF and PER colocalize only in the PDF LNvs (Helfrich-Förster, 1995), it is very likely that the short-period rhythms detected in disco1 and w;;gal1118/UAS-bax flies are PDF-independent. Indeed, a minority of the null pdf01 mutants display a weak activity rhythm with a 22–23-h period in DD (Renn et al., 1999).
Although the single PDF-negative LNv and the LNds show PER cycling in the w;;gal1118/UAS-bax flies, our failure to detect PER expression in identifiable LNs in the disco1 brains does not support them as a cellular basis for the weak short-period rhythm displayed by these genotypes. In contrast, the PER-expressing DNs are present in both the disco1 and w;;gal1118/UAS-bax flies. We show here that the DNs cyclically express PER in wild-type, disco1 and w;;gal1118/UAS-bax flies. Therefore, these neurons appear to be good candidates for generating the weak short-period rhythmicity detected in the disco1 and w;;gal1118/UAS-bax flies, as well as in the pdf01 mutants. The averaged activity plots of the disco1 (this study) and pdf01 (Renn et al., 1999) flies indicate that the majority of the individuals become only progressively arrhythmic, over several days after transfer to DD. In contrast, w;;gal1118/UAS-bax flies become arrhythmic more quickly, suggesting that the presence of PDF-depleted LNvs (although other gal1118 cells cannot be excluded) could be required for this very short-term rhythmicity.
Constitutive overexpression of PER using gal1118-driven UAS-per leads to the loss of locomotor activity and eclosion rhythms. Which are the cells responsible for this arrhythmicity? It would be rather surprising if the overexpression of PER in cells that do not normally express PER, like the PER- and PDF-negative s-LNv-like cells of the adult or the EH and gd1-2L cells of the larva, could have an effect on activity and eclosion rhythms. Therefore we believe that ectopic PER expression in the PER-negative gal1118 cells is very likely to be irrelevant to the arrhythmic phenotype. Among the gal1118 cells, three types of cells normally express PER: the PDF LNvs, the LNds and the DN1 + DN2. In heterozygous w;UAS-gfp/+;gal1118/+ flies, we have seen at least a 10-fold excess of GFP expression in the LNvs in comparison to the LNds (see Fig. 1), and DN1 + DN2 gal1118-positive neurons appear to be even weaker (see Fig. 2B). However, strong activity and eclosion rhythm defects are observed in the heterozygous w;;gal1118UAS-per19/++ flies. Although we cannot rule out the possibility that the weak gal1118-driven constitutive PER expression in the LNds (or even weaker expression in the gal1118 DN1 + DN2) is also involved, the gal1118 expression pattern suggests that most, if not all, of the behavioural defects are the consequences of PER overexpression in the PDF-expressing LNvs
The gal1118 UAS-per flies do not show long-term weak short-period activity rhythms in DD like those detected in the disco1 and w;;gal1118/UAS-bax flies, nor short-term rhythmicity during the first 2 days in DD like the disco1. A similar arrhythmicity was recently obtained by overexpressing PER with a tim-gal4 transgene (Kaneko et al., 2000), although tim-gal4 drives gene expression in many more PDF-negative clock cells in the brain (Kaneko, 1998). This suggests that most of the effect seen with the tim-gal4 driver stems from the lateral neurons. In contrast to the strong arrhythmicity in DD, the LD activity shows a clear lights-off anticipation with a wild-type phase, in both homozygous (Fig. 7) and heterozygous (not shown) genotypes, indicating clock function under these conditions. The LD rhythmicity may reflect light-induced cycling of clock components, such as TIM, in the neurons overexpressing PER. Both LD and DD behaviours indicate that PER overexpression in the LNvs does not produce the same effects on the circadian output as does their targeted ablation or the absence of PER expression. These differences could be explained by a dominant effect of the PDF-expressing LNvs on other putative brain pacemaker cells. In wild-type flies, the PDF LNvs could impose their 24-h oscillations and prevent the other oscillator(s) in producing the LD phase advance and DD weak short-period rhythm. In gal1118 UAS-per flies, saturating levels of PER in the PDF LNvs might be dominant over the contribution of other clock cells, and impose arrhythmic behaviour, possibly through the constitutive release of neurotransmitter.
The essentially arrhythmic eclosion phenotype of the disco1 mutant (Dushay et al., 1989) focused the control of this rhythm on the brain. The gal1118 UAS-per experiments strongly suggest that PER oscillations in the PDF LNvs are required for the generation of this developmentally related rhythm. The observation of cyclic PER expression in wild-type pupal prothoracic glands and the absence of eclosion rhythms in flies with detectable PER only in the LNvs has lead to the suggestion that PER expression in the prothoracic glands is involved in the control of eclosion rhythms (Emery et al., 1997). If it is the case, the present results indicate that both the LNs and the prothoracic glands oscillators would be required for eclosion rhythms. In the larval brain, the EH neurons project into the ring gland (McNabb et al., 1997), from which the pupal prothoracic glands are derived, and to the vicinity of the LNvs dorsal projections, suggesting that these neurons could talk to each other. However, flies ablated for the EH neurons show eclosion rhythms in DD (McNabb et al., 1997), indicating that these cells are not required for eclosion rhythmicity.
The absence of defects in the activity rhythms of the heterozygous w;(UAS-tntG or H)/+;gal1118/+ and homozygous w;UAS-tntG;gal1118 flies suggests that the LNvs do not use synapses with synaptobrevin-mediated exocytosis to transmit the circadian information to their target. While this report was in preparation, similar results were published by Kaneko et al. (2000) with a pdf-gal4 transgene. It has been recently shown that PDF is the main neurotransmitter for the activity rhythms (Renn et al., 1999), and that its levels circadianly fluctuate in the axon terminals of the small LNvs (Park et al., 2000). Taken together, these results indicate that the PDF-based neurotransmission does not require synaptobrevin (n-syb). Because spontaneous release of neurotransmitter at the embryonic neuromuscular junction is not abolished in the absence of n-syb (Sweeney et al., 1995; Deitcher et al., 1998), it is possible that PDF release depends on the same mechanisms of vesicle fusion. It will be interesting to see whether this property is shared by other neurons secreting neuropeptides, as for example the EH neurons of the larval brain (see McNabb et al., 1997) or the neurons that release the putative neuropeptide encoded by the amnesiac gene through a dynamin-dependent mechanism (Waddell et al., 2000). The slowly appearing arrhythmicity of the homozygous w;UAS-tntH;gal1118 is intriguing. Because it appears only with one UAS-tnt line, in flies carrying two copies of both gal1118 and UAS-tntH insertions, it might be related to nonspecific effects of the accumulation of very high levels of the toxin.
We have shown that the eye still displays wild-type-like circadian oscillations of PER when those oscillations are blocked in the LNs. Therefore, not only does the eye oscillator run in the absence of PER in the LNs (Zerr et al., 1990; Hardin et al., 1992) or in the brain (Cheng & Hardin, 1998), but it also behaves independently of the LN's oscillator. In the wild-type brain, our data indicate that PER cycles in both the DN1 + DN2 and DN3 groups of dorsal neurons as well as in the LNds and in a single PDF-negative LNv, in addition to the PDF-expressing LNvs. These cells still display PER oscillations in PDF LNv-ablated flies, indicating that the different sets of central brain PER-expressing neurons have the capacity to behave autonomously from the PDF LNvs. Moreover, at least the DN3s maintain independent PER cycling when the LNs' clock is blocked. How these central brain clock cells participate in the circadian system is not known, but the recent description of the PER neurons projections within the brain (Kaneko & Hall, 2000) should help to identify their targets. Autonomous PER circadian oscillations occur in many peripheral tissues of Drosophila (Giebultowicz & Hege, 1997; Plautz et al., 1997), but they dampen within a few days in DD (Hardin, 1994; Plautz et al., 1997), whereas the activity pacemaker is able to run for several weeks. It will be interesting to see whether this property is shared by all the central brain clock neurons or is specific to the LNs.
Acknowledgements
This work was supported by grants from CNRS (ATIPE ‘Développement’ and appel d'offres ‘Biologie cellulaire’) and Fondation pour la Recherche Médicale to F.R., and from NIH (NS31214) to P.E.H. F.R. is supported by INSERM. We thank Yuzhong Cheng for the UAS-per lines, Michel Boudinot for his help with the behavioural analysis software, Annie Lamouroux for comments on the manuscript and Jean-Didier Vincent for his continuous support. We are grateful to S. Gaumer and B. Mignotte (University of de Versailles/Saint-Quentin, France) for the UAS-bax line and to C. O'Kane (University of Cambridge, UK) for the UAS-tnt lines, as well as to R. Stanewsky (University of Regensburg, Germany) for the anti-PER antibody, K. Rao (University of West Florida, USA) for the anti-PDF antibody, S. McNabb (University of Washington, USA) for the anti-EH antibody and J-R. Martin (University of Orsay, France) for the anti-TeTxLC antibody.
Abbreviations
-
- AEBSF
-
- 4-(2 aminoethyl)-benzenesulfonylfluoride
-
- CC
-
- corpora cardiaca of the ring gland
-
- CT
-
- circadian time
-
- DD
-
- constant darkness
-
- DN
-
- dorsal neurons
-
- EH
-
- eclosion hormone
-
- gd1
-
- gal1118 dorsal 1
-
- gmbc
-
- ≈ 20 cells located underneath the calyces of the mushroom bodies
-
- gpi
-
- a small group of large-cell-body neurons located at the surface of the central dorsal brain and projecting into the pars intercerebralis
-
- LD
-
- light–dark
-
- l-LNv
-
- large ventral lateral neuron
-
- LN
-
- lateral neuron
-
- LNd
-
- dorsal lateral neuron
-
- LNv
-
- ventral lateral neuron
-
- NGS
-
- normal goat serum
-
- OX-PBS
-
- PBS and Thimerosal
-
- OX-PBS-T
-
- OX-PBS with 0.3% Triton X-100
-
- OX-PBS-T-N
-
- OX-PBS-T containing 5% NGS
-
- PBS
-
- phosphate-buffered saline
-
- PDF
-
- pigment dispersing factor
-
- per
-
- period gene
-
- POT
-
- posterior optic tract
-
- RT
-
- room temperature
-
- SCN
-
- suprachiasmatic nucleus
-
- s-LNv
-
- small ventral lateral neuron
-
- TeTxLC
-
- tetanus toxin light chain
-
- w;UAS-gfp;gal1118
-
- flies carrying two copies of the gal1118 and UAS-gfp insertions
-
- ZT
-
- Zeitgeber time.




