Eastern and Western Poor Cod (Trisopterus minutus capelanus) Populations in the Mediterranean Sea: Evidence from Allozyme and Minisatellite Loci
Abstract
Abstract. Nine allozyme and two minisatellite loci were used to investigate potential genetic differentiation among three samples of Mediterranean poor cod, Trisopterus minutus capelanus, from the Gulf of Lion, the Tuscan Archipelago and the Aegean Sea. Both types of markers showed consistent results, with FST values of 0.0262 and 0.0296 (P < 0.0015, after Bonferroni correction for multiple tests) for allozymes and minisatellites, respectively. Allele frequency heterogeneity tests between pairs of samples showed a clear separation between the two western Mediterranean samples (Gulf of Lion, Tuscan Archipelago) and the eastern one (Aegean Sea). The results indicate that at least two reproductively isolated populations of poor cod occur in the Mediterranean.
Problem
The Mediterranean poor cod (Trisopterus minutus capelanus Risso, 1826) is distributed from the coast of Morocco to the eastern coast of Spain, along the Italian peninsula, the Adriatic Sea, the Aegean Sea and eastwards to the coast of Israel (FAO, 1990). Average specimens are 15–20 cm long, with a maximum length of 30 cm (Svetovidov, 1948). The species is found at depths from 30 to 450 m (Biagi et al., 1990), with the highest abundance between 50 and 100 m (Politou & Papaconstantinou, 1991).
Another taxon of poor cod, Trisopterus minutus minutus (O. Müller, 1776), is found in the Atlantic. This classification is controversial, however, and recent allozyme analyses (Tirard et al., 1992; Mattiangeli et al., 2000) have suggested that the genetic separation between the two morphs is greater than sub-species level. The Atlantic poor cod is not considered here.
Although not of great commercial importance, the Mediterranean poor cod is one of the most abundant endemic fish species (from 3 to 10% of total catch biomass) (Biagi et al., 1990) and is regularly found in northern Mediterranean fish markets (FAO, 1990). Moreover, the abundance of Mediterranean poor cod makes it an interesting species for future development of commercial fisheries in areas such as the Aegean Sea (Politou & Papaconstantinou, 1991).
Most studies carried out on this fish have concentrated on biological aspects in local areas. In general, the samples investigated were either from the western part of the Mediterranean (eastern coast of Spain: Morte et al., 2001; the Tyrrhenian Sea: Sartor et al., 1990; Biagi et al., 1992; the Adriatic Sea: Froglia & Zoppini, 1981; Giannetti & Gramitto, 1988; Gramitto, 1999) or from the eastern part (the Aegean Sea: Politou & Papaconstantinou, 1991, 1994), without evaluating the relationships between the different areas. Recent allozyme analysis by Mattiangeli et al. (2000) suggested no genetic differentiation between western and eastern Mediterranean poor cod samples. However, east–west separation has been demonstrated in other Mediterranean marine species (Kotoulas et al., 1995; Borsa et al., 1997; Bahri-Sfar et al., 2000), and in the case of the Mediterranean poor cod the eastern sample used by Mattiangeli et al. (2000) was small (n = 20).
Different levels of genetic structure in marine fish populations may be revealed by the use of different molecular markers (e.g. walleye pollock, Theragra chalcogramma: Grant & Utter, 1980; Mulligan et al., 1992; cod, Gadus morhua: Mork et al., 1985; Pogson et al., 1995; Galvin et al., 1995b; Arnason, 1998). This underlines the advantage of using more than one type of molecular marker when evaluating levels of gene flow among natural populations. During this study, both allozyme and minisatellite loci were chosen to analyse the population structure of the Mediterranean poor cod. The large number of genetic studies using allozymes means that there is a huge body of literature against which new data can be evaluated (Shaklee & Bentzen, 1998). On the other hand, the low level of polymorphism detected can be a limitation when investigating population structure (Ferguson et al., 1995).
Mini- and microsatellite loci are known to be some of the most polymorphic DNA sequences, and they are therefore widely used in population analyses (Wright & Bentzen, 1994). The initial search for the loci and primers is time consuming and expensive, but once this step is carried out the primers can often be used with other closely related species, because the flanking sequences tend to be conserved in congeneric species (Shaklee & Bentzen, 1998).
Three PCR-amplifiable minisatellite loci, previously designed for whiting, Merlangius merlangius L. (Galvin et al., 1995a; McGregor et al., 1996), and subsequently modified and tested on Atlantic poor cod (Mattiangeli et al., 2002), suggested the possibility of VNTR (variable number tandem repeat)-based investigation of Mediterranean poor cod population structure.
Previous allozyme analysis of Tyrrhenian and Aegean Sea poor cod samples failed to detect genetic heterogeneity (Mattiangeli et al., 2000). If genetic differences do indeed occur, failure to detect them may have been due either to the small sample size from the Aegean Sea (n = 20), or to the low resolving power afforded by the type of marker chosen. Consequently, in order to address these issues, in the present study we combined the use of new molecular markers (minisatellite DNA loci) with an increased sample size from the Aegean in order to evaluate genetic differentiation between the eastern and western Mediterranean populations of poor cod.
Material and Methods
1. Samples investigated and DNA extraction
Three Mediterranean poor cod samples were collected from Gulf of Lion in July 1997, from the Tuscan Archipelago in May 1998 and from the Aegean Sea in autumn 1992 and 1998 (Fig. 1). Whole fish were frozen and individually stored immediately after catch, at either −20 °C or directly at −80 °C. Tissue samples (liver and muscle) for allozyme analysis were cut and kept frozen at −80 °C in individually numbered plastic bags, and part of the gills (approx. 1 cm3) were taken from frozen fish and stored in ethanol at room temperature for DNA analysis.
Map of the Mediterranean Sea showing the sampling locations.
Total genomic DNA was isolated from gills stored in ethanol. Approximately 0.5 cm of gill tissue was dried on tissue paper to remove excess ethanol, and genomic DNA was extracted by standard proteinase K digestion, phenol/chloroform purification and DNA precipitation as outlined in Sambrook et al. (1989).
2. Molecular marker analysis
The allozyme investigation includes both the results from the samples analysed by Mattiangeli et al. (2000) (100 fish from the Gulf of Lion, 60 fish from the Tuscan Archipelago and 20 fish from the Aegean Sea) and the results from 50 additional Aegean Sea specimens, which were analysed for the first time in the present study. Tissue enzyme extractions were carried out using partially frozen tissue samples (muscle and liver) which were mechanically homogenized in 0.3 ml of the tissue dilution buffer described by Aebersold et al. (1987), centrifuged at 10,000 rpm for 10 min at 2 °C, and subjected to horizontal electrophoresis using 11% starch gels. Gels were run on ceramic cooling plates in which water circulated at 4 °C. In order to combine results from the specimens investigated here with the results of Mattiangeli et al. (2000), the same nine loci (Table 1) and the same procedures were used (described in Mattiangeli et al., 2000).
| locus | allele frequencies | allele frequ. heterog. | FST (P) | dev. from H–W prop. | ||
|---|---|---|---|---|---|---|
| Gulf of Lion | Tuscan Arch. | Aegean Sea | ||||
| AAT-1* | (98) | (60) | (20 + 501) | n.s. | 0 | n.s. |
| 2 | 0.005 | 0 | 0 | (n.s.) | ||
| 1 | 0.995 | 1.000 | 1.000 | |||
| AAT-2* | (97) | (58) | (10 + 501) | n.s. | 0.0032 | n.s. |
| 3 | 0.031 | 0.078 | 0.042 | (n.s.) | ||
| 2 | 0.964 | 0.922 | 0.958 | |||
| 1 | 0.005 | 0 | 0 | |||
| AAT-3* | (95) | (59) | (20 + 501) | *** | 0.0891 | n.s. |
| 3 | 0 | 0.034 | 0.007 | (***) | ||
| 2 | 0.758 | 0.737 | 0.964 | |||
| 1 | 0.242 | 0.229 | 0.029 | |||
| ADA-3* | (89) | (60) | (20 + 441) | n.s. | 0.0158 | n.s. |
| 2 | 0.994 | 0.967 | 0.945 | (n.s.) | ||
| 1 | 0.006 | 0.033 | 0.055 | |||
| G3PDH-1* | (89) | (59) | (20 + 501) | ** | 0.0295 | n.s. |
| 4 | 0.022 | 0.017 | 0 | (*) | ||
| 3 | 0.191 | 0.203 | 0.343 | |||
| 2 | 0.787 | 0.780 | 0.636 | |||
| 1 | 0 | 0 | 0.021 | |||
| GPI-1* | (97) | (60) | (20 + 491) | n.s. | 0 | n.s. |
| 3 | 0.041 | 0.067 | 0.043 | (n.s) | ||
| 2 | 0.680 | 0.692 | 0.696 | |||
| 1 | 0.278 | 0.242 | 0.261 | |||
| GPI-2* | (99) | (60) | (20 + 501) | ** | 0.0080 | n.s. |
| 4 | 0.086 | 0.083 | 0.029 | (n.s.) | ||
| 3 | 0 | 0.008 | 0.007 | |||
| 2 | 0.914 | 0.892 | 0.893 | |||
| 1 | 0 | 0.017 | 0.071 | |||
| MDH-1* | (100) | (60) | (20 + 501) | n.s. | 0.0025 | n.s. |
| 2 | 0.980 | 0.983 | 1.000 | (n.s.) | ||
| 1 | 0.020 | 0.017 | 0 | |||
| PGM* | (100) | (60) | (19 + 501) | n.s. | 0.0007 | n.s. |
| 2 | 0.995 | 1.000 | 0.986 | (n.s.) | ||
| 1 | 0.005 | 0 | 0.014 | |||
| heterozyg. (obs.) | 0.166 | 0.204 | 0.145 | over all loci | ||
| heterozyg. (exp.) | 0.163 | 0.183 | 0.156 | *** | 0.0262 (***) | n.s. |
- The numbers of individuals scored are shown in parentheses, and the most cathodic allele is denoted allele 1. In the Aegean sample the allele frequencies reported were calculated after adding the new specimens (indicated by 1), sampled for the present study, to those analysed by Mattiangeli et al. (2000). For allele frequency heterogeneity, FST values and deviation from Hardy–Weinberg expected proportions at each locus and over all loci, statistical significance levels were corrected using the Bonferroni procedure: over all loci values of P ≤ 0.025, and at each locus values of P ≤ 0.0046, were considered statistically significant at the 5% level. Negative FST values are considered equal to zero; * is equivalent to P ≤ 0.05, ** to P ≤ 0.01 and *** to P ≤ 0.001, after Bonferroni correction.
Three minisatellite loci (Mmer-Amp1A, Mmer-Amp1B and Mmer-Amp2) have been shown to amplify in Atlantic poor cod, Trisopterus minutus minutus (Mattiangeli et al., 2002). Fifty fish from each Mediterranean poor cod sample were analysed using the same three minisatellite loci. Approximately 200 ng DNA were amplified in a volume of 20 μl containing 0.075 M Tris pH 9.0, 20 mM (NH4)2SO4, 0.01% Tween 20 (Reaction buffer IV, Advanced BiotechnologiesTM), 2.0 mM MgCl2, 0.25 mM dNTPs (PharmaciaTM), 1 μM of each primer – one of which was labelled with IRD800 (MWG-Biotech Ltd) – and 1U Taq Polymerase (Advanced BiotechnologiesTM). Amplifications were carried out using a Hybaid thermal cycler ‘Omni-E’ with an initial denaturation step at 95 °C for 2 min. The number of subsequent cycles varied according to the locus and consisted of denaturation at 95 °C for 1 min, annealing (see Results for the optimal temperature at each locus) for 1 min and extension at 72 °C for 1 min.
Prior to electrophoresis on 4% polyacrylamide (Long Ranger, FlowgenTM) gels (length 25 cm) on an automatic DNA sequencer (Licor 4200 series), PCR products were diluted 1:10 for Mmer-Amp1A and Mmer-Amp1B, and 1:20 for Mmer-Amp2, and mixed with formamide loading dye (1% bromophenol blue in formamide) in the ratio 1:2 PCR dilution to loading dye.
Separation was carried out by electrophoresis at 1200 V (max. 50 mA and 50 W) for approximately 2 h. Allele sizes were estimated by reference to a 200–2000 bp size ladder (microSTEP-13a, MicrozoneTM) using image analysis software (RFLPscan Plus V. 3.0, ScanalyticsTM). As an additional control, PCR products from two standard individuals of known genotype were loaded onto each gel every 10–15 lanes.
3. Statistical analyses
The same statistical procedures were used, where possible, for both allozyme and minisatellite data, in order to compare results from the two types of markers. Genotypes were tested against Hardy-Weinberg proportions using GENEPOP (Raymond & Rousset, 1995). Allele frequency heterogeneity at each locus, among all samples and between pairs of samples, was used to test if the allelic distribution was identical across samples using a Markov-chain approach (500 batches of 10,000 iterations) (GENEPOP). Tests for linkage disequilibrium between all pairs of loci, and Weir & Cockerham's (1984) FST and its departure from zero (i.e. no genetic differentiation) at each locus and over all loci, were calculated using GENEPOP. The Bonferroni procedure (Weir, 1990) was used to correct for multiple tests. RST (Slatkin, 1995), a measure of genetic differentiation based on the stepwise mutation model, was calculated for the minisatellite loci using Arlequin (Schneider et al., 2000). Genetic distances (DA; Nei et al., 1983) among samples were estimated using the program DISPAN (Ota, 1993).
Results
Acceptable PCR products were not obtained for Mmer-Amp1B in Mediterranean poor cod, so the use of this marker was discontinued. The optimal annealing temperatures and amplification cycles for the other two loci were: Mmer-Amp1A at 58 °C for 30 cycles, Mmer-Amp2 at 54 °C for 32 cycles (Figs. 2a and 2b).
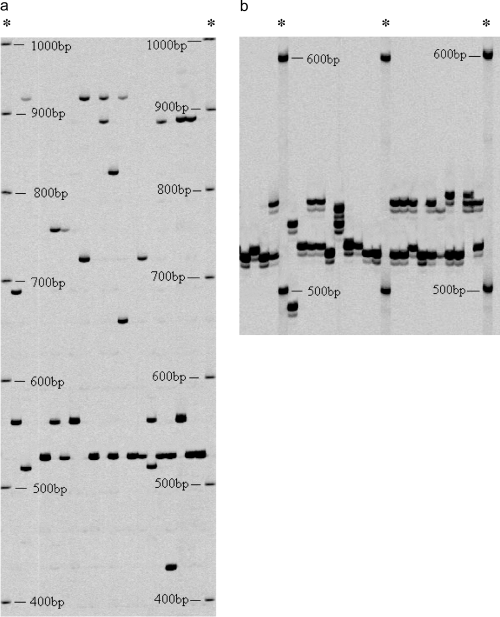
Portions of polyacrylamide gels showing examples of allelic variation at Mmer-Amp1A (a) and Mmer-Amp2 (b). Molecular weight markers are indicated in each gel by the symbol *.
Allele frequencies and observed and expected heterozygosities calculated from the allozyme and minisatellite analyses are shown in Tables 1 and 2, respectively. The Gulf of Lion sample showed the highest number of private alleles (variants that are unique to a single population; Slatkin, 1985) in both allozyme (two private alleles in AAT-1* and AAT-2*) and minisatellite analyses (10 private alleles between the two loci).
| locus | allele frequencies | allele frequ. heterog. | FST (P) | RST (P) | dev. from H-W prop. | ||
|---|---|---|---|---|---|---|---|
| Gulf of Lion | Tuscan Arch. | Aegean Sea | |||||
| Amp1A | (41) | (35) | (47) | ** | 0.0333 | 0.0423 | n.s. |
| 428 | 0.012 | 0 | 0 | (**) | (***) | ||
| 458 | 0 | 0 | 0.011 | ||||
| 492 | 0.012 | 0.057 | 0 | ||||
| 517 | 0.024 | 0.029 | 0.011 | ||||
| 524 | 0.452 | 0.614 | 0.296 | ||||
| 556 | 0.110 | 0.043 | 0.085 | ||||
| 579 | 0.012 | 0 | 0 | ||||
| 588 | 0 | 0 | 0.021 | ||||
| 627 | 0 | 0 | 0.011 | ||||
| 656 | 0.012 | 0 | 0.053 | ||||
| 688 | 0.012 | 0.029 | 0.074 | ||||
| 722 | 0.037 | 0.071 | 0.043 | ||||
| 757 | 0.037 | 0.057 | 0.117 | ||||
| 789 | 0.037 | 0 | 0.011 | ||||
| 819 | 0 | 0 | 0.032 | ||||
| 820 | 0.024 | 0 | 0 | ||||
| 887 | 0.085 | 0.043 | 0.149 | ||||
| 919 | 0.098 | 0.057 | 0.043 | ||||
| 951 | 0.012 | 0 | 0 | ||||
| 983 | 0 | 0 | 0.011 | ||||
| 1142 | 0 | 0 | 0.021 | ||||
| 1240 | 0.012 | 0 | 0 | ||||
| 1272 | 0.012 | 0 | 0 | ||||
| 1400 | 0 | 0 | 0.011 | ||||
| Amp2 | (40) | (40) | (47) | *** | 0.0256 | 0.0399 | n.s. |
| 492 | 0.025 | 0.038 | 0.021 | (n.s.) | (n.s.) | ||
| 495 | 0.038 | 0 | 0 | ||||
| 506 | 0.013 | 0 | 0 | ||||
| 509 | 0.013 | 0 | 0 | ||||
| 512 | 0.350 | 0.350 | 0.384 | ||||
| 515 | 0.472 | 0.436 | 0.245 | ||||
| 518 | 0.013 | 0 | 0 | ||||
| 524 | 0.013 | 0.038 | 0.043 | ||||
| 527 | 0.025 | 0.025 | 0 | ||||
| 530 | 0 | 0.038 | 0.021 | ||||
| 533 | 0.025 | 0.075 | 0.191 | ||||
| 536 | 0.013 | 0 | 0.074 | ||||
| 539 | 0 | 0 | 0.021 | ||||
| heterozyg (obs.) | 0.630 | 0.595 | 0.777 | over all loci | |||
| heterozyg (exp.) | 0.704 | 0.639 | 0.801 | *** | 0.0296 (**) | 0.0411 (***) | n.s. |
- The numbers of individuals genotyped are shown in parentheses. For allele frequency heterogeneity, FST and RST values and deviation from Hardy–Weinberg expected proportions at each locus and over all loci, statistical significance levels were corrected using the Bonferroni procedure: over all loci values of P ≤ 0.025, and at each locus P < 0.0046, were considered statistically significant at the 5% level. Negative FST values are considered equal to zero; * is equivalent to P ≤ 0.05, ** to P ≤ 0.01 and *** to P ≤ 0.001.
The intra-sample genotypic proportions from the allozyme and minisatellite analyses did not deviate significantly from Hardy–Weinberg expectations at any locus (Tables 1 and 2). There was no evidence of linkage disequilibrium between any pair of allozyme or minisatellite loci. The allele frequency heterogeneity test, over all loci (Fisher's method, GENEPOP), indicated highly significant genetic differentiation, both in the allozyme and minisatellite investigation (P < 0.0001, which is highly significant after Bonferroni correction; Tables 1 and 2). There was significant allele frequency heterogeneity at three allozyme loci (AAT-3*, G3PDH-1* and GPI-2*) out of nine, and at both minisatellite loci (Tables 1 and 2) (values of P < 0.0046 at each locus were considered significant after Bonferroni correction).
FST values, at each locus and over all loci, from both allozyme and minisatellite data are shown in Tables 1 and 2. The calculation of FST over all allozyme and minisatellite loci indicated that 2.62% and 2.96%, respectively, of the total genetic variability detected was due to differences among samples, while FST values at each locus were significantly different from zero at two allozyme loci (AAT-3* and G3PDH-1*) out of nine and at Mmer-Amp1A. The RST values calculated for the minisatellite data (Table 2) showed a higher level of differentiation than the FST values. The RST value over both minisatellite loci was 4.11%. However, only Mmer-Amp1A showed a significant RST value after Bonferroni correction.
Table 3 presents the significant results from the allele frequency heterogeneity test for all pairs of samples at each locus. At three allozyme loci (AAT-3*, G3PDH-1* and GPI-2*) and at both minisatellite loci the sample from the Aegean Sea showed significant heterogeneity versus either the Gulf of Lion or the Tuscan Archipelago sample, while no significant frequency difference was found between the two western samples at any locus.
| locus | samples | P |
|---|---|---|
| AAT-3* | Gulf of Lion vs Aegean Sea | *** |
| Tuscan Archipelago vs Aegean Sea | *** | |
| G3PDH-1* | Gulf of Lion vs Aegean Sea | ** |
| GPI-2* | Gulf of Lion vs Aegean Sea | *** |
| Mmer-Amp1A | Tuscan Archipelago vs Aegean Sea | * |
| Mmer-Amp2 | Gulf of Lion vs Aegean Sea | *** |
- Only sample pairs that showed significant heterogeneity, after Bonferroni correction for multiple tests (values of P ≤ 0.0015 were considered significant after Bonferroni correction), are shown; * is equivalent to P ≤ 0.05, ** to P ≤ 0.01 and *** to P ≤ 0.001, after Bonferroni correction.
Nei's genetic distance (DA) between samples from the western part of the Mediterranean Sea was much lower than the distances between these two samples and the Aegean Sea sample, both when calculated from allozyme and minisatellite allele frequencies (Table 4).
| Gulf of Lion | Tuscan Archipelago | Aegean Sea | |
|---|---|---|---|
| Gulf of Lion | – | 0.0879 | 0.1548 |
| Tuscan Archipelago | 0.0050 | – | 0.1363 |
| Aegean Sea | 0.0193 | 0.0141 | – |
Discussion
The minisatellite analysis presented here is the first reported investigation on the population structure of Mediterranean poor cod using DNA markers. Both types of molecular markers, allozymes and minisatellites, gave similar indications of population structure, as indicated by allele frequency heterogeneity and FST analyses.
It is not always the case that different types of molecular markers show consistent results. Recent studies on North Atlantic herring, Clupea harengus, excluding Norwegian fjord populations, using allozymes, mtDNA RFLPs and microsatellites on the same samples showed that the microsatellite data exhibited the highest level of divergence. They revealed significant genetic heterogeneity among samples, which appeared to be homogeneous when analysed with the other markers (Turan et al., 1998; Shaw et al., 1999).
On the other hand, other studies have demonstrated close agreement between allozymes and minisatellites. Laikre et al. (1995) used allozyme and minisatellite loci to show differentiation between four samples of brown trout (Salmo trutta), and the GST values were almost the same between the two types of marker (allozymes GST = 0.17, minisatellites GST = 0.16). Moreover, recent studies using different markers (allozymes: Allegrucci et al., 1997; mtDNA: Cesaroni et al., 1997; RAPDs: Caccone et al., 1997; microsatellites: Bahri-Sfar et al., 2000) on the same samples of sea bass (Dicentrarchus labrax) reported consistent results on the presence of an east–west differentiation in the Mediterranean Sea.
In the present study both allozyme and minisatellite data revealed a clear separation between the two western Mediterranean samples (Gulf of Lion and Tuscany Archipelago) and the eastern one (Aegean Sea) when the allele frequency heterogeneity was tested between pairs of samples (Table 3). Moreover, Nei's (1983) genetic distances (DA) among the Mediterranean samples (Table 4) clearly supported this separation. This result is in contrast with the allozyme analysis presented by Mattiangeli et al. (2000), where only 20 individuals were analysed in the sample from the Aegean Sea and no significant heterogeneity was found. Consequently, the increased number of individuals (from 20 to 70) in the eastern Mediterranean sample revealed an east–west separation in the allozyme analysis.
Restriction of gene flow around the Hellenic peninsula may explain the observed differentiation between the Aegean and Tyrrhenian Sea samples. There are cases where a geographic barrier can produce a similar pattern of population differentiation in different species (Avise, 1994). In an allozyme study on European anchovies, Engraulis encrasicolus, which are more migratory than Mediterranean poor cod, Bembo et al. (1996) found two stocks in the Adriatic Sea, but also indications of a distinction between the Aegean and Tyrrhenian Sea samples, as with the Mediterranean poor cod results here presented. Moreover, a recent study on sea bass (Bahri-Sfar et al., 2000) showed a significant difference between samples from the eastern and the western part of the Mediterranean Sea, indicating the unidirectional water circulation in the Siculo-Tunisian Strait as the cause for limited gene flow.
The homogeneity observed between the two samples from the western part of the Mediterranean (Gulf of Lion and Tuscan Archipelago) is consistent with the findings of Lenfant & Planes (1996), who cited currents as being responsible for the lack of differentiation between samples of white sea bream, Diplodus sargus, fished within the same areas.
The availability of only three samples of Mediterranean poor cod in the present study limits the description of the population structure of this fish. A more complete understanding will require a greater number of samples from several different locations, in particular the Adriatic Sea and the Spanish coast. The Adriatic Sea is known to have abundant quantities of this species (Froglia & Zoppini, 1981), and Svetovidov (1948) indicated some morphological differences in the specimens from this area compared with Tyrrhenian Sea specimens. Moreover, a clear separation between samples of sea bass (Dicentrarchus labrax) from the Aegean and the Adriatic Sea was shown by Bahri-Sfar et al. (2000). The authors suggested the presence of ‘sub-basins’ with limited gene flow at the larvae stage due to the presence of cyclonic circulation in the northern parts of the Adriatic and the Aegean Sea. In contrast to the sea bass, poor cod are relatively stationary as adults, and most of the gene flow occurs during the egg and larvae stage (Politou & Papaconstantinou, 1991). Therefore, a cyclonic circulation could indeed favour the differentiation of the poor cod in the Adriatic.
Several studies undertaken along the Spanish coast indicated the presence of genetic structure in other fish species (Diplodus sargus: Lenfant & Planes, 1996; Sardina pilchardus: Ramon & Castro, 1997), probably due to current systems which could also effect the distribution of Mediterranean poor cod.
Local fishery resources in the Mediterranean Sea are managed as independent units based only on national territorial waters (according to the EU Common Fishery Policy), an approach which does not take into account the genetic structure of the different fish species. Studies on the yield-per-recruit, based on estimates of growth and mortality parameters, by Politou & Papaconstantinou (1991) suggested that any increase in the commercial harvest of Mediterranean poor cod in the Aegean Sea would result in over-exploitation. The time required for population recovery in many marine fish, in particular gadoids, appears to be considerably longer than previously believed, despite the fecundity typical of these species (Hutchings, 2000). In view of these considerations, a deeper knowledge of the population structure of the Mediterranean poor cod could prove valuable.
Conclusions
Both types of markers – allozyme and minisatellite – used in this study gave consistent results, indicating independent recruitment in the western and eastern Mediterranean poor cod. Thus, they proved to be useful tools for the investigation of the genetic structure of this species, and open the possibility of further studies of Mediterranean poor cod population genetics.
Acknowledgements
Thanks are due to Dr Richard Milner (CEFAS, UK) for extensive help in co-ordinating the sampling, to Prof. M. Abbiati (University of Bologna, Italy) for the sample from the Tuscan Archipelago, to Dr A. Souplet (IFREMER, Sete, France) for the sample from the Gulf of Lion, to Dr Costas Papaconstantinou and Dr Celia Vassilopoulou (National Centre for Marine Research, Greece) for the Aegean Sea sample from 1992 and to Prof. Costas Triantaphyllidis (Aristotle University of Thessaloniki, Greece) for the Aegean Sea sample from 1998. Financial assistance from EC FAIR CT 95-0282 and DN98040015 (Directorate for Nature Management, Norway) are gratefully acknowledged. The authors wish to thank the two anonymous referees for their helpful comments.




