Molecular determinants for the action of general anesthetics at recombinant α2β3γ2γ-aminobutyric acidA receptors
Abstract
General anesthetics modulate the activity of ligand-gated ion channels including the GABAA receptor. Mutational studies mainly on the benzodiazepine-insensitive α2β1(M286W) and α6β3(N289M)γ2 GABAA receptors revealed that a serine in transmembrane domain 2 and a methionine in transmembrane domain 3 are essential for the action of most general anesthetics. We investigated whether these residues would similarly be relevant for their action at the benzodiazepine-sensitive GABA receptor subtype, α2β3γ2. We found that not only the N265M but also the M286W mutation nearly abolished the modulatory effect of etomidate. However, the anti-convulsant loreclezole, a structural homologue of etomidate, was inactive on the N265M mutant, but displayed normal modulatory activity on the M286W mutant. Both mutations did not affect the modulatory action of the neurosteroid alphaxalone. The direct action of alphaxalone, however, was dramatically increased in the M286W mutant to about twice the maximal GABA current but not significantly affected in the N265M mutant. These data demonstrate that the structural requirements for modulatory and direct actions of various general anesthetics are distinct. The molecular switches induced by these mutations can be exploited to identify the molecular determinants for the action of general anesthetics.
Abbreviations
-
- used
-
- ALPHAX, alphaxalone
-
- DMSO
-
- dimethyl sulphoxide
-
- ENFL
-
- enflurane
-
- ETOM
-
- etomidate
-
- HEK
-
- human embryonic kidney
-
- LOREC
-
- loreclezole
-
- PROP
-
- propofol
-
- tetraethyl ammonium chloride (TEACP); TM
-
- transmembrane.
General anesthetics have been widely used in clinical practice for more than a century. Their mechanism of action in the central nervous system is, however, only poorly understood. Initially, a nonspecific action of general anesthetics on the lipid bilayer of neuronal cells was proposed (Meyer 1901; Overton 1901). Over the past decade it became clear that general anesthetics modulate the activity of several recombinant ligand-gated ion channels (Krasowski and Harrison 1999). At surgical concentrations, most general anesthetics potentiate GABAA receptor-mediated responses. Some general anesthetics enhance glycine receptor-induced responses or inhibit the activity of nicotinic acetylcholine and ionotropic glutamate receptor subtypes (Franks and Lieb 1994; Harris et al. 1995). Thus, it is conceivable that general anesthetics may produce their clinical effects by enhancement of inhibitory and/or depression of excitatory synaptic transmission. Two-pore domain potassium channels, which generate voltage-independent background currents, are further potential targets (Patel et al. 1999; Gray et al. 2000; Sirois et al. 2000).
GABAA receptors are pentameric complexes in the plasma membrane, which constitute GABA-gated chloride channels. Currently seven different classes of subunits with mostly multiple variants (α1–6, β1–3, γ1–3, σ1–3, δ, ε, θ) are known. Most GABAA receptors are composed of α, β and γ subunits (Barnard et al. 1998) and contain binding sites for modulators such as benzodiazepines, barbiturates and␣neurosteroids.
The low affinity of general anesthetics for GABAA receptors has so far precluded a direct identification of specific␣binding sites. However, defined amino acid residues in the transmembrane regions 2 and 3 (TM2 and TM3) of GABAA receptor α and β subunits were shown to be critical for the action of general anesthetics (Krasowski and Harrison 1999). In an elegant study using chimeric receptors, Mihic et al. (1997) identified a serine residue in TM2 and a methionine residue in TM3 of the α and β subunits that are necessary for modulation of GABAA receptors by the volatile anesthetic enflurane and for ethanol. When the respective amino acids were mutated to residues present in the GABA receptor ρ1 subunit, which is insensitive to anesthetics [e.g. α2(S270I), β1(M286W)], the response of these receptors to enflurane was abolished. The same methionine residue in the TM3 domain of the β subunit was also found to be essential for the modulatory action of the intravenous anesthetic propofol (Krasowski et al. 1998). Independently, studying α6β3γ2 receptors, Belelli et al. (1997) reported that an asparagine residue present in the β2 and β3 subunits determines the sensitivity to etomidate. When this asparagine residue was mutated to serine [β3(N265S)] or to methionine [β3(N265M)], which are the residues observed in the naturally etomidate-insensitive β1 subunit and the Drosophila RDL receptor, respectively, the α6β3γ2 receptors lost their sensitivity for etomidate.
Until now, the TM2 and TM3 mutations mentioned above have been studied almost exclusively in recombinant α2β1/α2β1γ2 or α6β3γ2 receptors (Belelli et al. 1997; Krasowski et al. 1998). By studying αβ receptors it is possible to delineate the minimal subunit requirements for drug action. However, αβ receptors are unlikely to occur in the brain to any significant degree. α6βxγ2 receptors are restricted to the cerebellum and are therefore unlikely candidates for mediating the action of general anesthetics in vivo. Moreover, α6 knockout mice responded normally to the action of general anesthetics (Homanics et al. 1997). Thus, the functional consequences of the aforementioned point mutations in a major benzodiazepine-sensitive GABAA receptor subtype are currently not known. The involvement of the β3 subunit-containing receptors in general anesthesia in vivo was demonstrated in the β3 subunit-deficient mice, which showed an altered sensitivity to general anesthetics (Quinlan et al. 1998). We therefore set out to investigate the pharmacological properties of the β3(N265M) and β3(M286W) mutations in recombinant α2β3γ2 receptors which, together with α1β2γ2 receptors, represent about 80% of all benzodiazepine-sensitive GABAA receptor in brain (Benke et al. 1994).
Materials and methods
cDNAs and site-directed mutagenesis
Rat α2, β3, and γ2S GABAA receptor subunit cDNAs in the expression vector pBC12/CMV (Bertocci et al. 1991) were kindly provided by Dr P. Malherbe (Basel, Switzerland). For the β3 subunit, site-directed mutagenesis substituting an asparagine residue at position 265 with a methionine residue, β3(N265M), or a methionine residue at position 286 with a tryptophan residue, β3(M286W), was performed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) with complementary oligonucleotides spanning the mutations. For the β3(N265M) mutation, oligonucleotides RJR-1: 5′-GCTCACCATGACAACCATCATGACTCACCTTCGAGAGAC-3′ and RJR-2: 5′-GTCTCTCGAAGGTGAGTCATGATGGTTGTCATGGTGAGC-3′ were used (mutated codon underlined). For the β3(M286W) mutation the oligonucleotides were RJR-3: 5′-GCCATCGACATGTACCTGTGGGGTTGCTTCGTCTTTGTATTCC-3′ and RJR-4: 5′-GGAATACAAAGACGAAGCAACCCCACAGGTACATGTCGATGGC-3′. The mutated cDNAs were verified by double-stranded DNA sequencing. The numbering of amino acids is based on the mature subunit protein after cleavage of the signal peptide sequence.
Cell culture and transfection
Human embryonic kidney cells 293 (HEK 293) were grown in Dulbecco's modified Eagle medium (DMEM, Gibco-BRL Life Technologies, Basel, Switzerland) supplemented with 5% heat inactivated fetal calf serum (FCS, Gibco-BRL), 100 U/mL penicillin and 100 mg/mL streptomycin (both from Gibco-BRL, Life Technologies). Cells were transiently transfected with the␣rat␣cDNA combinations α2β3γ2S, α2β3(N265M)γ2S and α2β3(M286W)γ2S using the calcium phosphate precipitation technique (Chen and Okayama 1987), as described previously (Knoflach et al. 1992).
Electrophysiology and data analysis
The patch-clamp technique in the whole-cell configuration was used to record GABA-induced Cl– currents. Recording pipettes were pulled from borosilicate glass (Hilgenberg, Malsfeld, Germany) and had a resistance of 6–7 MΩ when filled with intracellular solution. The membrane potential was held at −60 mV. The current signals were amplified with an Axopatch-1D amplifier (Axon Instruments, Inc., Foster City, CA, USA), filtered by a 1 kHz four-pole Bessel low-pass filter. The analysis of data was performed using the pClamp data acquisition program set (pClamp 8.0, Axon Instruments) and FigP (Biosoft, Cambridge, UK).
The GABA dose–response curves were obtained by applying 5- s pulses of GABA every 1.5 min to the patch-clamped HEK 293 cells. The maximum current amplitudes from individual cells␣were first fitted separately using the equation I/Imax= 1/{1 + (EC50/[GABA])Hill}, where I is GABA-evoked current, Imax is the maximum of the fit, EC50 is the GABA concentration evoking the half-maximal response, and Hill is the Hill coefficient. The individual dose–response curves were then normalized to Imax, and the data replotted using the mean values for each concentration.
Drug application
GABA was applied in the presence or absence of drugs to the patch-clamped cell for 5 s every 1.5 min using a multibarrelled microapplicator pipette constructed from seven concentrically arranged glass tubes ending in a common tip. Air-tight syringes, containing the solutions, were connected to the microapplicator via Teflon tubes, in order to avoid loss of the anaesthetics due to evaporation or sticking to the tubes. Six of the tubes were used for drug delivery, and the seventh tube at the centre provided slow aspiration to prevent accumulation of drugs in the common tip and leakage from␣the inactive barrels. To allow a rapid shut-off of the drug application, a sucking pipette was placed in front of the tip of the drug-applicator, in order that the tested cell was positioned between the applicator and the suction. In this way, the solution coming from the application bathed the cell and was directly and rapidly sucked out by the suction.
Solutions and drugs
The recording pipettes were filled with an intracellular saline solution containing 120 mm CsCl, 20 mm tetraethyl ammonium chloride (TEACP), 1 mm CaCl2, 2 mm MgCl2, 11 mm EGTA, 10 mm HEPES, adjusted to pH 7.2 with CsOH. The cells, placed in a Petri-dish fitting into the recording chamber, were continuously superfused with a bath a solution using a second applicator. The bath solution contained 157 mm NaCl, 5.4 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2 and 5 mm HEPES, adjusted to pH 7.4 with NaOH. The experiments were performed at room temperature. GABA was from Sigma (St. Louis, Mo, USA), while etomidate and loreclezole were provided by Janssen Pharmaceutica (Beerse, Belgium). Propofol was obtained from Aldrich (Buchs, Switzerland), alphaxalone from Sigma (Buchs, Switzerland) and enflurane from Abbott Laboratories (Cham/Zug, Switzerland). GABA was dissolved in water and used at concentrations of 3 µm for α2β3γ2 and α2β3(M286W)γ2 receptors and of 30 µm for α2β3(N265M)γ2 receptor, which correspond to EC5 for all receptors. Stock solutions of the test compounds were prepared in 100% dimethyl sulphoxide (DMSO) and diluted 1000-fold before use. During the experiments, the final concentrations of DMSO in the bath solutions were not higher than 0.1%, which had no effect on GABA responses. The volatile enflurane was dissolved in the external solution. Fresh solutions were prepared every hour. Based on other studies in which a similar application method was used, we expect only a small loss of enflurane of approximately 10% and 15% (Antkowiak and Heck 1997; Banks and Pearce 1999), which would affect recordings from wild type and mutant receptors in the same way.
Results
GABA-sensitivity of wild-type and point-mutated recombinant GABAA-receptors
We introduced the two point mutations N265M and M286W located in transmembrane domains 2 (TM2) and 3 (TM3), respectively, into the rat GABAA receptor β3 subunit cDNA (Fig. 1). The wild-type and point-mutated β3 subunit cDNAs were coexpressed in HEK 293 cells with rat α2 and γ2 subunit cDNAs. The EC50 value for GABA for the wild-type receptor α2β3γ2 was 47 ± 5 µm(Fig. 2), which is similar to the value previously obtained in our laboratory, 74 ± 12 µm (Benson et al. 1998). The GABA dose–response curve for the α2β3(N265M)γ2 receptor was shifted to the right, with an EC50 value of 122 ± 10 µm, which is significantly different from wild-type (***p < 0.001) (Fig. 2). In contrast, the GABA dose–response curve for the α2β3(M286W)γ2 receptor was shifted to the left, with a significant decrease (***p < 0.001) of the EC50 (14 ± 2 µm) (Fig. 2). The Hill slopes for the GABA concentration-response relationships␣for both point-mutated β3 containing receptors (α2β3(N265M)γ2, Hill = 1.6 ± 0.2; α2β3(M286W)γ2, Hill = 1.3 ± 0.2) were not significantly different compared with wild-type α2β3γ2 receptors (Hill = 1.3 ± 0.1). The Imax values for all receptor combinations were not significantly different (2007 ± 530 pA for α2β3γ2; 1307 ± 330 pA for α2β3(N265M)γ2 and p = 0.27 compared to wild type; 1065 ± 290 pA for α2β3(M286W)γ2 and p = 0.13 compared to wild type). In the presence of the open channel blocker picrotoxin we observed an outward current of about 50 pA (48 ± 21 pA, n= 6). This leak current presumably results from spontaneous channel opening and corresponds to 10% of the GABA-induced inward current.
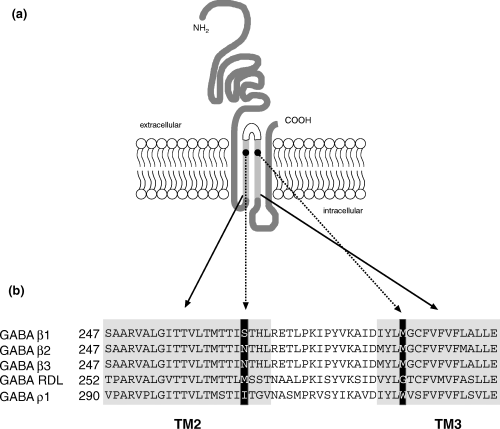
(a) Schematic representation of a subunit of the GABAA receptor, showing the four transmembrane domains (TM) and the localization of the two mutated amino acids in TM2 and TM3 (●). (b) Alignment of the amino acid sequences of the TM2 and TM3 domains of rat and Drosophila␣melanogaster GABA receptor subunits. In the black boxes are the amino acids in positions 265 and 286 which have been mutated in the β3 subunit, specifically the asparagine (N) in position 265 to methionine (M), the corresponding residue found in the RDL receptor from Drosophila, and the methionine (M) in position 286 to the corresponding tryptophane (W) in the GABA ρ1 receptor. Figure modified from Belelli et al. (1999).
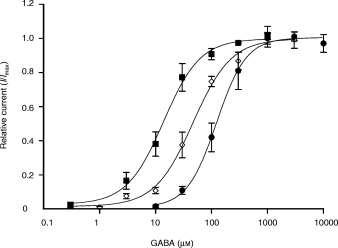
GABA dose–response curves for wild type and point-mutated GABAA receptor subtypes expressed in HEK 293 cells transiently transfected with the cDNAs coding for the wild-type or mutated β3 subunit in combination with the α2 and γ2 subunits. The data points and error bars represent the means and standard errors of 6–8␣experiments for the different GABA concentrations. ⋄, α2β3γ2, EC50 = 47 ± 5 µM (n = 7), Hill = 1.3 ± 0.1 (n = 7); ●, α2β3(N265M)γ2, EC50 = 122 ± 10 µM (n = 8), Hill = 1.6 ± 0.2 (n = 8); ▪, α2β3(M286W)γ2, EC50 = 14 ± 2 µM (n = 6), Hill = 1.3 ± 0.2 (n = 6).
Modulation of GABA-induced chloride currents by propofol
To assess the allosteric modulation of the GABA-induced currents of wild-type and point-mutated receptors by general anesthetics, GABA concentrations of 3 µm for α2β3γ2 and α2β3(M286W)γ2 receptors and 30 µm for α2β3(N265M)γ2 receptors were chosen, roughly corresponding to EC50 values. In cells expressing wild-type receptors, 1 µm and 10 µm propofol potentiated the GABA-induced chloride current by 1484 ± 400% and by 1953 ± 670%, respectively (Fig. 3). In contrast, 1 µm propofol induced only a very small potentiation of the GABA-evoked currents mediated by receptors containing the point-mutated β3(N265M) and β3(M286W) subunits [Fig. 3; 53 ± 10% for α2β3(N265M)γ2 and 51 ± 6% for α2β3(M286W)γ2]. With 10 µm propofol, potentiations of GABA-induced currents were still detectable in cells expressing mutated receptors (166 ± 25% for α2β3(M286W)γ2 and 155 ± 33% for α2β3(N265M)γ2). For both 1 µm and 10 µm propofol the potentiations induced at the mutated receptors were significantly smaller compared to those induced at the wild-type receptors (Fig. 3).
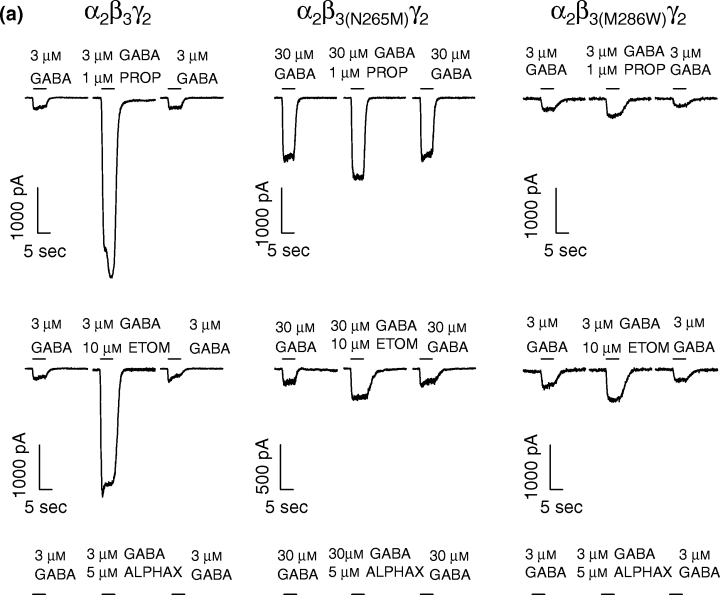
(a) Single traces of the effects of intravenous anesthetics propofol (PROP), etomidate (ETOM) and alphaxalone (ALPHAX) on the GABA-evoked currents are shown for the wild type α2β3γ2 and the␣point-mutated α2β3(N265M)γ2 and α2β3(M286W)γ2 GABAA receptors. All three compounds potentiate the current in the α2β3γ2 receptors. In both point-mutated receptors, propofol and etomidate display only a marginal potentiation of the GABA-evoked currents, while alphaxalone maintains its potentiating effect. (b) Potentiation of the GABA-evoked currents by propofol (1, 10 µm), etomidate (10 µm), loreclezole (10 µm) and alphaxalone (5 µm) for α2β3γ2, α2β3(N265M)γ2 and α2β3(M286W)γ2 GABAA receptors. The modulation of GABA-evoked currents is expressed relative to the control currents at the given GABA concentration (mean ± standard error, n= 3–9). Data were analysed by Student's t-test, *p < 0.05 and ***p < 0.001 as compared with the controls.
Modulation of GABA-induced chloride currents by etomidate
In cells expressing α2β3γ2 wild-type receptors, 10 µm etomidate strongly potentiated GABA-induced currents (947 ± 244%; Fig. 3). Both point mutations in the β3 subunit, N265M and M286W, resulted in a loss of sensitivity to etomidate (81 ± 41% and 38 ± 23% potentiation, respectively). The anti-convulsant loreclezole is a closely related analogue of etomidate and the two compounds are thought to share a common binding site on GABAA receptors (Wingrove et al. 1994; Belelli et al. 1997). Loreclezole (10 µm) induced a strong potentiation of the GABA-induced current in the wild-type receptors (505 ± 138%; Fig. 3), albeit smaller than the potentiation induced by etomidate (10 µm). In line with an earlier report (Wingrove et al. 1994), the point mutation N265M in the TM2 domain of the β3 subunit rendered the receptor insensitive to loreclezole. However, the substitution of the methionine to tryptophane in the TM3 domain did not affect the modulation of GABA-induced chloride currents by loreclezole.
Modulation of GABA-induced chloride currents by alphaxalone
Neurosteroids are known to potentiate chloride currents mediated by GABAA receptors (Puia et al. 1990; Lambert et al. 1995). Alphaxalone is a neurosteroid which can be classified as an intravenous anesthetic. As expected, 5 µm alphaxalone potentiated the current activated by GABA on cells expressing the α2β3γ2 receptor (420 ± 154%; Fig. 3). No significant change in the potentiation of the GABA response was observed in both point-mutated receptors [427 ± 120% for α2β3(N265M)γ2 and 230 ± 80% for α2β3(M286W)γ2].
Modulation of GABA-induced chloride currents by enflurane
To assess the sensitivity of the mutated receptors to volatile anesthetics, the modulatory action of enflurane was studied. Enflurane (1 mm) potentiated the GABA-evoked current 122 ± 14% in wild-type receptors, whereas both point-mutated receptors were significantly less sensitive to enflurane [23 ± 12% potentiation for α2β3(N265M)γ2 and 4 ± 2% for α2β3(M286W)γ2; Fig. 4].
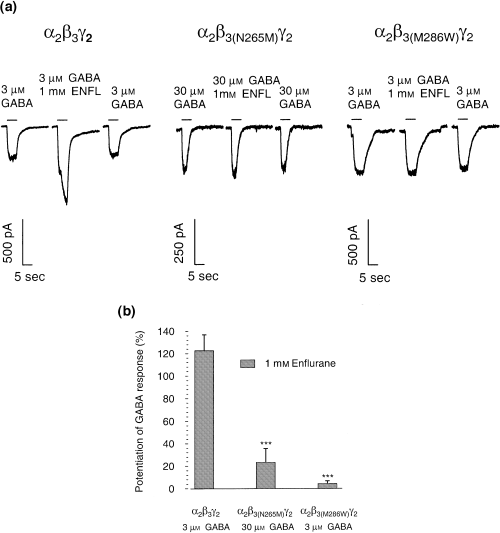
(a) Whole-cell current traces showing the effects of the volatile anesthetic enflurane (ENFL) on wild-type α2β3γ2 and␣point-mutated α2β3(N265M)γ2 and α2β3(M286W)γ2 GABAA receptors. In α2β3γ2 receptors enflurane potentiates the current, while in both point-mutated receptors potentiation is essentially abolished. (b) Bar histogram for the potentiation of the GABA-evoked currents by enflurane (1 mm) on α2β3γ2, α2β3(N265M)γ2 and α2β3(M286W)γ2 GABAA receptors. The modulation of GABA-evoked currents is expressed relative to the control currents at the given GABA concentration (mean ± standard error, n = 3–9). Data were analysed by Student's t-test, ***p < 0.001 as compared with the controls.
Direct activation of GABAA receptors by propofol
Propofol is able to directly activate GABAA receptors in the absence of GABA (Hales and Lambert 1988; Hales and Lambert 1991; Hara et al. 1993). In the present study, 10 µm propofol was applied to patch-clamped HEK 293 cells expressing either the wild-type α2β3γ2 receptors or receptors containing a point-mutated β3 subunit (Fig. 5). When applied to α2β3γ2 receptors in the absence of GABA, 10 µm propofol induced an inward current corresponding to 25 ± 7.4% of the maximal current evoked by GABA. The same concentration of propofol induced a very small current through the α2β3(N265M)γ2 receptor (2.1 ± 1.3%), which is statistically different from the wild-type control (***p < 0.001). The point mutation M286W apparently reduced the current induced by 10 µm propofol (14 ± 3.3%, Fig. 5, bar histogram), however, this decrease was not significant when compared to the wild-type receptor.
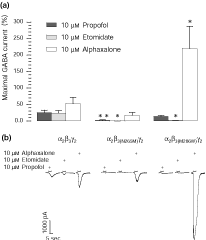
Direct activation of GABAA receptor-mediated Cl– currents by␣propofol (10 µm), etomidate (10 µm) and alphaxalone (10 µm) in α2β3γ2, α2β3(N265M)γ2 and α2β3(M286W)γ2 receptors. (a) The currents activated by the three compounds are displayed as a percentage of the maximal GABA response which was determined separately; bars show the percentage of the direct gating ± standard error, n = 3–8. Propofol still activates the Cl– current in α2β3(M286W)γ2 receptors, but not in α2β3(N265M)γ2 receptors. The direct action of etomidate is abolished in both point-mutated receptors, while alphaxalone activates the currents in both mutated receptors, with a significantly larger current in the α2β3(M286W)γ2 receptors. Data were analysed by Student's t-test, *p < 0.05 and **p < 0.01 as compared with the controls. (b) Selected traces showing the whole-cell Cl– currents activated by propofol, etomidate and alphaxalone in α2β3γ2, α2β3(N265M)γ2 and α2β3(M286W)γ2 receptors.
Direct activation of GABAA receptors by etomidate
Since etomidate also displays GABA-mimetic properties (Hill-Venning et al. 1997; Sanna et al. 1997), we investigated the direct action of etomidate on wild-type and mutated receptors expressed in the HEK 293 cells. 10 µm etomidate directly activated the α2β3γ2 receptors inducing an inward current corresponding to 24 ± 7.3% of the maximal GABA-evoked current (Fig. 5). Both point mutations in the β3 subunit prevented the direct activation of the GABAA receptors by etomidate (Fig. 5).
Direct activation of GABAA receptors by alphaxalone
Alphaxalone also exerts a direct effect on α2β3γ2 GABAA receptors (Fig. 5). In cells expressing wild-type receptors, 10 µm alphaxalone induced a chloride current corresponding to 52 ± 19% of the maximal GABA current. The point mutation N265M in the β3 subunit appeared to reduce the GABA mimetic action of alphaxalone (17 ± 9% of the maximal GABA current). However, this change was not statistically significant. In contrast, alphaxalone evoked a chloride current in cells expressing receptors containing the M286W mutation that was significantly larger than the current observed in the wild-type receptor (220 ± 67% of the␣maximal GABA current; *p < 0.05).
Discussion
In the present study, we assessed the phenotypic consequences of the N265M (TM2 domain) and M286W (TM3 domain) point mutations in the GABAA receptor β3 subunit on general anesthetic action in α2β3γ2 recombinant receptors, which represent one of the most common GABAA receptor subunit combinations in the CNS (Benke et al. 1994; Fritschy and Mohler 1995).
To test whether the introduction of the point mutations in either the TM2 or the TM3 domain alters the responsiveness of the respective α2β3γ2 receptors to GABA, GABA dose–response curves were obtained for the recombinant α2β3(N265M)γ2 and α2β3(M286W)γ2 receptors and compared to the α2β3γ2 receptor. The point mutation N265M in TM2 shifted the dose–response curve to the right with a significant increase of the EC50 value (Fig. 2). The point mutation M286W in the TM3 shifted the dose–response curve to the left with a significant decrease of the EC50 value for GABA (Fig. 2). Interestingly, Krasowski et al. (1998) reported that the GABA sensitivity of the α2β1 wild-type receptor (EC50 = 8.7 ± 0.4 µm) did not change when the M286W mutation was introduced (EC50 = 8.7 ± 0.4 µm). This difference to our results may be due to either the presence of the γ2 subunit in our experiments or due to the presence of the β3 subunit instead of the β1 subunit. In addition, Krasowski et al. (1998) used human subunits while we used rat subunits. The changes in the EC50 values might be explained by changes in the apparent affinity of the mutated receptors to GABA or a change in the kinetic properties of the chloride channel. A single channel analysis of the wild-type and mutated channels, combined with binding studies would allow to distinguish between the two possibilities.
Modulation by intravenous anesthetics (propofol, etomidate and alphaxalone) and by the anti-convulsant loreclezole
Propofol, etomidate and alphaxalone potentiated GABA-activated currents on recombinant α2β3γ2 GABAA receptors, in keeping with earlier findings on other subunit combinations (Belelli et al. 1997; Krasowski et al. 1998). The␣modulatory action of propofol and etomidate was significantly reduced by the N265M and M286W mutations, in line with results reported by Pistis et al. (1999) and Belelli et al. (1997) for the N265M mutation. In the α2β1 subunit combination (Krasowski et al. 1998), the action of propofol was abolished by the M286W mutation, but the action of etomidate remained unaffected. In contrast, our data demonstrate that the M286W mutation also abolished the modulatory action of etomidate on a major GABAA receptor subtype. With respect to the modulatory action, propofol and etomidate are equally affected by the two point mutations. Interestingly, while the N265M mutation abolished the action of loreclezole, in line with observations by Wingrove et al. (1994), we demonstrate here that the M286W mutation had no effect on loreclezole action. Thus, the modulatory action by the closely related compounds etomidate and loreclezole are differentially affected and can be separated. Neither the N265M nor the M286W mutation affected the modulatory action of alphaxalone. This corresponds to results obtained by Belelli et al. (1997) using another neurosteroid, 5α-pregnan-3α-ol-20-one, and by Krasowski et al. (1998). Furthermore, by using chimeric receptors Rick et al. (1998) showed that the TM2 and TM3 domains of the β subunit are not necessary for the modulatory effects of alphaxalone. These results indicate that the modulatory action of alphaxalone has structural requirements quite different from those of propofol and etomidate.
Modulation by volatile anesthetics: enflurane
On wild-type α2β3γ2 receptors enflurane had a potentiating effect, though smaller compared to the action of the other anesthetics tested and compared to other reports (Mihic et al. 1997). The relatively small potentiation we observed might be due to the fact that enflurane has a dual action on GABAA receptors, with maximal potentiations achieved at concentrations much lower (100 µm) than the one used in our study (1 mm) (Antkowiak and Heck 1997; Banks and Pearce 1999). In both point-mutated receptors, α2β3(N265M)γ2 and α2β3(M286W)γ2, the modulation by enflurane is significantly reduced, indicating that both amino acid residues in TM2 and TM3 are important for enflurane modulation of GABA-induced currents. In line with our results, Mihic et al. (1997) reported that in α2β1(M286W) receptors the action of enflurane was abolished. The action of enflurane on α2β1(S265M) receptors has not been determined, however, enflurane action was partially reduced on α1β1(S265I) receptors (Mihic et al. 1997) but not altered on α2β1(S265I) receptors (Krasowski and Harrison 2000). From those studies, it appears that the choice of the subunit composition influences the response of the mutated receptors to enflurane, depending on the presence of the α1 or α2 subunit. In any case, our results demonstrate that not only the M286W but also the N265M mutation strongly diminishes the action of the volatile anesthetic enflurane.
GABA-mimetic action of intravenous anesthetics
In addition to their modulatory action, propofol, etomidate and the neurosteroid alphaxalone also exert a direct GABA-mimetic action on α2β3γ2 receptors. In line with previous results (Krasowski et al. 1998; Pistis et al. 1999) the N265M mutation abolished the direct effect of propofol, while the M286W had no significant influence. In contrast, while the N265M mutation abolished the direct action of etomidate, similar to findings reported by McGurk et al. (1998), the M286W mutation also abolished the direct effect of etomidate, whereas Krasowski et al. (1998) reported only a reduction of the direct effect in α2β1(M286W) receptors. Thus, while N265 in TM2 is important for the direct effect of propofol and etomidate, M286 in TM3 appears to be essential for the direct action of etomidate but not necessarily of propofol, indicating that these two intravenous anesthetics have separate structural requirements concerning the amino acids involved in their GABA-mimetic action.
The neurosteroid alphaxalone displays a distinct behaviour compared to the other two intravenous anesthetics tested. To our knowledge, it has not been reported previously whether the two point mutations affect the direct action of alphaxalone. While the N265M mutation had no significant effect on the direct action of alphaxalone, surprisingly we found that alphaxalone directly activates the α2β3(M286W)γ2 receptors significantly stronger than the respective wild-type receptor. In any case, it is interesting to note that the β3(M286W) mutation has this dramatic effect on direct activation by alphaxalone but no effect on the modulatory action of alphaxalone. This demonstrates that the direct and modulatory actions of alphaxalone are structurally separable and dependent on distinct mechanisms. In a study on recombinant α1β1γ2 GABAA receptors, Sanna et al. (1997) suggested that alphaxalone directly activates GABAA receptors by interacting with sites localized on α1 or γ2, but not β1. In fact, they showed that the presence of β1 subunit reduces the sensitivity of the receptor to alphaxalone. Our finding suggests that the point mutation in the TM3 domain of the β3 subunit may induce a conformational change of the receptor, which might facilitate the interaction of alphaxalone with the site responsible for its direct agonist action. This site might be located on the α and/or γ subunits.
In this report, we have tested the effects of point mutations in TM2 and TM3 of the β subunit of several anesthetics on one of the major GABAA receptor subtypes. The mutated residues may either form part of a binding site for general anesthetics, or the mutations may induce conformational changes that modify the transduction by the receptor. Previous studies had been performed with subunit combinations unlikely to occur in the brain (α2β1/α2β1γ2) or expressed in locations unlikely to be relevant for general anesthesia (α6β3γ2). In several central aspects outlined above, our study provided results that differed from previously published research, demonstrating that extrapolation of the results obtained with unusual subunit combinations to major GABAA receptor subtypes should be done with caution. It is therefore important to determine the effects of mutations on general anesthetic action on major GABAA receptor subtypes.
The modulatory and/or direct effects of general anesthetics on GABAA receptors support the idea that they are potential targets for mediating the clinical actions of these drugs. It is thus of interest to characterize the action of general anesthetics on the molecular level by studying the effects of specific point mutations on the pharmacological properties of GABAA receptors. Our studies demonstrate that amino acid residues in TM2 and TM3, respectively, may differentially regulate the type of action (modulatory or direct) of general anesthetic agents and the actions elicited by chemically unrelated anesthetic agents.




