Disruption of tubulin polymerization and cell proliferation by 1-naphthylarsonic acid
Abstract
Arsenical compounds exhibit a differential toxicity to cancer cells. Microtubules are a primary target of a number of anticancer drugs, such as arsenical compounds. The interaction of 1-NAA (1-naphthylarsonic acid) has been investigated on microtubule polymerization under in vitro and cellular conditions. Microtubules were extracted from sheep brain. Transmission electron microscopy was used to show microtubule structure in the presence of 1-NAA. Computational docking method was applied for the discovery of ligand-binding sites on the microtubular proteins. Proliferation of HeLa cells and HF2 (human foreskin fibroblasts) was measured by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] assay method following their incubation with 1-NAA. Fluorescence microscopic labelling was done with the help of α-tubulin monoclonal antibody and Tunel kit was used to investigate the apoptotic effects of 1-NAA on the HeLa cells. 1-NAA inhibits the tubulin polymerization by the formation of abnormal polymers having high affinity to the inner cell wall.
Abbreviations:
-
- As2O3
-
- arsenic trioxide
-
- DAPI
-
- 4′,6-diamidino-2-phenylindole
-
- DMA
-
- dimethylarsinic acid
-
- FBS
-
- fetal bovine serum
-
- HF2
-
- human foreskin fibroblasts
-
- MAP
-
- microtubule-associated protein
-
- MTT
-
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
-
- 1-NAA
-
- 1-naphthylarsonic acid
-
- PI
-
- propidium iodide
1. Introduction
Tubulin, the major building block of microtubules is present in almost all eukaryotic cells. It is a heterodimer consisting of α- and β-tubulin. Both subunits have an approximate molecular mass of 55 kDa and share considerable homology. The α-/β-tubulins bind head to tail during the construction of protofilaments, forming a cylindrical polymer. Microtubules can switch between assembling/disassembling both in vivo and in vitro. This unequilibrated behaviour, known as dynamic instability, is based on the hydrolysis of GTP by tubulin subunits (McNally, 1996; Desai and Mitchison, 1997; Hirokawa et al., 1998; Nogales, 2000). Microtubule dynamics are regulated by MAPs (microtubule-associated proteins). Several classes of MAPs act by stabilizing microtubules (Sloboda et al., 1976; Cleveland et al., 1977; Parysek et al., 1984; Wiche et al., 1991). Tau protein is a neuronal MAP that forms cross-bridges between microtubules. This protein promotes microtubule growth and nucleation, in vitro and in vivo, and is regulated by phosphorylation and dephosphorylation. Tau's C- and N-termini differentially affect the microtubule growth and nucleation (Hirokawa et al., 1988; Aizawa et al., 1990; West et al., 1991; Brandt and Lee, 1993; Chau et al., 1998; Makrides et al., 2004).
Since microtubules are the target of anti-mitotic compounds, they play crucial role in cell division and cancer therapy (Jordan and Wilson, 2004; André and Meille, 2006; Attard et al., 2006; Pasquier and Kavallaris, 2008). While arsenic and relative compounds are poisonous, there are abundant elements in nature and have been used therapeutically for >2400 years in Oriental medicine (Forkner and Scott, 1931). As2O3 (arsenic trioxide) exhibits differential toxicity to cancer cells, while it is an effective drug for the treatment of APL (acute promyelocytic leukaemia) (Jackson and Grainge, 1975; Cuzick et al., 1982; Buchner, 1997; Chan and Huff, 1997; Huff et al., 1999; Li and Broome, 1999; Waxman and Anderson, 2001; Miller Jr et al., 2002; Rojewski et al., 2004). Ling et al. (2002) showed that As2O3 induces M-phase arrest due to interaction with tubulin, resulting in their polymerization. They have also found that tubulin polymerization by As2O3 did not affect GTP binding to β-tubulin (Ling et al., 2002). However, these results are not in agreement with those of Li and Broome (1999), who have reported that As2O3 caused blockade of GTP binding to β-tubulin and thus inhibited tubulin polymerization. Organo-arsenical compounds have lower toxicity than As2O3 as an inorganic molecule (Kaise et al., 1988); for example, DMA (dimethylarsinic acid), an anticancer agent, disrupts mitotic spindle and induces mitotic arrest in cultured mammalian cells (Kawata et al., 2001). Moreover, 1-NAA (1-naphthylarsonic acid) is an organo—arsenic compound, which contains greater hydrophobic moieties than that of DMA.
In the present study, the interaction and toxicity of 1-NAA has been examined in relation to tubulin dimers in cancerous and normal cells.
2. Materials and methods
2.1. Chemicals
1-NAA was prepared in the Laboratory of Macromolecules, Institute of Biochemistry and Biophysics, University of Tehran. Human cervical carcinoma cells (HeLa, NCBI C115) and human fetal foreskin fibroblasts (HF2; NCBI C190) were obtained by National Cell Bank of Iran, Pasteur Institute of Iran, Tehran, Iran. Pipes and EDTA were purchased from Merck. GTP, ATP, EGTA, magnesium sulfate, monoclonal anti-α-tubulin antibody, anti-mouse IgG FITC, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide], Trypan Blue, trypsin, PBS, Triton X-100, formaldehyde, BSA, DAPI (4′,6-diamidino-2-phenylindole), PBST, uranyl acetate were purchased from Sigma. Phosphocellulose p11 was purchased from Whatman. Formvar carbon-coated copper grids (Pelco), PI (propidium iodide), RPMI 1640 (Gibco) and FBS (fetal bovine serum; Seromed) were also used.
2.2. Equipment
Turbidometry was conducted by UV-Vis spectrophotometer (Varian Cary Bio100). Electron micrographs were taken with a transmission electron microscope (Model 902A, Zeiss). Cell micrographs were prepared by inverted and fluorescence microscopy (Zeiss). MTT colorimetric assay was measured with an ELISA reader (Stat Fax, model 2100, Awareness Technology Inc.). Microtubules were observed after staining by fluorescence microscopy (Axioskop 40, Zeiss).
2.3. Purification of microtubule protein from sheep brain
Sheep brain microtubule protein was purified by a 2 cycle assembly/disassembly process using PEM buffer (containing 100 mM Pipes, pH 6.9, 2 mM MgSO4 and 1 mM EGTA) and 0.2 mM GTP. The cold homogenized centrifuge pellet was frozen with liquid nitrogen and stored at −70°C. Tubulin concentration was ∼2.5 mg/ml (Mahinpour et al., 2009).
2.4. Tubulin assembly
Tubulin concentration was determined by Bradford method at 595 nm (Ashford et al., 1998) and 280 nm (Marshall and Williams, 1993) with a UV-Vis spectrophotometer. Tubules were assembled in PEM buffer (pH 6.9) and PMG buffers (containing 80 mM Pipes, pH 6.9, 1 mM EGTA, 2 mM MgSO4 and 3.4 M glycerin). The assembly was initiated by adding GTP at appropriate concentrations at 37°C, and measured by the turbidity at 350 nm after 30 min. A range of concentrations of 1-NAA (0.5–2 mM) was tested on tubulin polymerization at a constant concentration of GTP at 37°C for 10 min. Colchicine was used as a positive control.
To examine whether 1-NAA affected polymer interaction or the activity of MAPs, polymerization was allowed to progress up to the maximum of the elongation phase and small amounts of 1-NAA were added to the solution without a change in the temperature or the concentration of the tubulin solution.
2.5. Electron microscopy
Samples for transmission electron microscopy were prepared by negative staining using uranyl acetate on formvar carbon-coated copper grid, and examined in a TEM (Hitachi Hu-12A) operating at 75 kV (Mahinpour et al., 2009).
2.6. Docking
The 3D tubulin structure was chosen for computing by HEX 4.1 protein docking software (Ritchie, 2003). Blind docking was performed with Tau protein as the first ligand. The whole experiment was performed on the shape and electrostatic relationship. Docking results were generated by HEX, indicating the lowest energy as the best complex of tubulin-ligand. Post-processing refinement was a Newton-like minimization based on an OPLS force-field parameter. The results were saved as a pdb file. As a second run of docking process, 1-NAA was used as a ligand and the same procedure was performed.
2.7. Cell proliferation assay
Proliferation of HeLa and fibroblast cells was measured using the MTT assay. Briefly, the cells were plated into a 96-well microtitre plate (Griener) at 2 × 104 cells/well in RPMI 1640 medium with 10% FBS. The cells were incubated in 1-NAA (0.7–5 mM) at 37°C for 24, 48 and 72 h in a humidified air atmosphere with 5% CO2. The medium over the cells was removed, 100 μl of MTT (5 mg/ml in PBS) was added to each well, which was incubated for 3 h at 37°C in air with 5% CO2. The attenuance (D) was recorded on an ELISA reader (Stat Fax, 2100).
2.8. Immunocytochemical assay
Cells were fixed with formaldehyde for 15 min and washed with PBS solution. They were incubated for 10 min in PBS containing 0.25% Triton X-100 and washed for 5 min. Cells were blocked with 1% BSA in PBST for 30 min and incubated in the diluted anti-α-tubulin in PBS (1:100) (Ling et al., 2002) at room temperature for 1 h. After washing thrice with PBS solution, the cells were incubated with FITC-conjugated secondary antibody (1:500) (Ling et al., 2002) in dark room for 1 h. The cells were stained with DAPI in PBS (1 μg/ml) and PI in medium (1 μg/ml). Microtubules were observed by fluorescence microscopy.
2.9. Flow cytometry
To follow apoptosis due to exposure to 1-NAA, an APO-BRDU™ kit with dual colour flow cytometry was used after 24 and 48 h incubation of HeLa cells in the presence of 1-NAA. Positive control cells were incubated with 40 μg/ml etoposide, an anti-tumour drug.
3. Results
3.1. Tubulin assembly
Administration of 1-NAA (0.5–2 mM) caused a decrease in tubulin polymerization, but an increase in nucleation time at 37°C in the presence of 1 mM GTP (Figure 1). As illustrated in plots 5 and 6, two concentrations of colchicine completely inhibited the polymerization.
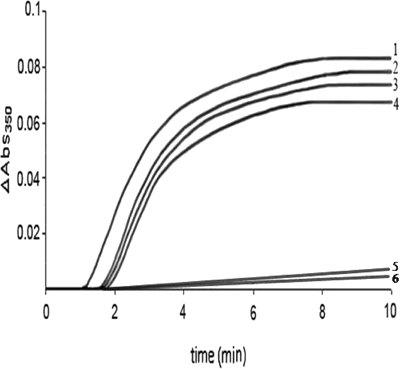
Effect of 1-NAA on tubulin polymerization in the presence of 1 mM GTP
Plot 1, 0 mM; plot 2, 0.5 mM; plot 3, 1.5 mM; plot 4, 2 mM of 1-NAA. Plots 5 and 6 show the effect of 0.1 mM and 0.5 mM colchicine respectively as a positive control.
Based on the results showed in Figure 1, we investigated in which step of polymerization 1-NAA interacts with microtubule protein. Therefore, 1-NAA was added to the sample 280 s after the initiation of tubulin polymerization under normal conditions (Figure 2, plot 2). Attenuance dropped to a similar level as the microtubule sample incubated with 1-NAA at the inception (Figure 2, plot 1). Electron micrographs of the tubulin polymers produced upon interaction of 1-NAA were in short oligomer lengths, but apparently linked head to tail, forming an apparent long or polymer-like molecule (Figures 3A and 3B).
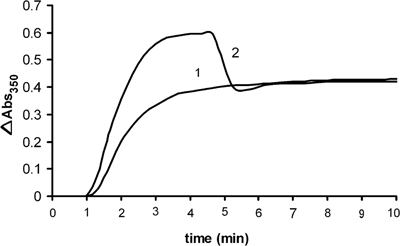
Comparison of equilibrium state of tubulin polymerization in the presence of 1 mM GTP incubated in the presence of (1) 1 mM of 1-NAA at 37°C, (2) after late addition of 1 mM 1-NAA to tubulin solution, i.e. following 280 s of polymerization and incubation at 37°C
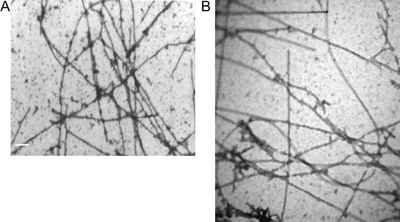
Electron micrographs of (A) microtubule polymerization in the presence of 1-NAA, scale bar=500 nm, (B) microtubule polymerization in normal conditions, in the presence of 1 mM GTP without 1-NAA, scale bar=500 nm
3.2. Docking
In exploring changes in the microtubule length due to the interaction of 1-NAA, docking was applied by the HEX4 software. 1-NAA binds to the binding site of tau protein on the α-subunit (Figure 4). Deformation in the secondary structure and conformational changes occurred in the α-tubulin subunit, which affected tubulin polymerization.
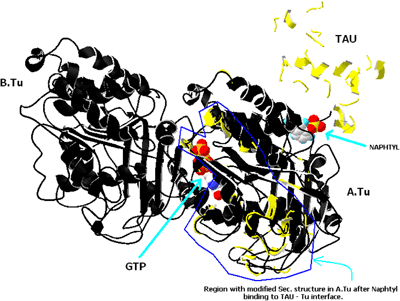
Structural changes in tau protein after ligand binding on α-tubulin in the presence of 1-NAA (the conformational variation from normal is shown in yellow)
3.3. Cell proliferation assay
The effect of 0.7–5 mM 1-NAA on proliferation of HeLa cells is shown in Figure 5(A). An increase in toxicity (up to 84%) occurred with increasing concentrations of 1-NAA after 24, 48 and 72 h, although the difference in toxicity at 0.7–5 mM at 48 and 72 h was not significantly different. 1-NAA increased cell death, with cytotoxicity affecting up to 96% of HF2 cells (Figure 5B). Inhibiting tubulin polymerization with 1-NAA results in abnormal microtubules in the human fibroblasts (Kawata et al., 2001).
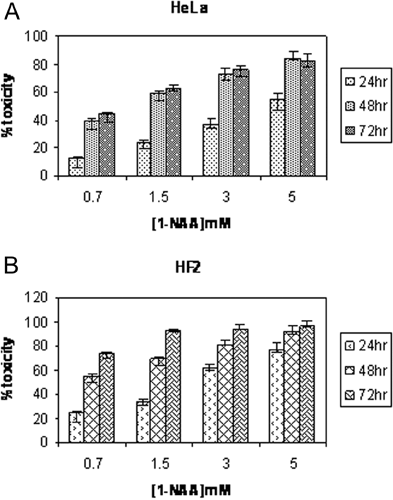
Toxicity effects of 0.7–5 mM 1-NAA on (A) the HeLa cells and (B) HF2 cells after 24, 48 and 72 h of incubation (P<0.05)
3.4. Immunocytochemical studies
HeLa cell microtubules labelled with anti-α-tubulin antibody in the presence of 1-NAA (LC50 for 24 h=4.4 mM) showed that microtubule-1-NAA were localized in the central area of the cells (Figure 6a1), with the cytoskeletal microtubules apparently being pushed towards the cell membrane (Figure 6b1). Most of the cells were affected (Figures 6a2 and 6b2).
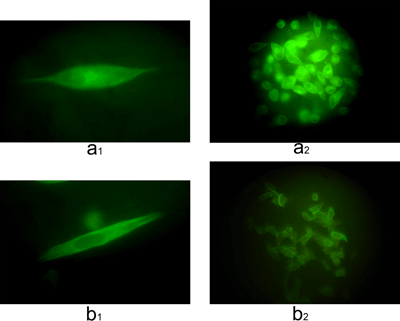
Fluorescence micrographs of HeLa cells incubated with monoclonal anti-α-tubulin antibody and stained with FITC
(a) In the absence of 1-NAA, (a1) a single cell (magnification: × 1000), (a2) a population of cells (magnification: × 630). (b) After incubation for 24 h in the presence of 1-NAA (LC50), (b1) a single cell (magnification: × 1000) and (b2) population of cells (magnification: × 630).
3.5. Flow cytometry
The effect of 1-NAA on the HeLa cell apoptosis followed by flowcytometry showed no significant change (Figures 7A–7F) at 24 h but >2-fold increase after 48 h treatment compared with that of 24 h (2.27±1.19 compared with 1.13±0.33). Apoptosis in negative controls was <1% at 24 and 48 h (Figures 7A and 7B). HeLa cells showed evidence of some necrosis after PI staining (Figure 8).
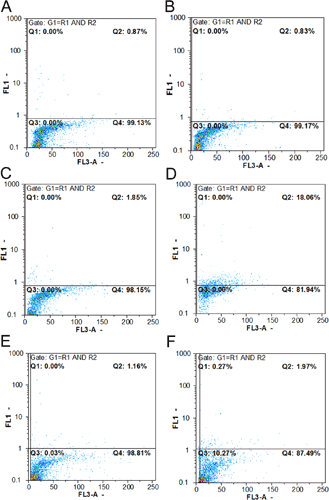
Flow cytometric results of HeLa cells by the Tunel method
(A) Negative control after 24 h of incubation, (B) negative control at 48 h of incubation, (C) positive control after 24 h of incubation (HeLa cells incubated with 40 mg/ml etoposide), (D) positive control, 48 h of incubation, (E) in the presence of 4.4 mM 1-NAA (LC50: 24 h), (F) in the presence of 1.2 mM 1-NAA (LC50: 48 h).
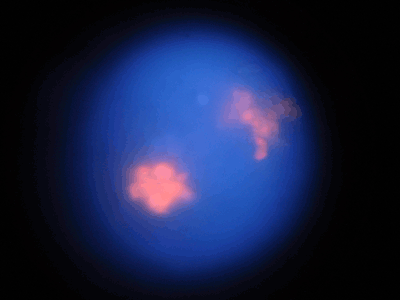
Fluorescence micrographs of HeLa cells stained with PI after incubation for 24 h in the presence of 1-NAA (magnification: × 630)
4. Discussion
At 0.5–2 mM, 1-NAA interferes with tubulin nucleation. Microtubule depolymerization occurred when the same concentration of ligand (1 mM) was added to polymerization solution at the end of elongation phase. Regarding the major role of tau protein in the nucleation phase (Hirokawa et al., 1988; Aizawa et al., 1990; West et al., 1991; Brandt and Lee 1993; Chau et al., 1998; Makrides et al., 2004), it seems that 1-NAA influences microtubule structure close to the tau-binding site. Moreover, electron micrographs of tubulin assembly in the presence of 1 mM ligand (1-NAA) showed short tiny filaments that joined together longitudinally. We believe that turbidometric observation of microtubule solution was in accord with electron microscopy (Figures 1 and 3).
The structural alteration of tubulin dimer-tau protein by interaction with 1-NAA was investigated by the simulation of ligand-protein with the help of HEX4 software. The location of tubulin-1-NAA interaction is on α-tubulin, adjacent to tau protein (Figure 4). Interestingly, the tau protein becomes structurally deformed and conformational changes of α-tubulin were also observed. To check whether these structures can separate centrosomes for the initiation of mitosis, the fibroblast and HeLa cells were incubated with 1-NAA. In the presence of 1-NAA, the cytoskeletal microtubules concentrated at the inner membrane of the cell, they were generally absent from the centre of cell with respect to controls (Figures 6A and 6B). 1-NAA therefore alters microtubule polymerization, resulting in the formation of abnormal polymers with high affinity to the inner cell wall. Li and Broome (1999) have shown that the major process of cell death due to As2O3 was necrosis. But flowcytometry of HeLa cells in the presence of 1-NAA supported the necrotic mechanism by showing an insignificant amount of apoptosis. Moreover, it seems that 1-NAA-induced apoptosis is time dependent. We suggest that short microtubules in the cytoplasm do not have sufficient opportunity to join together for them to be detected by fluorescence microscopy. Therefore, only a fraction of cytoskeletal microtubules remain tubular and long enough at inner cell membrane sites (Figures 6a1 and 6b1). Other organo-arsenical compounds related to 1-NAA should be synthesized and tested to elucidate the mechanism of action on the cellular organization of tubulin, tau and MAPs in order to find a highly effective and less toxic anti-mitotic drug.
Author contribution
Roya Mahinpour did the practical work and wrote the paper. Gholamhossein Riazi was the supervisor and corresponding author. Mohammad A. Shokrgozar was the co-supervisor and corresponding author. Mohammad N. Sarbolouki was another co-supervisor. Shahin Ahmadian was a consulting Professor and contributed to the electron microscopy. Ali A. Moosavi-Movahedi was a consulting Professor and edited the paper prior to submission. Hamid Hadi Alijanvand contributed to the bioinformatic work. Kayhan Azadmanesh contributed to flow cytometry. Masoumeh Douraghi contributed to flow cytometry and edited to the paper. Maryam Heidari contributed to the practical work. Zahra Naghdi Gheshlaghi contributed to the practical work.
Acknowledgements
We thank the University of Tehran and National Cell Bank of Iran, Pasteur Institute of Iran for helpful cooperation.
Funding
This work was supported by the University of Tehran and National Cell Bank of Iran, Pasteur Institute of Iran.




