Receptors for complement C5a. The importance of C5aR and the enigmatic role of C5L2
Abstract
Complement component C5a is one of the most potent inflammatory chemoattractants and has been implicated in the pathogenesis of numerous inflammatory diseases. C5a binds two receptors, C5aR and C5L2. Most of the C5a functional effects occur through C5aR, and the pharmaceutical industry has focused on this receptor for the development of new anti-inflammatory therapies. We used a novel approach to generate and test therapeutics that target C5aR. We created human C5aR knock-in mice, and used neutrophils from these to immunize wild-type mice. This yielded high-affinity blocking mAbs to human C5aR. We tested these anti-human C5aR mAbs in mouse models of inflammation, using the human C5aR knock-in mice. These antibodies completely prevented disease onset and were also able to reverse established disease in the K/B × N arthritis model. The physiological role of the other C5a receptor, C5L2 is still unclear, and our studies with blocking mAbs to human C5L2 have failed to demonstrate a clear functional role in signaling to C5a. The development of effective mAbs to human C5aR is an alternative approach to drug development, for this highly attractive target.
The complement system plays an essential role in innate immunity, and production of one of the pathway components, C5a, is particularly important for recruitment of immune cells and effective clearance of infectious agents. However, the detrimental effects of uncontrolled C5a production have been implicated in various pathological conditions making C5a and its receptors attractive targets for therapeutic intervention for inflammatory diseases. Mice genetically deficient in one of the receptors for C5a (C5aR) and antagonists that inhibit C5a signaling have established that the blocking C5a or its receptors holds enormous promise for treatment of various autoimmune diseases and acute inflammatory conditions. This review will outline recent findings on the biology of the two receptors for C5a, C5aR and C5L2, and the rationale for development of new anti-inflammatory agents that inhibit C5aR or C5L2 function.
THE COMPLEMENT SYSTEM AND THE GENERATION OF C5A
The complement system is an ancient defense mechanism that facilitates phagocytosis and clearance of pathogens. It is a biochemical cascade, comprising more than 20 serum proteins that normally circulate as inactive forms. Complement can be activated by four different pathways: the classical pathway, the alternative pathway, the lectin pathway1 and, the recently discovered, extrinsic protease pathway.2 Regardless of how the pathway is activated, the cascade leads to the generation of one of the most important effector molecules, C5a. The classical pathway is initiated by C1q binding to immune complexes of complement fixing IgG1 and IgM on the surface of pathogens. The lectin pathway is activated independently of immunoglobulin, by serum protein mannose-binding lectin, which directly recognizes carbohydrate structures on bacterial and viral surfaces. The lectin pathway is very similar to the classical pathway except for the initial recognition and activation step, and, importantly, all the pathways converge to generate C5 convertase. C5 convertase from the classical and alternative pathways (C4bC2aC3b and C3bC3bBb, respectively) cleaves C5 into C5a and C5b. C5b binds to C3b and initiates the formation of the membrane attack complex, which leads to cell lysis and bacterial killing (reviewed in Ricklin and Lambris1 and Ward3). Recently, there have been additional activation pathways identified, which give rise to C5a. An extrinsic protease pathway involves direct cleavage of C3 and C5 by a series of proteolytic enzymes such as kallikrein4 and thrombin.2 Thrombin can directly activate and cleave C5 to yield biologically active C5a in mice genetically deficient in C3, in which C5 convertase cannot be formed.2 The final products of complement activation—C3a, C4a and C5a—were referred to as anaphylatoxins because of their ability to trigger degranulation of mast cells, basophils and neutrophils. Of these, C5a is the most effective mediator of leukocyte degranulation or chemotaxis, and represents one of the most potent inflammatory molecules produced during immune responses.
C5a STRUCTURE AND FUNCTION
Nuclear magnetic resonance spectroscopy shows the structure of human C5a to be a four-helix bundle; the four α-helical segments juxtapose in an antiparallel topology, which are stabilized by three disulfide bonds (Cys21-Cys47, Cys22-Cys54 and Cys34-Cys55) and connected by three peptide loops.5, 6, 7 The flexible carboxyl terminal tail (aa 69–74 MQLGR) forms a helical turn that is connected by a short loop (reviewed in Monk et al.8), which is important for effector functions of C5a. When this region is missing, C5a still binds to the receptor but loses agonistic activity.9 This and other data support a ‘two-site binding’ model for C5a–C5aR interaction.10 The model suggests that the N terminus and disulphide-linked core region of C5a interact with a ‘recognition site’ that includes both the N terminus and the third extracellular loop of C5aR, and the C-terminal region of C5a fits into a binding pocket around the fifth transmembrane region, called the ‘effector site.’10, 11 This model explains why C5a without the C-terminal region loses full effector function, even though it binds to the receptor.
C5a is one of the most potent inflammatory peptides and shows diverse activities on many cell types. Depending on the cell type, C5a signaling can lead to various outcomes including phagocytosis, degranulation, H2O2 production, granule enzyme release, delay or enhancement of apoptosis, chemokine and cytokine production, and chemotaxis. Table 1 summarizes the pleiotropic functions of C5a for different cell types.
Once C5a is cleaved from C5, plasma enzyme carboxypeptidases rapidly metabolize C5a by removing the C-terminal arginine to form ‘C5a desArg.’29, 30 C5a desArg has a reduced potency, ∼10–1000 times compared with C5a depending on the its function.31 The Kd of C5a and C5a desArg binding to C5aR is estimated at 1 and 412–660 nm, respectively.32, 33 Both C5a and C5a desArg are cleared from body fluids very quickly. When 125I-C5a or 125I-C5a desArg are injected intravenously into adult rabbits, more than 50% of the injected radioactivity for both mediators is cleared from the circulation within 2 min and accumulates in highly vascularized organs such as lung, liver and spleen.34 This rapid removal of C5a from circulation is believed to be due to the binding of C5a to receptors for C5a on leukocytes. When purified granulocytes or monocytes were added to complement-activated plasma in vitro, up to 80% of C5a was removed from plasma and C5aR-blocking antibody inhibited C5a clearance.35 Notably, when C5aR internalization and recycling in granulocytes was blocked by treating leukocytes with the ionophore monensin, C5a was still cleared from plasma indicating that rather than ligand-receptor internalization, binding of C5a to the receptor on leukocytes is important in C5a removal from the circulation.35
RECEPTORS FOR C5a: C5aR AND C5L2
The potent inflammatory functions of C5a (see Table 1) indicate that inhibition of this ligand, or its receptor(s), might alleviate certain inflammatory conditions. There are two receptors known to bind to C5a; C5aR (CD88) and C5L2 (GPR77). Both are seventh transmembrane proteins and their genes are located on chromosome 19, q13.33–13.34 (human) directly neighboring each other. They cluster together with the genes for other closely related chemoattractant receptors such as formyl-peptide receptors FPRH1 and FPRH2.36 C5aR was the first anaphylatoxin receptor to be cloned, in 1991.37, 38 In contrast, C5L2 was cloned much later, in 2000, by PCR amplification using degenerate primers based on amino-acid sequences known to be conserved in chemoattractant receptors.39 The C5L2 sequence shows closest identity to that of C5aR (58%) and C3aR (55%).40, 41
C5a and C5a desArg have been reported as ligands for C5L2.41 Okinaga et al.33 performed competitive ligand-binding assays with 125I-C5a in cell lines transfected with human C5aR or C5L2. They showed that C5a bound to both C5aR and C5L2 with high affinity (Kd 3.4 and 2.5 nm, respectively). C5a desArg bound to C5aR with greatly reduced affinity (660 nM) compared with C5a, but it bound to C5L2 with affinity as high as C5a (12 nM). Whether C3a and C3a desArg serve as ligands for C5L2 is uncertain, as different laboratories have produced conflicting data.33, 42, 43
The functional role of a receptor is intimately associated with the cell types upon which it is expressed. C5aR was initially thought to be expressed mainly on leukocytes such as neutrophils, eosinophils, basophils, monocytes, dendritic cells and mast cells, but it is now established that C5aR can be widely expressed, on both immune and nonimmune cells. Reported nonimmune cell that express C5aR include vascular endothelial cells,44 cardiomyocytes,45 astrocytes,46 microglia,47 neural stem cells,48 oligodendrocytes,49 synoviocytes,50 articular chondrocytes,51 renal glomerular mesangial cells,52 hepatic kupfer cells and stimulated hepatocytes,53, 54, 55 bronchial epithelial cells56 and keratinocytes.57 C5aR shows a dramatic pattern of regulation, especially in tissues from mice undergoing the cecal ligation/puncture sepsis model.58 C5aR immunoreactivity was strikingly increased in lung, liver, kidney and heart in the cecal ligation/puncture model.
C5L2 is expressed at much lower levels on both immune and nonimmune cells, compared with C5aR. C5L2 is expressed on neutrophils, macrophages,59 immature dendritic cells39 and some nonimmune type cells such as adipocytes and skin fibroblasts,60 as well as adrenal gland, spinal cord, thyroid, liver, lung, spleen, brain and heart.61 In our studies, we have raised highly specific monoclonal antibodies (mAbs) to human C5L2 and confirmed that C5L2 in humans is expressed on neutrophils and other nonlymphoid cells, but at levels considerably less than that of C5aR (H Lee and CR Mackay, unpublished). These mAbs should be useful in establishing the precise pattern of C5L2 expression and regulation in tissues, particularly where expression may deviate from that of C5aR, such as in adipose tissue.
C5a RECEPTORS: STRUCTURE AND SIGNALING PROPERTIES
Both C5aR and C5L2 are seven transmembrane proteins that belong to rhodopsin-like family. Although these two receptors share structural similarity, there are several important differences. C5aR signaling pathways have been studied extensively. C5aR couples to pertussis toxin-sensitive Giα2, Giα362 or pertussis toxin-insensitive Gα1663 and initiates several downstream signaling pathways (reviewed in Johswich and Klos64). Signaling of C5aR involves intracellular calcium mobilization, and activation of different pathways such as phosphatidylinositol-bisphosphate-3-kinase/Akt (also known as protein kinase-B; PKB),65 Ras/B-Raf/mitogen-activated protein kinase/extracellular signal-related kinase,66 phospholipase D (PLD),67 protein kinase C,66 p21-activated kinases, which are downstream effectors of cdc42 and rac GTPases,68 signal transducers and activators of transcription, sphingosine kinase69 and NF-κB.70 In neutrophils, C5a activates phosphatidylinositol-bisphosphate-3-kinase to phosphorylate Akt, and subsequently Bad protein is phosphorylated and prevents pro-apoptotic caspase 9 activation resulting in inhibition of apoptosis.65 C5a protects neurons from glutamate-induced apoptosis through mitogen-activated protein kinase-mediated regulation of caspase 3.71 PLD activated by C5a produces phosphatidic acid and diglyceride in neutrophils. PLD is known to control many neutrophil functions, such as the oxidative response, degranulation, chemotaxis and protease release.72 C5a also results in extracellular signal-related kinase phosphorylation downstream of RAS/RAF signaling and causes nicotinamide adenine dinucleotide phosphate (reduced form) oxidase assembly and results in reactive oxygen species production in rat alveolar macrophages.73 Mitogen-activated protein kinase inhibitor PD98059 and SB203580 and phosphatidylinositol-bisphosphate-3-kinase inhibitor wortmannin and LY294002 inhibit C5a-induced migration of macrophages,74 indicating an important role for the mitogen-activated protein kinase and phosphatidylinositol-bisphosphate-3-kinase pathways in chemotaxis. C5a stimulates the generation of sphingosine-1-phosphate and sphingosine kinase-1 activity in human macrophages, and knocking down sphingosine kinase-1 using antisense oligonucleotides abolishes C5a-induced intracellular calcium signals, degranulation, cytokine production and chemotaxis.69 In neutrophils, C5a activates IκBα, which suppresses NF-κB, whereas in macrophages C5a actuates NF-κB and leads to cytokine and chemokine production (reviewed in Guo et al.75). In human HEK293 cells, C5a induces signal transducers and activators of transcription phosphorylation both at Tyr705 and Ser727 residues through the activation of PTX-insensitive Gα16 protein, and Ras/Raf/MEK/extracellular signal-related kinase and c-Src/JAK pathways are involved.76
DISSECTING THE ROLE OF THE ENIGMATIC C5a RECEPTOR, C5L2
Unlike C5aR, C5L2 has been regarded, until recently, as a nonfunctional decoy receptor. Okinaga et al.33 showed that when the murine B cell line L1.2-expressing C5L2 was treated with C5a, there was no mobilization of intracellular calcium, extracellular signal-related kinase phosphorylation or receptor internalization, in contrast to C5aR expressing cells, which responded to C5a as expected. Moreover, they examined mRNA expression after C5a treatment of bone marrow cells from C5aR-deficient mice to probe effects of C5L2 signaling on transcription and found no detectable C5a-mediated changes in gene expression. Similarly, RBL-2H3 cells transfected with C5L2 did not show any intracellular calcium mobilization, degranulation or receptor internalization upon C5a binding,41 and HL-60 and U937 cell lines, which endogenously express C5L2 showed similar results in calcium mobilization.42 It has been suggested that the inability of C5L2 to signal is due to the change in a highly conserved ‘DRY (Asp-Arg-Tyr) motif’ located at amino-acid residues 137–139 near the third transmembrane domain, which is highly conserved in many GPCRs. The central arginine residue of this motif has been shown to be important in receptor coupling to G proteins.77 C5L2 has DLC (Asp-Leu-Cys) in the motif and when central leucine was mutated to arginine, C5a could induce a small increase in intracellular calcium levels in 293 T cells co-expressing Gα16, suggesting that leucine at residue 132 in C5L2 contributes to G protein uncoupling.33 Other differences in the C5L2 structure, compared with C5aR, are the lack of the NPXXY (Asn-Pro-X-X-Tyr) motif in the seventh transmembrane domain and a shorter third intracellular loop lacking the conserved basic region and serine/threonine residues, which are thought to be potential protein kinase C phosphorylation sites41 (reviewed in Monk et al.8).
In 2005, Gao et al.61 reported that in a rat model of sepsis following cecal ligation/puncture, mRNA and protein levels for C5L2 increased in lung and liver and blocking C5L2 with an anti-C5L2 antibody dramatically increased the level of the inflammatory cytokine IL-6 in serum. Similar results were obtained by Gerard et al.78 from mice genetically deficient in C5L2. They found that mice lacking C5L2 showed enhanced responses to both C5a and C5a desArg, suggesting an anti-inflammatory function for C5L2.
All of the above data supported the notion that C5L2 is a nonsignaling receptor and that it serves as a decoy, similar to a number of other nonsignaling decoy chemoattractant receptors. However, recent data now suggest a functional positive regulatory role of C5L2 for C5a signalling.59 Using another C5L2-deficient mouse strain, Chen et al. showed that these mice were hypersensitive to lipopolysaccharide-induced septic shock and showed reduced airway hyperresponsiveness and inflammation in an ovalbumin-induced model. We have attempted to resolve these discrepancies through another approach—use of C5L2 antagonistic mAbs. We produced a mAb that completely antagonizes C5a binding to C5L2 transfectants. In our hands, the anti-C5L2 mAb did not significantly inhibit neutrophil chemotaxis to C5a (H Lee, Y Lim and CR Mackay, unpublished). Moreover, anti-C5aR mAbs completely blocked neutrophil chemotaxis to C5a, suggesting that C5aR was the predominant, if not the only, receptor for chemotactic responses to C5a. Likewise in a phagocytosis assay in vitro, anti-C5aR mAb completely blocked neutrophil phagocytosis, whereas anti-C5L2 mAb showed no effect (unpublished). Hopefully, mAbs will be developed that recognize and block mouse C5L2, and these should help resolve the in vivo relevance of this receptor.
ROLE OF C5a AND ITS RECEPTORS IN INFLAMMATORY DISEASES
As C5a is such a potent pro-inflammatory mediator, it is very important to control expression to allow rapid responses to pathogens, but at the same time protection for the host against unregulated overactivity.1 When this tightly regulated balance is disrupted, overproduction of C5a can occur, leading to uncontrolled inflammation. Excessive production of C5a can downregulate immune responses in some leukocytes, and, at the same time, it can overactivate other cell types. For example, when high levels (10–100 nM) of C5a are present in plasma, neutrophils become functionally paralyzed and demonstrate defective phagocytosis and reduced generation of H2O2, loss of chemotactic responses to C5a and end up losing bacteria-killing effect.79, 80, 81 On the other hand, high levels of C5a potentiate macrophage responses to lipopolysaccharide, to produce excessive levels of pro-inflammatory mediators such as TNF-α and various other chemokines82 leading to uncontrolled inflammation. On endothelial cells, high levels of C5a leads to enhanced production of pro-inflammatory mediators such as IL-8, IL-1β and RANTES,83 and tissue factor, which serves as a cofactor for blood coagulation.84
Overproduced C5a or upregulated C5aR expression has been implicated in the pathogenesis of many inflammatory conditions, autoimmune and neurodegenerative diseases. These include rheumatoid arthritis,85, 86, 87 respiratory distress syndrome,88 inflammatory bowel diseases,89 glomerulonephritis,90 systemic lupus erythematosus,91 ischemia/reperfusion injury,92 chronic obstructive pulmonary disease,93 sepsis,79 multiple sclerosis,94 asthma and allergy,95 atherosclerosis,96 xenograft rejection,97 hemorhagic shock98, 99 and antiphospholipid syndrome.100
DEVELOPMENT OF INHIBITORS OF C5a/C5aR SIGNALING
Over the past decades, even before the discovery of C5aR, many groups have tried to develop highly potent and specific small molecule antagonists to block C5a function. This has turned out to be surprisingly difficult, probably because C5a has a relatively high molecular weight (>10 000) and blocking large protein–protein interactions with small molecules can be difficult, especially if the interaction involves multiple sites. Nevertheless, in recent years a few potent antagonists for C5aR have been developed101 including nonpeptidic small molecules,102, 103 C5a mutants,104 short peptides105 and cyclic peptides,106, 107, 108 mAbs and antibody fragments.109, 110, 111, 112
NON-PEPTIDE C5aR ANTAGONISTS
Nonpeptide small molecular weight antagonists that can be easily administered orally have been developed. Merck identified series of chemical compounds, which were effective antagonists for C5aR but because of unwanted agonistic activity, they were not developed further.102 Mitsubishi Pharma Corporation (Yokohama, Japan) developed an orally active nonpeptide C5aR antagonist W-54011. W-54011 inhibits 125I-labeled C5a to human neutrophils, intracellular calcium mobilization, chemotaxis and generation of reactive superoxide species without showing agonistic activity up to 10 μM. Neurogen reported a nonpeptide antagonist NDT9520492, which contains a three-ring structure103 and a similar compound NGD 2000-1 was tested in human clinical trials for the treatment of rheumatoid arthritis. Phase I studies of NGD 2000-1 showed that it inhibited cytochrome P450 (3A4), a metabolic enzyme found in the liver, which metabolizes many drugs (reviewed in Monk et al.8 and Sumichika113). Development of NGD 2000-1 has been abandoned.
C5a MUTANTS AND ANTAGONISTIC PEPTIDES FOR C5aR
C5a1–71T1M,C27S,Q71C monomer (C5aRAM; CGS 27913) was developed by modifying the C-terminal region of human C5a.104 C5aRAM and its dimer C5aRAD (CGS 32359) inhibit 125I-C5a binding to human neutrophils, C5a-induced intracellular calcium mobilization, CD11b integrin upregulation, superoxide generation, lysozyme release and chemotaxis. In vivo, C5aRAM inhibited C5a-induced dermal edema in rabbits and C5a-induced neutropenia in micropigs.104 Moreover, in a porcine model of surgical revascularization, when given intravenous (i.v.) injection before surgical procedure, C5aRAD significantly inhibited neutrophil activation and reduced infarct size.114
A novel recombinant C5aR antagonist ΔpIII-A8 was discovered by Heller et al.115 by screening human C5a phage libraries in which the C terminus of C5a desArg was mutated. It inhibits binding of 125I-hC5a to differentiated U-937 cells, C5a-induced chemotaxis and lysosomal enzyme release without agonistic activity. In the reverse passive Arthus reaction, i.v. injection of ΔpIII-A8 significantly inhibited polymorphonuclear leukocyte accumulation in the peritoneum, skin and the lung. In a model of intestinal ischemia/reperfusion injury, ΔpIII-A8 decreased tissue injury by reducing bowel wall edema, hemorrhage and pulmonary microvascular dysfunction.115
Peptide analogues of the C terminus of C5a were generated based on structure/activity studies. N-MePhe-Lys-Pro-D-cha-Trp-D-Arg (C-089) is a full antagonist and blocks C5a-induced degranulation and GTPase activity in human neutrophils.105 Taylor's group used C-089 as a structural template and modified it to generate potent and selective C5a antagonists. A cyclic peptide, Phe-[Orn-Pro-D-Cha-Trp-Arg] (F-[OPdChaWR]), which showed high affinity for C5aR and inhibited 125I-C5a binding and C5a-mediated myeloperoxidase release from human neutrophils.106, 107 They acetylated F-[OPdChaWR] to derive a new antagonist, AcF-[OPdChaWR], which inhibits 125I-C5a binding to intact polymorphonuclear leukocytes with an IC50 of 20 nM108 (reviewed in Monk et al.8 and Sumichika113). It inhibited C5a- and lipopolysaccharide-induced neutropenia and the reverse passive Arthus reaction in rats.116 In a rat model of immune-mediated monoarticular arthritis, oral administration of AcF-[OPdChaWR] significantly reduced the severity of pathology such as swelling of knee, histopathology, gait disturbance and serum and intra-articular levels of inflammatory cytokines.117 AcF-[OPdChaWR] also reduces pathological symptoms in other animal models of inflammatory diseases, such as ischemia/reperfusion injury,118, 119 inflammatory bowel diseases,89 hemorrhagic shock,98 lupus nephritis120 and sepsis.121 This compound has been evaluated in human clinical trials.122
THERAPEUTIC mAbs TO C5aR
Monoclonal antibodies constitute a rapidly growing class of therapeutics due, in part, to their predictable pharmacokinetic properties, their high success rate in the clinic123 and their ability to antagonize large protein–protein interactions. Moreover, developments in the production, humanization and engineering of mAbs124 has resulted in more than 150 antibodies advancing to clinical development125 with 22 antibodies have been approved by the FDA for therapeutic application.123 Recently, an antibody against complement component C5 (Eculizumab; soliris) was approved by the FDA and represents the first complement-targeting drug that had been given marketing authorization.1
We have undertaken an extensive effort to develop therapeutic mAbs to hC5aR. mAbs to C5aR were raised using two approaches. First, we used a well-established approach126 for raising mAbs to chemoattractant receptors, using L1.2 cells (a murine B cell lymphoma line) expressing very high levels of hC5aR (∼80 000 receptors per cell).109 In a second and perhaps more innovative approach, we immunized wild-type mice with neutrophils from hC5aR knock-in (KI) mice.109 In this approach, wild-type mice should mount a focused response to hC5aR, since it will be the only antigen seen as foreign, and is expressed at very high levels on neutrophils, up to 200 000 molecules per cell.127 Both approaches were successful in generating highly specific mAbs to hC5aR. mAbs generated through both of these approaches completely blocked C5a binding to its receptor, intracellular calcium mobilization and leukocyte chemotaxis, and showed no signs of agonist activity.109
Recent studies by us and others suggest a critical role for the second extracellular loop of the chemoattractant receptors in ligand binding,109, 128 receptor activation129 and even in antagonist binding.130 Of the many mAbs generated by us against hC5aR, all of the most potent inhibitors of C5a binding or function mapped to a very specific region in the second extracellular loop of C5aR.109 The location of this region is shown in a predicted three-dimensional structural model of hC5aR (Figure 1), generated using the rhodopsin structure as a template and alignment of conserved C5aR residues. Similar mapping of function-blocking mAbs to the second extracellular loop have been reported for CCR5.131
Use of hC5aR KI mice provides an excellent example of how transgenic mice can be used for the in vivo evaluation of pharmacological agents such as therapeutic mAbs or small molecules. Use of human molecule KI mice is particularly useful for preclinical development of drugs that display selectivity for the human target. In our studies with anti-C5aR mAbs, we used hC5aR KI mice in a model of inflammatory arthritis. K/B × N inflammatory arthritis mediated by serum transfer was induced in hC5aR KI mice, and the preventative and therapeutic effect of mAb 7F3 were readily demonstrable.109 The very rapid reversal of inflammation was likely due to blocking C5a and C5aR signaling and preventing neutrophil migration to the joint, rather than neutrophil depletion from the blood. C5aR is also expressed by mast cells and macrophages, both of which play a role in the K/B × N serum transfer model of arthritis. To our knowledge, there is no other treatment that is as effective at reversing inflammation in this model, besides anti-C5 treatment. However, anti-C5 blocking mAb required a much higher amount of mAb (∼40 mg kg−1)132 compared with anti-C5aR mAb (as low as ∼1 mg kg−1). The reason for this may presumably relate to the high concentration (∼170 μg ml−1) of C5 that is normally present in blood and tissue fluids.133 Blocking C5aR not only prevented synovitis, but also cartilage degradation in the K/B × N arthritis model.109 Blocking C5aR might provide additional benefit to rheumatoid arthritis patients, over its anti-inflammatory effects, as Onuma et al. reported that C5aR is expressed on human articular chondrocytes, and expression levels were significantly higher in rheumatoid arthritis patients than in control groups (26% in rheumatoid arthritis, 9.0% in osteoarthritis and 6.9% in normal). Moreover, IL-1β, which is known to have a catabolic effect on chondrocyte metabolism,134, 135 significantly enhances expression of C5aR on chondrocytes in rheumatoid arthritis and normal samples.51 Also, it has been reported that local production of almost all of the complement components are found in joint tissue,136 and levels of C5aR expression are also upregulated in inflammatory and proliferative synovial tissues.85 These data suggest that C5aR may be directly involved in joint destruction in rheumatoid arthritis patients. A model summarizing the likely steps in the pathogenesis of rheumatoid arthritis, and the involvement of C5a and its receptors, is shown in Figure 2.
Conclusions
C5a and its receptors are attractive targets for therapeutic intervention in numerous inflammatory diseases. The appropriate indications for C5a–C5aR inhibition will emerge with the relevant human clinical trials, although studies in rodents suggest that antagonists of C5aR will find utility in a wide range of inflammatory diseases, particularly rheumatoid arthritis. The most effective way to inhibit C5aR is still to be resolved. We have developed blocking mAbs, since small molecule inhibitors have proven difficult for this receptor. Hopefully, anti-C5aR mAbs will enter human clinical trials in the coming years.
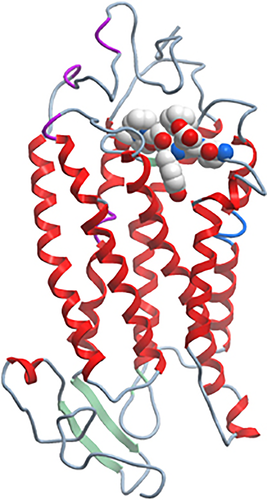
Molecular representation of the surface of human C5aR with the binding site for inhibitory monoclonal antibodies (mAbs). The small region in the second extracellular loop of C5aR (EEYFPP) was the main target for blocking and inhibitory mAbs.109 The picture was kindly provided by Søren Berg Padkjær (Novo Nordisk A/S) and was produced using the program MolSoft BrowserPro using a homology model based on alignment of C5aR sequence with rhodopsin. C5aR backbone (schematic), Epitope ‘EEYFPP’ (spacefilling). It is likely that this region in the second extracellular loop of C5aR plays a critical role, either as a second binding site for C5a and/or as a region that transmits a conformational change in receptor structure, that results in receptor signaling.
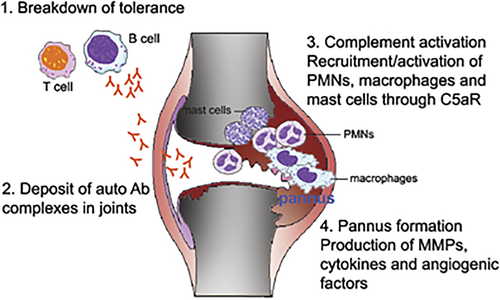
Steps in the pathogenesis of rheumatoid arthritis, and the role of C5a and C5aR. Steps in the pathogenesis of rheumatoid arthritis may include the following: 1. Breakdown of T-cell and B-cell tolerance. This could occur due to a variety of genetic and environmental conditions, but ultimately results in the production of autoantibodies directed to self-proteins. 2. Deposit of antibody complexes in synovial tissue. 3. Activation of the complement pathway. In the K/B × N model of inflammatory arthritis, autoantibody deposition activates the alternative complement pathway, which leads to C5a production. C5a production serves to activate mast cells, and to recruit inflammatory cells to the joint, such as polymorphonuclear leukocytes (PMNs), mast cells and macrophages. 4. In human rheumatoid arthritis, the synovial membrane becomes hyperplastic and ultimately develops into a ‘pannus,’ which destroys the articular cartilage and underlying bone. The release of angiogenic factors, such as VEGF or angiogenic chemokines contributes to new blood vessel growth and continued inflammation. Release of inflammatory cytokines such as TNF, IL-1 and matrix metaloproteinases (MMPs) contribute to joint inflammation and destruction.
| Cell type | Activity |
|---|---|
| Neutrophils | Enhanced expression of adhesion molecules12 |
| Chemotaxis13 | |
| Oxidative burst (O2 consumption)14 | |
| Phagocytosis14 | |
| Release of granule enzymes15 | |
| Delayed apoptosis16 | |
| Eosinophils | Release of granule enzymes17, 18 |
| Chemotaxis19 | |
| Basophils | Histamine release20 |
| Mast cells | Histamine secretion20 |
| Chemotaxis21 | |
| Plasmacytoid dendritic cells | Chemotaxis22 |
| Macrophages/monocytes | Chemotaxis23 |
| Cytokine release24 | |
| Thymocytes | Enhances apoptosis25 |
| Endothelium | Vasodilation26 |
| Chemokine release26 | |
| Hepatocytes | Enhanced regeneration27 |
| Microglia | Chemotaxis28 |





