Sex Differences in the Effects of Inherited Bitter Thiourea Sensitivity on Body Weight in 4–6-Year-Old Children
Abstract
Previous studies have shown that inherited taste blindness to bitter compounds like 6-n-propylthiouracil (PROP) may be a risk factor for obesity, but this literature has been highly controversial. The objectives of this study were (i) to confirm findings that show an interaction between PROP status and sex on BMI z-score, and (ii) to determine if sex also interacts with variations in TAS2R38 (phenylthiocarbamide (PTC) genotype) to influence weight status in 4–6 year olds. Also, we tested whether nontaster children consumed more fat and total energy at laboratory-based meals. Seventy-two ethnically diverse children who ranged in weight status were classified as tasters (N = 52) or nontasters (N = 20) using a standard PROP screening solution. Anthropometric measures were taken, and at the end of each visit, children ate ad libitum from test meals intended for exploratory purposes. Genomic DNA was extracted from saliva and alleles at TAS2R38 were genotyped for A49P polymorphisms. In 75.8% of children, PTC genotype predicted PROP phenotype, whereas in 24.4%, genotype did not predict phenotype. PROP nontaster males had higher BMI z-scores than taster-males and females in both groups (P < 0.05), but due to a three-way interaction between PROP phenotype, TAS2R38 genotype, and sex, this relationship was only true for children who were homozygous for the bitter-insensitive allele (P < 0.0005). There were no differences in test-meal intake as a function of PROP phenotype or TAS2R38 genotype. These results suggest that the TAS2R38 variation, PROP phenotype, and sex interact to impact obesity risk in children. Future studies should be done to determine how this trait influences energy balance.
Introduction
Approximately 30% of US adults and children are genetically insensitive to the bitter taste of thiourea compounds like phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP), and these individuals have been termed “nontasters” (1,2). The ability to taste these compounds is associated with perception of a broad range of basic tastes and textures in the diet, with nontasters generally exhibiting lower responses than tasters (for review see refs. 3,4). This reduced responsiveness in nontasters is in part due to a reduced number of fungiform papillae on the anterior tongue (1,5). Because these variations in inherited taste response may impact food preference and intake (4,6), public health researchers are interested in studying this trait.
The relationship between the PROP/PTC taste phenotypes and obesity is controversial, and some (7,8,9,10), but not all (11,12,13,14) reports suggest that nontasters have increased BMIs. Early reports from Fisher et al., noted that PTC tasters tended to be thinner (ectomorphs), whereas nontasters tended to have heavier body types (endomorphs) (15). More recent studies in adults (8,10) and children (9) have noted similar moderate-to-weak associations between PROP status and BMI, suggesting that nontasters may be prone to obesity at various stages of development. In the latter study, no differences in BMI z-score were seen between taster groups overall, but when children were analyzed by sex, nontaster males showed the expected relationship—they were heavier—whereas the opposite pattern was seen in females. Questions remain about these findings, however, primarily because large epidemiological studies in female breast cancer patients (11,13), and another done in a community sample of both sexes (12) failed to find any relationship between PROP status and body weight. Also, Lumeng et al. (14) used identical PROP classification methods to those employed by Keller and Tepper (9), but found conflicting results in a cohort of low-income, 3–6-year-old children. Nontasters actually had lower BMI z-scores than tasters. These results were not analyzed separately in males and females, so it is unclear if sex differentially influenced the relationship between PROP status and body weight in Lumeng et al. (14), as reported elsewhere (9). Other proposed reasons for inconsistencies across studies are differences in scaling methods (16) and lack of body weight variation in cohorts (4).
The mechanism linking PROP/PTC status to body weight is not known, but one suggestion is that it may influence the discriminability (17,18,19) and palatability (20,21,22,23) of dietary fat. Excessive intake of high-fat foods is a risk factor for the development of obesity (24). The ability to detect fat in foods is attributed in part to textural cues (e.g., creaminess, oiliness) which are perceived via trigeminal nerve fibers surrounding the taste buds on the anterior tongue. Because nontasters have fewer taste buds receiving trigeminal input, they have decreased ability to discriminate textural cues from food (5). Tepper and Nurse (18) reported that nontasters had decreased abilities to discriminate differences in fat content between high- (40%) and low- (10%) fat salad dressings. But, nontasters reported liking the higher fat dressing better, even though they were unable to discriminate differences between the two (22). To speculate on a mechanism, it is possible that nontasters need higher levels of fat in foods to not only detect it, but also to achieve the same level of satisfaction that a taster would receive with a lower fat level. Several other studies have found that nontaster adults like higher fat foods such as ice cream, doughnuts, bacon, mayonnaise, and cheeses (18), and nontaster children report higher liking of cheese (21,25) and in females, only whole fat milk (21). Still, other studies do not support the above relationships (13,26,27), and the topic remains controversial.
In order to address some of these controversial findings, genetically informative studies are needed to measure the strength of the associations between food intake, body weight, PROP status, and the TAS2R38 gene. In 2003, Kim et al. (28) discovered the TAS2R38 gene (taste receptor, type 2, member 38) on chromosome 7q that predicts most of the variance in human ability to taste PTC and a less robust but significant proportion of the ability to taste the chemically similar compound PROP (29). TAS2R38 was identified as part of a family of G-protein coupled bitter-taste receptors. Two haplotypes constitute the majority of variation in this gene: AVI and PAV. The nontaster haplotype is AVI and it has an alanine at position 49 (A49P): rs713598, a valine at position 262 (V262A): rs1726866, and an isoleucine at position 296 (I296V): rs10246939 (rs notations refer to reference SNP IDs from the NCBI Single Nucleotide Polymorphism database, located at http:www.ncbi.nlm.nih.govSNP and the haplotype structure is detailed at http:www.hapmap.orgindex.html). The PAV haplotype is associated with the taster phenotype. To date, few studies have been performed to elucidate the relationships between variation at TAS2R38 and diet (30,31,32), and none have reported significant associations between this genotype and obesity.
Our hypothesis is that predicted associations between PROP phenotype, TAS2R38 genotype, and diet may be more apparent in children because taste is a key determinant of children's food choices (33), and is arguably a more salient predictor of intake for children than for adults (30). Effects that have been moderate to undetectable in adults may be stronger in children, and may represent a risk factor for obesity that warrants attention as children develop. In the present study, the primary objectives were to confirm previous associations between PROP status and body weight that were collected from a largely white, higher socioeconomic status cohort in a group of lower-income children from diverse ethnic backgrounds. In addition, the association between the TAS2R38 genotype and children's level of overweight was assessed in order to better understand the relationship between the PROP phenotype, TAS2R38 variation, and obesity. An exploratory objective tested the prediction that nontasters (assessed by both phenotype and genotype) would consume more total energy and a higher percentage of energy from fat at multi-item, ad libitum test meals.
Methods and Procedures
Participants
Seventy-two 4–6-year-old children (mean age 5.23 ± 0.77 years) from the New York metropolitan area participated in this study. Parents responded to an advertisement for the “Child Taste and Body Weight Study,” that was posted on a popular internet website. The participants were representative of the ethnic breakdown of upper Manhattan: 33.3% African American, 31.9% Hispanic, 19.4% white, 2.8% Asian/East Asian, and 12.5% parentally identified as “other.” Four percentage of the children were underweight (<5% BMI-for-age), 60% were normal weight (5th–85th percentile BMI-for-age), 11% were overweight (85th–95th percentile BMI-for-age), and 25% were considered obese (>95th percentile BMI-for-age) (34). Approximately 20% of the parents reported that their family earned <$20,000 per year. Children were eligible to participate if they were healthy and not on any medications, had been to school, and had no learning disabilities, or food allergies.
Families received monetary compensation for participating in the study, and children received a small toy prize following each session. This study was approved by the Institutional Review Board of St Luke's Roosevelt Hospital. All parents provided written consent for their children to participate.
Experimental procedures
Children attended the Child Taste and Eating Laboratory at St Luke's Roosevelt Hospital for four dinner visits to complete this cross-sectional study. Four visits were required to complete other study assessments that will not be reported in this article. Each visit lasted 1 h, with 30 min at the end devoted to consumption of a self-selected, multi-item, ad libitum dinner. On the first visit, parents (97% of whom were mothers) completed study questionnaires to assess children's usual food intake and parental feeding characteristics, assessed by the Child Feeding Questionnaire (35) (reported elsewhere). Children were screened for PROP status, anthropometric measures were taken, and saliva was collected for extraction of DNA.
PROP taste test
Children were classified as “tasters” or “nontasters” by having them sip and spit a solution of 0.56 mmol/l PROP (6-propyl-2-thiouracil; Aldrich Chemical, Milwaukee, WI) in distilled water and perform a “forced choice” procedure according to methods from Mennella et al. (30). If the drink tasted like “water” or “nothing,” children were instructed to give it to Big Bird. If the drink tasted “bad,” “yucky,” or “bitter,” children were instructed to give the drink to Oscar the Grouch so he could throw it in his trash. This method of classifying children with a screening solution has shown good test-retest reliability in a similar age group (r = 0.92; P < 0.001) (21).
Anthropometric measures
Anthropometric measures (weight and height) were performed by a trained researcher. Children were weighed and measured in stocking feet and light clothing on a standard balance scale and stadiometer, respectively. Height and weight were converted to BMI = kg/m2, and BMI z-scores, using the Centers for Disease Control and Prevention growth charts conversion program for SAS version 9.0. (SAS, Cary, NC) (36).
Dinner (test-meal)
In the last 30 min of each session, children ate ad libitum from a test meal of foods that were chosen because they are familiar and palatable to most children and have been used in past studies in this age group (37). Dinner consisted of macaroni and cheese (12% fat; Kraft Foods, Northfield, IL), baby carrots, canned green beans (Del Monte, Kingsburg, CA), mozzarella cheese sticks (66% fat; Polly-O, Kraft Foods), green grapes, graham crackers (23% fat; Nabisco, Kraft Foods), chocolate pudding (11% fat; Jello, Kraft Foods), whole milk, fruit punch (Apple & Eve, Rosalyn, NY), caffeine free cola (Coca-Cola Atlanta, GA), apple juice (Apple & Eve), chocolate milk (66% fat; Nesquik, Nestle, Glendale, CA), or fruit juice (Capri Sun, Kraft Foods). Standard portion sizes of each food were provided on serving trays, with main entrées served on plates. If children finished a serving, they were asked whether they wanted additional portions. Researchers read nonfood related books to children during the meal. Children were allowed 30 min to finish their meal.
Total energy and macronutrient consumption for the four meals was computed by first calculating the difference between the pre- and postweights of all foods and beverages eaten. Food label information was used to calculate total calories, and percentage of calories from fat, protein, and carbohydrates for each food. Using this information, SPSS statistical software (Version 16.0, Chicago, IL) was used to create scripts for calculating individual food and total meal energy and macronutrients.
DNA collection and genotyping
Saliva samples of ∼4 ml total volume were collected and purified according to manufacturer's instructions with Oragene DNA Self Collection Kits (DNA Genotek, Ontario, Canada). Of 72 children, DNA extraction was not possible in six due to inactivated DNA kits. Attempts to recontact these children were unsuccessful. There were no differences in main outcome variables between children who had genotyping performed and those who did not.
PCR was used to amplify DNA fragments in 20 µl reaction volumes with 100 ng genomic DNA, 1× reaction buffer (Boehringer Mannheim, Mannheim, Germany) containing [MgCl2] 1.5 mmol/l, 0.25 mmol/l each dNTP, 100 ng of each PCR primer (available upon request), and 1 U Taq polymerase. All thermocycling was performed with 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C, for 30 s, and extension at 72 °C for 30 s. Amplicons of ∼200 base pair were amplified from genomic DNA using a biotin labeled primer (available upon request) and subsequently purified using streptavidin beads (Amersham Biosciences, Uppsala, Sweden). Genotyping of TAS2R38 A49P and I296V was performed by pyrosequencing according to the manufacturer's recommended protocol (PSQ96 Biotage, Westborough, MA).
In this study, A49P and I296V were in perfect linkage disequilibrium, so results are presented for A49P only. Similar to groupings used by Mennella et al. (30), and for ease of comparing across studies, children homozygous for the bitter-insensitive allele at A49P were classified as AA, heterozygous for the bitter-insensitive allele as AP, and those homozygous for the bitter-sensitive allele as PP.
Statistics
Power analysis. Prior to the experiment, a power analysis was conducted using previous data (9) to assume an effect size of 0.5. For the primary study objective, it was determined that 75 subjects would allow us to detect differences between PROP taster groups as small as 10 BMI % units (or differences in BMI z-score of about 0.5 units). In the present study, we had 80% power to detect differences in BMI z-score as a function of PROP phenotype and TAS2R38 genotype. Also, for the exploratory objectives, we had 80% power to detect a difference in meal energy intake between PROP taster groups as small as 97 kcal, but we were not adequately powered to test for interactions (PROP status × sex) and (TAS2R38 genotype × sex) in the test meal.
Statistical procedures. Binomial χ2 was used to test for differences in dichotomous variables (e.g., sex) between tasters and nontasters. Independent samples t-tests were done to test for differences in BMI z-score as a function of PROP status (tasters vs. nontasters) and TAS2R38 genotype (AA vs. AP & PP). Two- and three-way ANOVA were used to test the interaction of PROP phenotype/TAS2R38 genotype with sex (independent variables) on BMI z-score and energy intake (dependent variables). When appropriate, child age, income, and ethnicity were used as covariates in these models. In performing the analyses using TAS2R38 genotype as the independent variable, AA children (homozygous for the bitter-insensitive allele) were compared to AP and PP (hetero- and homozygous for the bitter-sensitive allele). The AP and PP were combined because these genotypes are most likely “tasters.”
χ2-analyses were done to test the frequency of PROP taster and nontaster occurrence at sites A49P and I296V in the TAS2R38 genotype. As exploratory analyses, independent samples t-tests and one- and two-way ANOVAs were done using PROP status, sex, and TAS2R38 genotype as the independent variables and mean energy intake and percentage macronutrients as dependent variables. These were only analyzed for children who attended at least three out of four meals.
For all statistical tests, a P value of <0.05 was the cutoff for significance. All hypotheses were two-tailed. Descriptive statistics are reported as means ± s.d. Primary analyses were done using SPSS, version 16.0 (SPSS, Chicago, IL) for Windows XP. SAS version 9.0 was used to perform Tukey's post hoc tests on significant interactions.
Results
Overall, 71.2% of the children were PROP tasters, and 28.8% were nontasters by phenotype. This breakdown is similar to that seen in other studies of US adults (2) and children (9,21). Reported family income did not vary as a function of BMI z-score or other main outcome variables (P values ranging from 0.70 to 0.90). There were no differences between phenotypic PROP tasters and nontasters for age, sex, or BMI z-score. χ2-tests found no difference in the frequency of PROP tasters and nontasters across ethnic or sex groups (Table 1).
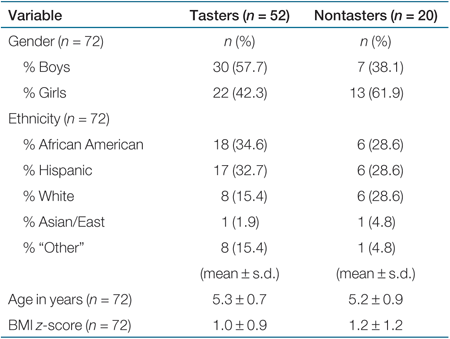 |
PROP phenotype: TAS2R38 genotype associations
In this cohort, A49P and I296V were in perfect linkage disequilibrium, so data are presented at A49P only. Children who were PROP nontasters by phenotype were more likely to have the AA genotype at TAS2R38 A49P, whereas children who were PROP tasters were more likely to have AP or PP at this single-nucleotide polymorphism (χ = 12.08; P = 0.002) (Table 2). There were no differences in ethnic group distribution (P = 0.8) or frequency of males and females at A49P (P = 0.3).
 |
In 50 (75.8%) of the children, TAS2R38 genotype predicted PROP phenotype (concordant), but for the remaining 16 (24.4%) children, TAS2R38 genotype did not predict PROP phenotype (discordant). For the 16 discordant children, 6 (37.5%) were classified as nontasters of PROP (phenotype) but carried the AP or PP genotype at TAS2R38, whereas 10 (62.5%) were tasters of PROP, but carried the AA genotype. White children were more likely to be concordant than nonwhite children (P < 0.05). There were no differences in concordance vs. discordance status as a function of sex (P = 0.3). Regardless of sex, concordant children had greater BMI z-scores than discordant children, with means equal to 1.2 ± 1.1 and 0.3 ± 0.4, respectively (t = 2.9;P < 0.05).
BMI and taster status
There were no overall differences in BMI z-score as a function of phenotypic PROP taster status (degrees of freedom (1,71); F = 1.34; P = 0.27). Also, there were no differences in BMI z-score between children who were homozygous for the bitter-insensitive allele (AA) and those who were either heterozygous, or homozygous for the bitter-sensitive allele (AP and PP) (degrees of freedom (1,65); F = 1.75; P = 0.16).
There was a significant interaction between PROP phenotype and sex on BMI z-score (degrees of freedom (1,71); F = 3.27; P < 0.05). Nontaster males had mean BMI z-scores of (1.9 ± 2.8), nearly double that of nontaster females (0.9 ± 2.0) and taster-males (0.9 ± 1.4), but not significantly higher than taster females (1.2 ± 1.6) (1). However, due to a significant three-way interaction between PROP phenotype, TAS2R38 genotype, and sex (degrees of freedom (3,64); F = 9.8, P < 0.0005), the effects of PROP phenotype on BMI z-score were only true for children who have the AA genotype. According to Tukey's tests, PROP nontaster males with the AA genotype had mean BMI z-scores = 2.6 ± 0.9, and this was significantly greater than PROP nontaster females with the AA genotype (mean BMI z-score = 0.8 ± 0.8) (1).
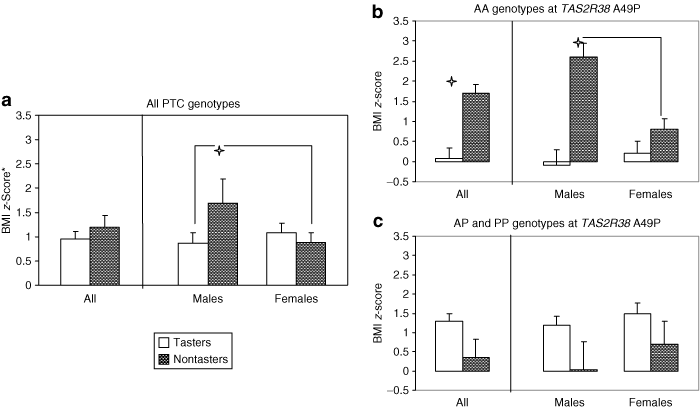
Children's BMI z-scores as a function of PROP status and TAS2R38 genotype. (a) Mean (±s.e.) BMI z-scores for taster and nontaster children. PROP nontaster males (n = 7) had higher BMI z-scores than PROP taster males (n = 30) and PROP nontaster (n = 13) and PROP taster (n = 22) females. However, this is only true for children with the AA genotype at TAS2R38. (b) For children with AA genotype only, PROP nontasters (n = 14) have higher BMI z-scores than PROP tasters (n = 11), (P < 0.05), and the interaction between PROP status and sex is significant for AA children (P < 0.005), according to Tukey's post hoc test. (c) Children with AP or PP genotypes only. There were no significant differences in BMI z-score between PROP nontasters (n = 5) and PROP tasters (n = 36) with the AP and PP genotypes. The interaction between PROP status and sex was also not significant for the AP and PP genotypes.
Energy intake and taster status
Total energy intakes as a function of PROP status, TAS2R38 genotype, and sex are displayed in Table 3. There were no significant differences across PROP taster groups, TAS2R38 genotypes, or interactions between the two for total energy intake, or for percent fat intakes (data not shown) (P values ranging from 0.14 to 0.73). Both age (r = 0.25; P < 0.05) and BMI z-score were positively associated with mean calories consumed across meals (r = 0.27; P < 0.05). All dependent variables are presented after adjusting for BMI z-score and age, even though results did not change after making these adjustments (Table 3).
 |
Discussion
This is the first study to demonstrate sex-related differences in BMI z-score as a function of variation in PROP phenotype and the TAS2R38 genotype. PROP-nontaster males who were homozygous for the bitter-insensitive TAS2R38 A49 allele had higher BMI z-scores than PROP-nontaster females who were homozygous for this allele. The present study also provides additional evidence that PROP taste sensitivity is associated with differences in weight status (7,8,9,10), although the results could only be interpreted when also considering the interactions between sex, TAS2R38 genotype, and PROP phenotype. Males who were nontasters by both measures (TAS2R38 genotype and PROP phenotype) had the highest BMI z-scores, with values that were over two standard deviations above the mean for children this age. These results support and further clarify previously reported interactions between PROP phenotype and sex on child BMI z-score (9). The present findings underscore the importance of considering both the PROP phenotype and TAS2R38 genotype in understanding the relationship between variations in bitter-taste sensitivity and obesity risk. At present, it is unclear why PROP/PTC status influences body weight differently in male and female children, but future studies are warranted to determine if these relationships are maintained into adulthood.
The prediction that nontasters have higher body weights is not new, but recent inconsistencies in the literature have made the idea highly controversial. Differences in PROP testing methodology (16) and lack of body weight variation in the cohorts (3) have been suggested as reasons for these inconsistencies. Null findings are probably not due to a lack of power because studies that have reported positive findings (9,10,20,38) have had fewer subjects than the large epidemiological cohorts studied by Drewnowski et al. (11,12,13). The present findings most closely agree with Tepper and Nurse who reported that nontaster young men had higher BMIs, but no such relationship was reported in women (22). In a follow-up study that assessed cognitive factors, nontaster females who reported low dietary restraint had significantly higher BMIs than medium and supertasters, only after level of dietary disinhibition (emotional eating) was controlled (10). Still, other studies have found no effect of PROP status on body weight (12,13,27), including Drewnowski et al. who tested only women and controlled for cognitive factors in the cohort (13). To add to this confusion, Lumeng et al. (14) studied low-income children and found that tasters, not nontasters, had higher BMI z-scores. The Lumeng study used similar PROP testing methods as the present study, but all the children were low-income, compared to only about 20% in our cohort. The ethnic breakdown of children in the two studies differed as well, with whites representing 50% of the cohort in the Lumeng study, but only 19% of children in the present study. Additionally, the interactions between TAS2R38 genotype, PROP phenotype, and sex were not tested in Lumeng et al. (14), so it is not possible to compare directly across the two studies. The factors affecting body weight may differ between males and females, with environmental factors such as maternal eating styles playing a greater role for females than males (39). This idea is supported by twin studies that have found higher heritability estimates for eating behaviors (40) and food intake (41,42,43) in males than in females. Further studies should be done to determine whether genetic contributions to overall obesity risk are also greater in males than in females.
Similar to other studies (29), variation at TAS2R38 was not perfectly correlated to the ability to taste PROP. In the present study, the PTC genotype predicted about 75% of the phenotypes assessed with PROP. As noted in Bufe et al. (29), PROP is a chemically related compound to PTC, but the ability to taste PROP at higher concentrations may be influenced by genes other than TAS2R38, along with environmental influences that have yet to be identified. Although PROP may not be as suitable a proxy for PTC as once suggested (44), the fact that some studies in adults have shown associations between PROP status and BMI that were not shown with the TAS2R38 genotype (45) suggest that measurement of both might be important to accurately interpret results.
Discordant children who lack consistency between TAS2R38 genotype and PROP phenotype may be an important group to study in future investigations. In the present study, two groups of discordant children were identified—taster phenotypes with nontaster genotypes and nontaster phenotypes with taster genotypes. It is reasonable to believe that these groups could differ on a wide number of factors, even though they did not show differences in BMI z-score or energy intake in the present study. Most notably, the psychophysical procedure used to measure PROP status was relatively insensitive, so it is possible that some children were simply misclassified by phenotype. Other possible explanations for this discordance include environmental influences on oral pathology that can impact taste functioning, the existence of other bitter-taste receptors that may also impact PROP status (46), or as suggested by Bufe et al. (29), a different unidentified gene that contributes to the perception of PROP. In addition, otitis media is a common childhood disease that can impact oral pathology and this might be another explanation for the discordance between phenotype and genotype. Moreover, because African American and Hispanic children were more likely to be discordant than white children, the pattern of linkage disequilibrium in TAS2R38 may vary as a function of ethnic group such that another functional variant for PROP sensitivity may be in linkage disequilibrium with A49P and I296V in whites but not African Americans or Hispanics.
Unlike Tepper et al. (44) who found no association between TAS2R38 alleles and obesity prevalence in a genetically isolated population of adults from southern Italy, and Timpson et al. (32) who published similar findings in elderly British women, our study found that the TAS2R38 genotype interacted with PROP status to predict differences in child BMI. It is plausible that the extent to which genes influence the expression of complex phenotypes like eating behavior and obesity may differ in childhood and adulthood, and in some instances, genetic influences from TAS2R38 variation may be apparent at one developmental stage but not another (30). Prospective studies are needed to better understand the respective roles of both the TAS2R38 genotype and PROP phenotype on body weight across time.
This study also began to explore the empirical prediction that nontaster males would consume more total energy and a higher percentage of energy from fat at ad libitum multi-item meals. No previous studies in children have examined laboratory food intake as a function of PROP status, although a study in 7–11-year-old children used 3-day food recalls to show that nontasters reported greater daily energy intake (38). In the present study, we observed no differences across meals in total energy intake or macronutrient selection as a function of PROP phenotype, TAS2R38 genotype, or their interaction. However, it is important to note that we were only powered to look at main effects between PROP taster groups and not interactions between taster groups and sex. The test meal portion of the study was an exploratory aim designed to generate baseline intake to be used in planning follow-up studies. The meal-intake data highlight the challenges that exist when studying food intake in children, particularly in studying the effects of genetic taste variations which likely explain only a small portion of this complex behavior. Typical intake in children is highly variable on a meal-to-meal basis (47). Goran noted that imbalances in energy consumption as small as 2% can result in obesity over time (48), so designing studies that are sensitive enough to detect small changes in energy intake across groups is important.
Strengths and limitations
There were several strengths to this study. There is a lack of observational food intake data from children this age, and even though results of the meal variables were not significant, the descriptive nature of these data is helpful to investigators planning future studies with this age group. Second, children were from diverse ethnic backgrounds, with <20% whites. Few studies have been done to characterize the effects of the PROP phenotype or TAS2R38 genotype in nonwhite, US populations, so the present study is a helpful addition. Finally, the consideration of both PROP phenotype and TAS2R38 genotype, and their interactions, on weight status is a significant advance in the field that will hopefully clarify some of the previous inconsistencies.
Several limitations of this study should also be discussed. Foremost, the sample size is small, particularly with respect to the nontaster males. In addition, we saw a lower proportion of nontaster males in this study than seen in previous studies (9,21). Despite our attempts to oversample nontasters, we were unable to successfully recruit additional nontaster males. The method used to classify PROP taster status in this study was a simple-screening procedure designed specifically for children (30), however it lacks the sensitivity of standard threshold procedures or validated suprathreshold scaling techniques. Although this method is highly reliable (Spearman's rho = 0.92), it contains no “standard” against which the perceived bitterness of PROP can be compared, so the opportunity to misclassify PROP status based on chance is high. Moreover, the use of a test meal to measure food intake is limited by the variety of foods that are served and may not represent children's usual dietary intakes. The foods selected for this study were chosen because they are familiar to most children, and they represent a number of the taste and textural qualities that might be influenced by PROP status (e.g., fattiness, sweet). However, not all children had identical levels of prior exposure to the foods before the study. Additionally, consuming meals away from home in a new location may also influence what and how much children eat (49). Also, highly restrictive feeding environments have been shown to encourage intake of foods that are restricted in the home (50), although it is doubtful this played a large role in the present study as adjusting for parental restriction did not change primary outcomes. Finally, the study was cross-sectional, so observing change in BMI z-score across time was not possible.
Conclusions
This study further suggests that inherited sensitivity to bitter thiourea compounds, a stable and reliably measured trait, influences weight status differently in male and female children. Body weight is a complex phenotype for which bitter taste genetics likely explains only a small portion. Before PROP/PTC status can be used to identify children who may be at risk for obesity, developing a better understanding of how it impacts body weight regulation is necessary. That some nontaster males may be at risk for carrying excess weight at certain stages of development warrants further study to determine the mechanism behind this relationship and the potential long-term health consequences that are involved.
Acknowledgments
This research was supported by NIH grant K01DK068008 (K.L.K.). Also, the work was made possible by the Obesity Research Center Grant (NIH grant 5P30DK026687-27). Additional funding from this study came from DK52431, DK63608, and DK26687 (W.K.C.). The authors kindly thank John C. Thornton, Ph.D., for advice on statistical analyses.
Disclosure
The authors declared no conflict of interest.





