Trends in and Patterns of Obesity Reduction Medication Use in an Insured Cohort
Abstract
Several prescription medications are approved to treat obesity, yet little is known about their use in the United States. Our objective was to describe recent trends and patterns of obesity reduction medication use in an insured US population. From among ∼4.2 million persons enrolled in two Blue Cross and Blue Shield plans, we obtained all medical and pharmacy claims for 86,804 persons who took an obesity reduction medication anytime during 2002–2005. Overall, obesity reduction medication use decreased significantly over time from 1% in 2002 to 0.7% in 2005 (P for trend <0.001), which was most notable for the newer medications (orlistat and sibutramine). Few (range: 11–18%) used these medications longer than 3 months regardless of whether they were Federal Drug Administration (FDA)-approved for long-term use or not. More than half (57%) of obesity reduction medication users also took narcotics and 38% took antidepressants. Few sympathomimetic users had potential serious contraindications prior to medication initiation, including cardiovascular diseases (2.4%), schizophrenia (2.5%), and age >65 (1.2%). Despite the high prevalence of obesity, obesity reduction medication use was low and decreased significantly from 2002 through 2005. Prescribers of these agents should be aware of approved durations, potential contraindications, and consider screening for depression and substance abuse.
Obesity and overweight remain highly prevalent (1,2), contribute strongly to morbidity and mortality (2,3,4), yet continue to remain underdiagnosed and undertreated (5,6). Despite a large body of literature addressing the modest efficacy of the Federal Drug Administration (FDA)–approved obesity reduction medications (7), few US studies have evaluated their use across >1 state (8,9). No studies have discussed recent trends or patterns of use in the United States, including when such drugs may be contraindicated. Therefore, we conducted a nonconcurrent prospective cohort study to evaluate patterns and trends in obesity reduction medication use, focusing specifically on safety concerns such as use in contraindicated settings and length of use of these medications.
Methods and Procedures
Identification of study subjects
We collaborated with two Blue Cross and Blue Shield plans that fully covered obesity reduction medications during the study period. From these plans, enrolling ∼4.2 million persons, we included persons who had a paid (or denied) claim for orlistat, sibutramine, phentermine, benzphetamine, diethylpropion, mazindol, methamphetamine, phendimetrazine, phenmetrazine, or phentermine resin during 2002–2005. We excluded subjects aged <12 years old or with <6 months of continuous health plan enrollment in order to describe more accurate use of these medications over time, leaving 86,804 persons for this study.
Data collection
For included study members, the plan provided the following: (i) enrollment files that included administrative data (i.e., gender, state, and enrollment periods); (ii) benefits information indicating medical and pharmacy coverage; and (iii) adjudicated in-patient, outpatient, and pharmacy claims records containing International Classification of Diseases, Ninth Revision codes, and Current Procedural Terminology and National Drug Codes. Claims data were used to determine comorbidity, medication use, and obesity diagnosis.
Nonobesity medications were determined using the Cerner 2005 Multum Lexicon (10) that groups each National Drug Code into one of 218 Multum therapeutic categories such as β-blockers. When describing patterns of use, we defined “continuous” use of a medication as no gaps in use of >14 days to account for subjects who did not refill their medications exactly on time.
A list of potential contraindications for each obesity reduction medication was developed by one author (a general internist) using the Physicians' Desk Reference (11) and was reviewed by a second author (a general internist experienced in obesity research). The contraindications could include medical diagnoses or prescription use, and were determined using International Classification of Diseases, Ninth Revision codes alone and/or grouped into distinct disease categories using the “Expanded Diagnosis Cluster” tool of the Johns Hopkins University adjusted clinical groups software (12), National Drug Code alone and/or grouped into distinct prescription drug categories using the “prescription morbidity group” tool of the adjusted clinical groups software (12).
Statistical analyses
We used descriptive statistics including proportions and means with standard deviations to describe obesity reduction medication users and their patterns of use from 2002 to 2005. We grouped all obesity medications besides phentermine, sibutramine, and orlistat into an “other” category because these numbers were small, had similar mechanisms of action and contraindications, and we noticed no major differences by group. The “ptrend” command in STATA was used to evaluate trends in medication use over time. Analyses were performed using STATA, Intercooled Version 8.0 (Stata Corporation, College Station, TX).
Results
Characteristics of obesity reduction medication users
During 2002–2005, orlistat was the most commonly used medication (39.4%), followed closely by phentermine (39.1%) and sibutramine (32.2%). The other older sympathomimetic medications were rarely used (8%). Most subjects were female (78%). The mean age was 49 years old. They were enrolled with an average of 39 months in their insurance plan. Less than a quarter had a claims diagnosis of obesity. Eight percent of subjects took two of these medications simultaneously. The top five classes of medications used by this sample were as follows: narcotic analgesics (57%), macrolides (46%), upper respiratory agents such as loratadine (41%), antidepressants (38%), and nonsteroidal anti-inflammatory drugs (37%). The top three narcotics used were hydrocodone, propoxyphene, and codeine alone or in combination. Use of these three narcotics ranged from 7 to 17% of obesity reduction medication users, and the mean days' supply in 2002 ranged from 21 to 66 days. In a comparison group of diabetic adults who were 6 plus months enrolled in the plan but not taking an obesity reduction medication, 15% were taking narcotics and 7% were taking antidepressants.
Trends in and patterns of use
Overall, obesity reduction medication use significantly decreased over time from 1% (n = 45,765 unique users) in 2002 to 0.7% (n = 28,835) in 2005 (P for trend <0.001). This was most notable for the newer medications (sibutramine and orlistat). The other sympathomimetics had relatively stable use during this time period (see 1).
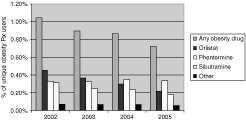
Trends in obesity reduction medication use by year. Other refers to other sympathomimetics including benzphetamine, diethylpropion, mazindol, methamphetamine, phendimetrazine, phenmetrazine, or phentermine resin. The denominators used to calculate these percentages were the number of plan members with medical and prescription coverage for at least 1 month that year: 4,373,183 in 2002; 4,277,865 in 2003; 3,994,977 in 2004; and 3,993,687 in 2005. Obesity reduction medication use decreased significantly over time for obesity reduction medications overall, sibutramine, and orlistat (P value for trend was <0.001). Phentermine and other sympathomimetics stayed relatively the same; the P value for trend was also significant for these medications (P < 0.001), yet is likely due to small differences given the large number of people analyzed.
The majority of obesity reduction medication users (74–79%) took these medications for ≤3 months even allowing for a 2-week gap in medication use (see Table 1 in Supplementary Appendix 1 online). However, a substantial minority (14–17%) were using phentermine and the older sympathomimetics >3 months. The obesity reduction medication users averaged 3–4 episodes of use from 2002 to 2005, with the average length of each episode lasting slightly more than a month.
Potential contraindications
Overall, up to 10% of subjects with a potential contraindication were started on an obesity reduction medication in 2004 (see Table 2 in Supplementary Appendix 1 online). Of particular concern were the more serious, yet less frequent contraindications found in sympathomimetic users, including 2.4% with a prior diagnosis of cardiovascular disease (mainly driven by nitrate use at 0.7% and ischemic heart disease at 1.1%), 2.5% with a prior diagnosis of schizophrenia, 2.0% taking a seizure medication concurrently, and 1.2% over age 65.
Discussion
Although obesity prevalence has remained high (2), obesity reduction medication use was very low overall and decreased over time from 1% in 2002 to 0.7% in 2005. Of those who took obesity reduction medications, most used them <3 months (range: 74–79%) raising the question of long-term effectiveness in real-world settings. However, a notable subset (range: 14–17%) took phentermine and other short-term sympathomimetics longer than 3 months raising the issue of potential safety concerns (13). The FDA recommends short-term use of these medications, which is generally translated as 12 weeks. Few subjects (up to 2.5%) had serious prior contraindications to use.
Several limitations deserve mention. First, our study may be less generalizable to the uninsured public, across other geographic locations such as the Southern United States, or to obese individuals with less comorbidity. Second, diagnoses reported in claims records underestimate actual diagnoses (14). We may therefore have underestimated potential contraindications noted by International Classification of Diseases, Ninth Revision diagnosis codes. Third, we only included people with both medical and prescription coverage as opposed to either coverage. This may have caused the prevalence of obesity reduction medications to be slightly overestimated due to undercounting of the denominator. However, a sensitivity analysis using individuals with prescription or medical coverage as the denominator produced similar results. Lastly, we did not have BMI; therefore, we were unable to assess obesity reduction medication use in obese persons, variations in trends of medication use by BMI, or medication effectiveness. We suspect that we would have seen similar trends in use because population level data suggest a relatively constant or increasing rate of obesity during this time period (2).
Since 1980, only one study has evaluated national trends of obesity reduction prescriptions in the United States through 2001 (9). Our results on trends in use from 2002 to 2005 update the prior study (9). We found decreased use of these medications overall, which were mostly led by decreased use of the newer agents (sibutramine and orlistat). We found relatively stable use of the older medications (phentermine and other sympathomimetics). In Stafford's article based on physician survey data (9), a downward trend was reported for all medications except orlistat that stayed relatively constant but had just been introduced into the market in 1999. Now that orlistat can be bought without a prescription, we may see increased use of this medication in the future. The overall downward trend may be problematic in light of the high prevalence of obesity in the United States and may be due to (i) increased use of bariatric surgery to treat obesity (8); and (ii) hesitancy of both physicians and patients to use these medications due to their modest weight loss effects, the need for long-term use to maintain weight loss, drug side effects, lingering concerns of valvular disease as a side effect, and the expense of the newer medications (7,8).
We also report several novel findings. First, we found that narcotic analgesics were the most frequently prescribed medication among plan members taking obesity reduction prescriptions. Whether this suggests that obese individuals who take obesity reduction prescriptions have higher rates of osteoarthritis and joint pain, or whether they are at higher risk for potential substance abuse is unclear. They also had high rates of antidepressant use (about 25%), both compared with diabetic adults taking antidepressants using our data (7%) and with the depression prevalence of 16% from a national survey of the general US population (15). Because substance use has been associated with depression (16), providers may need to pay particular attention to depressive symptoms among obesity reduction medication users.
Second, phentermine and other sympathomimetics were used for >3 months in a substantial number of individuals (14–17%). This suggests a potentially inappropriate use of these medications that have not been tested or FDA-approved for long-term safety. Also, 8% of subjects were taking >1 obesity reduction medication simultaneously. Although this may make sense given that weight regain is common after stopping the medications and only modestly effective when used as monotherapy, the risk of adverse side effects (e.g., pulmonary hypertension) may increase with longer or simultaneous use.
Third, few people had potential serious contraindications prior to starting any of the sympathomimetics. This proportion is slightly less than proportions seen with medications used to treat other chronic diseases such as diabetes (17). The risk of side effects of these medications should be weighed against the risks associated with obesity on an individual basis.
Future research should focus on (i) barriers to obesity reduction medication use by patients and providers; (ii) safety/complication rates in those with potential contraindications, those using >1 obesity reduction medication and/or longer than recommended use; and (iii) ways to better inform providers and patients regarding the FDA-recommended intervals for these agents until more safety data are available. Additionally, providers should consider screening for depression and monitoring for signs of substance abuse.
Disclosure
We did receive funding for this grant from Pfizer, Johnson and Johnson, and GlaxoSmithKline. I do not believe that this is a true conflict of interest because none of these companies have any FDA-approved obesity reduction medications. In the interest of full disclosure, we do mention it, however.
SUPPLEMENTARY MATERIAL
Acknowledgments
This study was funded by unrestricted research grants from Ethicon Endo-Surgery, Inc. (a Johnson & Johnson company); Pfizer, Inc.; and GlaxoSmithKline. In-kind support was provided by the Blue Cross and Blue Shield Association and the seven local Blue Cross and Blue Shield plans participating in this project. The funding and collaborating organizations were kept informed of the study's progress and shared their expertise on certain aspects of the study. Also, preliminary findings were shared with them, and they were invited to review the manuscript. However, they did not have any direct role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation or approval of the manuscript. We thank the Blue Cross and Blue Shield plans and the many staff members at these sites who actively contributed to this study by providing data and expert advice regarding use of these data. These organizations included Blue Cross and Blue Shield of Tennessee, Highmark Blue Cross and Blue Shield (of Pennsylvania), Blue Cross and Blue Shield of Michigan, Blue Cross and Blue Shield of North Carolina, Independence Blue Cross (of Pennsylvania), Wellmark Blue Cross and Blue Shield of Iowa, Wellmark Blue Cross and Blue Shield of South Dakota, and Hawaii Medical Service Association. We also are grateful to Hsien-Yen Chang, Johns Hopkins Bloomberg School of Public Health, for his technical assistance.
Supplementary material is linked to the online version of the paper at http:www.nature.comoby
Supplementary Appendix 1.





