Ethnicity and Weight Status Affect the Accuracy of Proxy Indices of Insulin Sensitivit
Abstract
This study tested the hypotheses that correlations between direct measures of insulin sensitivity and proxy indices of insulin sensitivity derived from fasting values, (i) would not be affected by ethnicity, and (ii) would be stronger in overweight vs. weight-reduced states. We further hypothesized that associations between proxy indices and fat distribution would be similar to those between directly measured insulin sensitivity and fat distribution. Testing was performed in weight-stable conditions in 59 African-American (AA) and 62 white-American (WA) overweight, premenopausal women before and after a weight loss intervention. Subjects were retested 1 year following weight loss. Proxy indices were correlated against the insulin sensitivity index SI determined via minimal modeling. Fat distribution was assessed using computed tomography. Correlations between Si and proxy indices were consistently stronger among overweight women (r = 0.44–0.52) vs. weight-reduced women (r = 0.18–0.32), and among AA (r = 0.49–0.56, baseline; 0.24–0.36, weight-reduced) vs. WA (r = 0.38–0.46, baseline; 0.19–0.31, weight-reduced). Among subjects who regained >3 kg after 1 year, correlations between SI and proxy indices were similar to those observed at baseline, whereas correlations were weak among women who maintained their reduced body weight. SI and all proxy indices were similarly correlated with intra-abdominal adipose tissue (IAAT) at baseline, but not after weight loss. In conclusion, correlations between SI and proxy indices were affected by both ethnicity and weight status. If proxy indices are used in multiethnic populations, or in populations including both lean and overweight/obese subjects, data should be interpreted with caution.
Introduction
The associations between insulin resistance, obesity, and its associated comorbidities, such as type 2 diabetes and cardiovascular disease, are well established (1,2). This has led to the development of numerous methods to evaluate insulin sensitivity. The hyperinsulinemic-euglycemic clamp technique (3) and the frequently sampled, intravenous glucose tolerance test (FSIGT) (4) are common methods used to directly measure insulin sensitivity.
The cost and time constraints of direct measures of insulin sensitivity make the use of proxy indices derived from simple equations using fasting glucose and insulin concentrations highly appealing to researchers and clinicians. These proxy indices are believed to primarily reflect insulin suppression of hepatic glucose production (hepatic insulin sensitivity) as opposed to whole-body insulin sensitivity (5). Commonly used proxy indices are the homeostasis model assessment of insulin resistance (HOMA-IR) (6), the quantitative insulin sensitivity check index (QUICKI) (7), and the fasting glucose-to-insulin ratio (FGIR) (8). Fasting plasma insulin is also frequently used as a proxy index.
The influence of ethnicity on the predictive ability of proxy indices is not clear. It is well established that African Americans (AAs) have lower whole-body insulin sensitivity than white Americans (WAs), independent of adiposity, diet, and physical activity (9). The physiological basis for this ethnic difference has yet to be explained. Because lower insulin sensitivity among AA vs. WA may have a different biological basis than the obesity-related depression in insulin sensitivity, it is not known whether proxy indices are equally valid in AA and WA.
Likewise, weight status may influence the accuracy of proxy indices. Correlation analyses from cross-sectional studies of nondiabetic populations suggest the relationships between direct measures of insulin sensitivity and proxy indices are stronger in overweight and obese populations than in normal-weight populations (10,11,12,13). The influence of weight status on the relationship between direct measures of insulin sensitivity and proxy indices has not been evaluated in a weight loss intervention study.
Research has shown that insulin sensitivity assessed via direct measures is influenced by body fat distribution, with intra-abdominal adipose tissue (IAAT) linked to greater decrements in insulin sensitivity compared to subcutaneous abdominal adipose tissue (SAAT) (14,15). However, in some cases, SAAT has been reported to provide a stronger, or at least an equal, association with insulin sensitivity compared to IAAT (16,17). Weight status may determine which adipose depot is more strongly correlated with insulin sensitivity (18). The effect of weight status on the relationship between adipose distribution and insulin sensitivity has not been reported in a longitudinal weight loss study, nor has it been established whether proxy indices are congruous with reported associations between fat distribution and direct measures of insulin sensitivity.
This study was designed to investigate associations between insulin sensitivity as determined from a FSIGT with minimal modeling (the insulin sensitivity index, SI) and proxy indices before and after a weight loss intervention among healthy, overweight, AA and WA premenopausal women. We hypothesized that correlations between SI and proxy indices, (i) would be similar in AA and WA, and (ii) would be stronger in the overweight vs. weight-reduced state. We further hypothesized that proxy indices and SI would show similar associations with fat distribution. Results from this study not only shed light on major methodological issues in obesity and diabetes research, but they also lend insight into the mechanisms of the development of hepatic and peripheral insulin resistance.
Methods and Procedures
Subjects
Subjects were healthy, premenopausal AA and WA women originally recruited as part of a weight loss study. For study inclusion, women were required to be overweight (BMI 27–30 kg/m2), 20–41 years of age with a history of regular menstrual cycles, normoglycemic, and sedentary. All women were nonsmokers and were not taking medications known to affect body composition or metabolism. All data were collected during three in-patient stays (baseline, postweight loss, and 1-year follow-up) at the University of Alabama at Birmingham (UAB) and the General Clinical Research Center (GCRC). Testing was conducted in the follicular phase of the menstrual cycle. Only data from 59 AA and 62 WA women who completed the FSIGT at baseline and after weight loss were included in this study. Data from 95 women were available for 1-year follow-up analyses. This study was approved by the UAB Institutional Review Board for Human Use. Subject consent was obtained before all testing.
Protocol
Before baseline assessment, all women completed 4 weeks of supervised weight maintenance, during which subjects consumed their own foods for 2 weeks and GCRC-provided foods for the following 2 weeks. Subjects were weighed three to five times weekly, and diets were adjusted as needed to ensure weight stability. For the weight loss intervention, women were randomized to one of three groups: weight loss by low-energy diet with no exercise; weight loss by low-energy diet plus aerobic exercise; or weight loss by low-energy diet plus resistance exercise. Insulin sensitivity did not differ among groups in either the overweight or weight-reduced states; thus all groups were combined. The weight loss diet consisted of 800–1000 kcal/d and was followed until subjects achieved a target BMI of <25 kg/m2. Postweight loss assessment was conducted following another 4-week period of supervised weight maintenance, in the weight-reduced state. During the year after weight loss, subjects were encouraged to attend weight maintenance classes and to continue their assigned exercise program (if applicable). Metabolic testing at the 1-year follow-up assessment was conducted following 4 weeks of supervised weight maintenance.
Assessment of insulin sensitivity
Insulin sensitivity was directly assessed using a FSIGT and mathematical modeling (4,19), following an overnight fast. The average of three blood samples collected over a 20-min period was used for assessment of fasting glucose and insulin. Additional insulin and glucose values were obtained by frequent blood samples collected throughout a 4-h period after glucose injection (50% dextrose, 11.4 g/m2) at time “zero” and bolus insulin administration (0.02 units/kg) at time “20.” These values were entered into the MINMOD computer program (version 3.0, Richard N. Bergman) for determination of SI (4,19). Glucose and insulin were measured using an Ektachem DTII analyzer (Johnson and Johnson, Rochester, NY) and radioactive immunoassay materials obtained from Linco Research (St. Charles, MO), respectively.
The following proxy indices were evaluated:

Body composition and fat distribution
Total fat mass was measured via dual-energy X-ray absorptiometry using a GE Lunar Prodigy densitometer (GE LUNAR Radiation, Madison, WI). Total abdominal adipose tissue (TAAT), IAAT, SAAT, superficial SAAT, and deep SAAT were assessed by computed tomography with a HiLight/Advantage Scanner (General Electric, Milwaukee, WI), as previously described (20,21).
Statistical analyses
Descriptive statistics (mean ± s.d.) for all variables of interest at baseline and after weight loss were determined for each ethnic group. A two-group t-test was used to compare age by ethnicity. A two-way repeated measures ANOVA was used to compare all other variables before and after weight loss and by ethnicity. The relationships between SI and proxy indices at the baseline, postweight loss, and 1-year follow-up periods were assessed for each ethnic group using Pearson correlations. Partial correlations, adjusting for ethnicity, were used to assess relationships between insulin sensitivity and fat distribution at baseline and after weight loss. The distributions for SI and FGIR deviated from normal, and were log10-transformed. Statistical analyses were performed using the SAS software package (version 9.1; SAS Institute, Cary, NC). The one-sample runs test of randomness was used to determine whether correlations of proxy indices, as a group, were different between the overweight and weight-reduced states and between AA and WA (22). All statistical analyses were two-sided with a 5% significance level.
Results
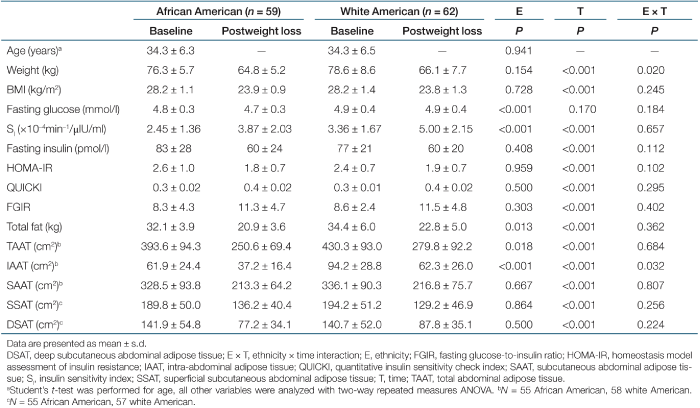
Descriptive characteristics for subjects at baseline and after weight loss by ethnicity are shown in Table 1, as are measures of insulin sensitivity and fat distribution. Weight loss did not have a significant effect on fasting glucose. All subjects became significantly more insulin sensitive with weight loss (P < 0.001 for all indices of insulin action). Likewise, measures of total and regional fat distribution were significantly reduced after weight loss (P < 0.001 for all). Overall, AA women had significantly lower fasting glucose (P < 0.001), SI (P < 0.001), total body fat (P = 0.0133), TAAT (P = 0.0182), and IAAT (P < 0.001) than WA. There were no ethnic differences in the proxy indices. For weight and IAAT, there was a significant interaction between ethnicity and time, indicating that WA had a larger reduction in weight (P = 0.0196) and IAAT (P = 0.0315) than AA.
 |
Correlations by ethnicity and weight status
Pearson correlations between SI and proxy indices at baseline and postweight loss, and for change in insulin sensitivity, are presented by ethnicity in Table 2. The magnitude of correlation coefficients for proxy indices vs. SI was significantly larger among AA vs. WA (P < 0.05). Correlations of change in proxy indices with change in SI were statistically significant in AA (with the exception of FGIR) but not in WA.
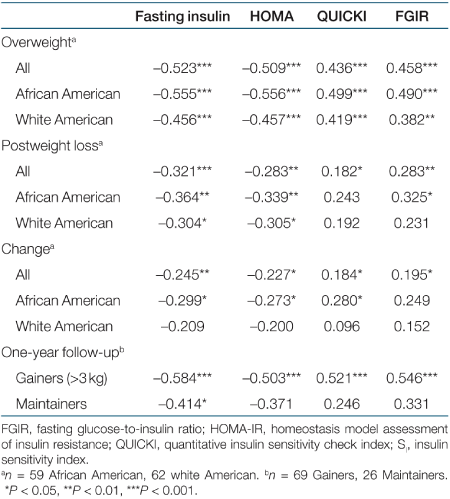 |
Among all subjects, correlations between SI and proxy indices (Table 2) were significantly stronger in the overweight state than after weight loss (P < 0.05). Adjusting postweight loss correlations for exercise group did not change the correlations (data not shown). Correlation coefficients for change in proxy indices vs. change in SI were generally lower than those for cross-sectional associations at baseline and postweight loss.
One-year follow-up
To further investigate the influence of adiposity on associations between SI and proxy indices, we compared data from subjects who gained >3 kg during a 1-year follow-up period with data from subjects who maintained their weight within 3 kg over this time (Table 2). Correlations between SI and the proxy indices among the women who regained >3 kg were stronger than were correlations among those who maintained their body weight. With the exception of fasting insulin, correlations in subjects who maintained their weight were not statistically significant.
Insulin sensitivity and regional fat distribution
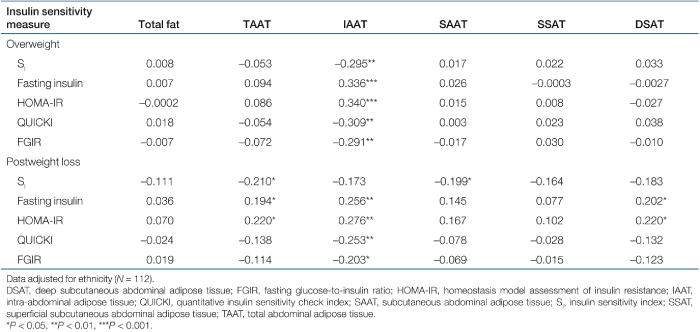
Partial correlations, adjusted for ethnicity, of SI and proxy indices with measures of fat distribution at baseline and after weight loss are shown in Table 3. At baseline, SI was significantly associated with IAAT. After weight loss, the association between SI and IAAT merely approached significance, whereas associations with TAAT and SAAT were significant. In contrast, the proxy indices remained significantly associated with IAAT regardless of weight status.
 |
Discussion
The present study was designed to determine whether the predictive ability of proxy indices to reflect insulin sensitivity would be affected by race or weight status in a weight loss intervention. Results indicated that associations between SI and proxy indices were stronger in AA vs. WA, and were stronger in the overweight vs. weight-reduced state. Additionally, weight status affected the associations of regional fat distribution with SI, but not those with the proxy indices. These findings indicate that subject population has a significant effect on the utility of proxy indices to reflect insulin sensitivity as measured using robust methodology.
Generally, AA have lower insulin sensitivity than WA (9). In our study, this ethnic difference was apparent with SI, a measure of both hepatic and peripheral insulin sensitivity (whole-body insulin sensitivity) (23), but not with the proxy indices (Table 1). Whether lower insulin sensitivity among AA vs. WA is because of lower hepatic or peripheral insulin sensitivity, or both, is not known. It is possible that ethnic differences in the hepatic and peripheral contribution to whole-body insulin sensitivity explain the poor ability of proxy indices to detect ethnic differences in whole-body insulin sensitivity. Alternatively, it is possible that lower insulin sensitivity among AA vs. WA is apparent only during a dynamic test such as the FSIGT, or under conditions of elevated insulin, such as the euglycemic-hyperinsulinemic clamp. In any case, robust techniques are recommended to investigate ethnic differences in insulin sensitivity.
Regardless of weight status, correlations between SI and the proxy indices were larger in AA vs. WA (Table 2), suggesting that whole-body insulin sensitivity is better reflected by proxy indices in AA than in WA. In contrast to our results, Mather et al. (12) did not find an influence of race on the correlations between clamp-derived insulin sensitivity (using an insulin dosage of either 40 or 120 mU/m2·min) and proxy indices derived from fasting values. The contrasting results of the studies may be due to differences in the technique used to measure insulin sensitivity or to the subject population. The FSIGT is a dynamic, physiological test that involves the subject's own pancreatic insulin response to a single glucose bolus (23). In contrast, the euglycemic-hyperinsulinemic clamp measures insulin sensitivity in a nonphysiological condition involving infusion of both glucose and insulin to achieve “clamped” circulating concentrations of both. In addition, the cohort investigated by Mather et al. (12) was a widely heterogeneous group, including lean, obese, and diabetic men and women. It is unclear why proxy indices in AA would have better correlations with SI than WA. Further research is needed to discern whether there is indeed an influence of ethnicity on the association between proxy indices and whole-body insulin sensitivity, and if so, the mechanism leading to such an influence. Regardless, researchers should be aware that proxy indices may reflect different physiologic processes in AA and WA.
In accordance with several cross-sectional studies (7,10,11,12,13), we found correlations between SI and fasting proxy indices to be greater in an overweight state than in a normal-weight state (Table 2), and for the first time, demonstrated this effect of weight status in a weight loss intervention study. To further examine the influence of body weight change on the predictive ability of proxy indices, we examined correlations within subjects who regained >3 kg 1 year after weight loss and within subjects who remained in the normal-weight state. Correlation coefficients between SI and proxy indices in women who gained >3 kg increased to magnitudes similar to those observed in the original overweight state (Table 2). In contrast, with the exception of fasting insulin, correlations in women who maintained a normal body weight remained relatively low and not statistically significant. Our longitudinal study design, thus, rules out the possibility that inherent metabolic differences between overweight and lean subject populations are responsible for the stronger correlations in obese vs. normal-weight states.
The decrease in magnitude of the correlations of SI with the proxy indices from the overweight to the weight-reduced state may reflect changes with weight loss in tissue-specific contributions to insulin sensitivity. Paquot et al. (24) showed that obese nondiabetic subjects have greater hepatic insulin resistance compared to lean controls. It is likely that, in the overweight state, compared to the weight-reduced state, a larger proportion of whole-body insulin sensitivity, and therefore SI, is of a hepatic nature, and consequently correlates more strongly with proxy indices, which likewise primarily reflect hepatic insulin sensitivity. Greater hepatic insulin resistance in the overweight state may occur secondary to the accumulation of IAAT (25,26), which may affect hepatic insulin sensitivity through releasing free fatty acids or adipokines (27,28,29).
We hypothesized that associations between proxy indices and fat distribution would be similar to those between SI and fat distribution. Instead we found that weight status influenced the congruence of proxy indices to SI in their associations with fat distribution. In the overweight state, both SI and the proxy indices were significantly associated with IAAT (Table 3). However, in the weight-reduced state, SI was significantly associated only with TAAT and SAAT, whereas the proxy indices remained significantly associated with IAAT. These observations suggest that the proxy indices, which reflect hepatic insulin sensitivity to a large degree, are primarily influenced by IAAT, regardless of weight status. In contrast, whole-body insulin sensitivity, which primarily reflects skeletal muscle glucose uptake, may be affected by IAAT only after IAAT reaches a certain threshold (30,31). In support of this hypothesis, clamp-derived insulin sensitivity was correlated with IAAT only in obese subjects, among whom the contribution of the liver to whole-body insulin sensitivity likely was greater (18). We show for the first time that the relationship of IAAT with whole-body insulin sensitivity is attenuated with weight loss. Taken together, these observations indicate that proxy indices of insulin sensitivity and direct measures of insulin sensitivity reflect different processes, and are influenced to a different degree by IAAT.
The longitudinal nature of our study allowed us to evaluate the ability of proxy indices to predict changes in insulin sensitivity. Correlations between changes in SI and changes in proxy indices were relatively low (Table 2). This was particularly evident within WA, among whom changes in none of the proxy indices was associated with change in SI. Our data support those of Hays et al. (32) and Duncan et al. (33), who did not find changes in proxy indices to be accurate indicators of change in clamp- or FSIGT-derived insulin sensitivity in healthy adults. These studies, as well as ours, suggest that robust measures of insulin sensitivity are necessary in longitudinal or interventional studies involving changes in body weight or metabolism.
Strengths of this study included the homogeneity of the subject population regarding baseline BMI, and the longitudinal design, which allowed for evaluating the accuracy of proxy indices with weight loss and weight regain in a single cohort. A limitation of our study was that our subjects did not undergo a 2-h oral glucose tolerance test. Thus we were only able to evaluate proxy indices derived from fasting insulin and glucose concentrations. It is likely that indices derived from a 2-h oral glucose tolerance test would have yielded different results, as these indices reflect both peripheral and hepatic insulin sensitivity (34). Furthermore, our findings may be limited to normoglycemic, premenopausal women.
In summary, results indicated that proxy indices of insulin sensitivity derived from fasting values of glucose and insulin predicted SI with greater accuracy in AA vs. WA women, and in the overweight vs. weight-reduced state. Changes in associations between SI and proxy indices with weight status were attributed to IAAT, the loss of which likely resulted in shifts in the contributions of hepatic and peripheral tissue to SI. Thus, proxy indices, such as fasting insulin, HOMA-IR, QUICKI, or FGIR, may accurately reflect whole-body insulin sensitivity only in overweight populations. In studies involving multiethnic or normal-weight populations, or in interventions involving weight change, the use of such proxy indices is not recommended.
Acknowledgment
This work was supported by grants from the National Institutes of Health (P30-DK56336, M01-RR-00032, R01-DK49779, and R01-DK51684). Food was provided by Nestlé Food Co., Solon, OH (Stouffer's Lean Cuisine) and HJ Heinz Co., Pittsburgh, PA (Smart Ones).
Disclosure
The authors declared no conflict of interest.





