Increasing Heritability of BMI and Stronger Associations With the FTO Gene Over Childhood
Abstract
The growing evidence of health risks associated with the rise in childhood obesity adds to the urgency of understanding the determinants of BMI. Twin analyses on repeated assessments of BMI in a longitudinal sample of >7,000 children indicated that the genetic influence on BMI becomes progressively stronger, with heritability increasing from 0.48 at age 4 to 0.78 at age 11. In the same large twin sample, the association between a common variant in the FTO gene and BMI increased in parallel with the rise in heritability, going from R2 < 0.001 at age 4 to R2 = 0.01 at age 11. These findings suggest that expression of FTO may become stronger throughout childhood. Increases in heritability may also be due to children increasingly selecting environments correlated with their genetic propensities.
Introduction
Twin research has been pivotal in identifying the substantial genetic influence on individual differences in BMI (1,2,3,4,5). However, the genetic effect size has varied markedly between studies, perhaps in part because of age differences. For a broad range of traits such as externalizing behavior problems, social attitudes, intelligence quotient, anxiety, and depression, heritability increases from childhood to early adulthood (6). There is also evidence for developmental increases in heritability for exercise (7), sports participation (8), and eating attitudes and behaviors (9). However, the evidence is mixed for age changes in the heritability of BMI. Birthweight is ∼40% heritable (10); moderate heritability has been reported at age 4 (ref. 11) and high heritability at age 10 (ref. 5). In adolescence, heritability estimates have been twice as high (ranging from 70 to 90%) (4), but the heritability of BMI in adulthood is generally lower, and there is evidence that it decreases over the life span (e.g., ref. 12); a recent study found decreases in heritability from 12 to 16 years (13). In contrast, research into BMI in a sample of 1,412 twin pairs showed that in three age groups (8–10, 11–13, and 14–16 years), heritability increased from 0.67 to 0.88 to 0.93, respectively (3).
Changes in heritability over the life span could be due to genetic or environmental changes. It is possible that different genes influence a trait at different developmental stages or the same genes could have a larger impact on a trait as it develops. In the case of obesity, heritability could change because individuals at genetic risk for obesity increasingly select obesogenic environments correlated with their genetic propensities. Twin studies can evaluate developmental continuity for genetic and environmental influences using multivariate longitudinal genetic analysis, which considers not only the variance of traits considered one at a time but also the covariance among traits. It yields a statistic called the genetic correlation, which can be roughly interpreted as the likelihood that genes found to be associated with one trait will also be associated with the other trait (or the same trait at a different time) (14). If the genetic correlation of BMI measured at two ages is low, then the probability of the same gene being associated at both ages is also low, and this is independent of the heritability of BMI at each age. However, twin studies do not provide information about genetic continuity of specific genes; developmental molecular genetic studies are the definitive test of genetic continuity.
Relatively few twin studies have assessed heritability longitudinally to determine the continuity of genetic influence throughout development. A small longitudinal study in adolescence from age 11 to 17 years produced high genetic correlations (0.79–0.97), indicating that the same genetic factors were at play in BMI across adolescence (3). Two recent studies of Dutch children aged 3–12 years and Australian adolescents aged 12–16 years also indicated significant genetic continuity across childhood and adolescence (13,15).
If the heritability of BMI increases over childhood, and there is strong genetic continuity for BMI, this suggests that there should be a progressively stronger association with BMI-linked gene variants. The FTO gene is the first well-replicated genetic correlate of BMI; it was recently associated with BMI in 14 populations (16) and has also been replicated in a large sample of 10-year-old UK twins (17). The finding continues to be replicated in both children and adults (e.g., refs. 18,19,20,21). Two of the original samples were children aged 7–11 and 14 years. The difference in BMI scores between the two homozygote groups (TT vs. AA genotypes) increased in the first sample from ages 7 to 11 years, although in the second sample the difference in BMI scores is much smaller, even though the children were older (14 years) (16). We found a difference of 0.73 BMI points between the two homozygote groups in 10-year-old twins (17).
In this study, we used longitudinal data on BMI in a large UK-representative sample of twins to test the hypotheses that (i) heritability would increase from age 4 to 11, (ii) the association between a common variant (rs9939609) in the FTO gene and BMI would increase in parallel, and (iii) information about the genetic correlations between ages would predict the likelihood of the FTO gene associated at one age also being associated at other ages.
Methods and Procedures
Sample
The sample was the Twins Early Development Study (TEDS), a study of twins born in the United Kingdom between 1994 and 1996 (ref. 22). The TEDS sample has been shown to be reasonably representative of the UK population (22). All twins and parents involved in TEDS provide informed consent for each stage of the study. Ethical approval for TEDS has been provided by the King's College London, ethics committee.
Response rates based on active families were 65, 63, 65, and 77%, respectively, at the four ages. For the purposes of the current study, we excluded from the analyses families in which at least one member of the twin pair had a specific medical syndrome or was an extreme outlier for perinatal problems such as extreme low birth weight. In order to avoid the disproportionate influence of extreme outliers in the individual differences analyses, we further excluded twin pairs in which at least one twin scored more than 3 s.d. below or above the mean for BMI at each age. The final sample included 3,582 twin pairs (1,422 MZ, 2,160 DZ) at 4 years of age; 3,148 twin pairs (1,336 MZ, 1,812 DZ) at 7 years of age; 3,573 twin pairs (1,483 MZ, 2,090 DZ) at 10 years of age; and 4,251 twin pairs (1,526 MZ, 2,725 DZ) at 11 years of age. N values spilt by sex and zygosity can be found in Supplementary Table S1 online.
The mean ages of the twins when questionnaires were returned were 4.05 (s.d. = 0.16), 7.05 (s.d. = 0.25), 9.93 (s.d. = 0.87), and 11.25 (s.d. = 0.68), respectively. Zygosity was assessed through a parent questionnaire of physical similarity, which has been shown to be >95% accurate when compared to DNA testing (23). For cases where zygosity was unclear from this questionnaire, DNA testing was conducted.
Measures
Height and weight data were collected by postal questionnaire from the families when their children were 4, 7, 10, and 11 years of age. BMI was calculated from the height and weight data (BMI = weight (kg)/(height (m)2)) and converted to BMI z-scores. BMI z-scores take into consideration the child's age and sex. They were based on 1990 UK growth reference curves (24) and were calculated using the program lmsGrowth (http:homepage.mac.comtjcole). All measures were residualized for age and sex effects using a regression procedure. Standardized residuals were used because the age and sex of twins is perfectly correlated across pairs, and variation within age at the time of testing and variation within sex could contribute to the correlation between twins, and thus be misrepresented as environmental influences shared by the twins (25). The final BMI scores were normally distributed with skewness <0.1 (see Supplementary Figure S1 online).
The means and s.d. for the raw BMI scores (i.e., unadjusted for age and sex) are presented in Supplementary Table S2 online. The results of a 2×2 (sex by zygosity) ANOVA, shown in Supplementary Table S2 online, indicate a significant main effect of zygosity at 10 years (P = 0.041, η2 = 0.001) and significant main effects of sex at all ages (4 years: P = 0.005, η2 = 0.002; 7 years: P = 0.005, η2 = 0.002; 10 years: P < 0.001, η2 = 0.008; 11 years: P < 0.001, η2 = 0.003). However, as is apparent from the mean scores and the effect sizes (η2), these differences are very small and account for <1% of the variance and are significant only because of the large sample size. There was a significant interaction between sex and zygosity at 7 years (P = 0.017, η2 = 0.002), but again the effect size of this significant effect is very small.
FTO. Single-nucleotide polymorphism rs9939609, which lies within the first intron of the FTO gene was genotyped in house using Taqman. The following sequence was used: GGTTCCTTGCGACTGCTGTGAA TTT[A/T]GTGATGCACTTGGATAGTCTCTGTT. One randomly selected member of a twin pair was selected to be in this sample. TEDS DNA has been extracted from buccal mucosa cells using a procedure described in Freeman et al. (26). This extraction procedure typically yields 60–70 µg of DNA resuspended in 1 ml of TE buffer, quantified by UV absorption and electrophoretic methods to confirm quantity and quality. The samples are then individually bar-coded and managed using a sophisticated sample-tracking database, which provides information about the location, quantity, and real-time usage of the DNA samples. Genotyping data and BMI data were available for 2,844 individuals at 4 years of age, 2,820 at 7 years of age, 2,870 at 10 years of age, and 2,882 at 11 years of age.
Twin analyses
Twinning provides a naturally occurring, quasi-experimental test of genetic influence. Identical twins are 100% genetically similar, whereas nonidentical twins are on average only 50% similar for segregating genes. At a crude level, this means that if a trait is influenced by genetics then within-pair resemblance for that trait should be higher in identical twins than in nonidentical twins. The twin method makes two important assumptions. The first is that MZ twins and DZ twins will have equally similar environments. This means that greater MZ similarity is interpreted as greater genetic influence. The second assumption is that results from twins are generalizable to the rest of the population. Discussion and defense of these assumptions can be found elsewhere (e.g., refs. 27,28,29,30).
An approximation of the genetic and environmental influences on a quantitative trait—in this case BMI—can be obtained by comparing intraclass correlations for MZ and DZ twins. Intraclass correlations provide a measure of the degree of relationship by asking what proportion of the variance is between-subjects variance. Structural equation model-fitting analyses provide more detailed estimates of genetic and environmental effect sizes that make assumptions explicit, test the fit of the entire model to the data, test the relative fit of alternative models, and provide confidence intervals for the parameter estimates (14). Mx software for structural equation modeling was used to perform standard model-fitting analyses using raw data (31). Two fit indices are reported: χ2 and Akaike's information criterion (32). Sex limitation analyses were performed to allow for both qualitative and quantitative sex differences (33) (Supplementary Table S3 online).
Longitudinal analyses. Prospective longitudinal analysis for BMI was performed using a Cholesky decomposition model (14). A Cholesky decomposition model was fit to raw data to test for common and independent genetic and environmental effects on variance and covariance in BMI at 4, 7, 10, and 11 years. Estimates from the Cholesky model can be transformed to obtain genetic, shared and nonshared environmental correlations between BMI at 4, 7, 10, and 11 years. The genetic correlation estimates the extent to which the same genetic effects operate across ages.
FTO analyses
FTO genotyping was carried out for one randomly selected member of each twin pair. We verified that the FTO polymorphism did not deviate significantly from Hardy-Weinberg equilibrium using an exact test, implemented in R (34) (P = 0.361). Association analyses were performed between FTO genotype and BMI using linear models to represent additive genetic effects, and ANOVA to test for genotypic (nonadditive) effects. We then compared the two models using ANOVA to see whether the nonadditive model provides a significantly better fit. This method is one method endorsed by Balding (35). The association analyses we performed are available as an R package from O.S.P.D. ([email protected]). This association package is designed for single-nucleotide polymorphism association analyses in singleton data sets. Association analyses were performed cross-sectionally on the longitudinal TEDS sample; therefore, the sample size varies slightly between the ages. The samples at each age remain representative of the whole TEDS sample and the general population.
Limitations
A general limitation is the use of the twin method. The validity of the twin method is discussed in detail elsewhere (14,27,28,29,30), but it is worth noting that adoption and family studies, which make different assumptions, support the findings from twin studies, showing moderate-to-high heritability for BMI (3). Ultimately, the most convincing evidence will come from molecular genetic studies that identify the genes responsible for heritability of BMI in childhood, as in the example of FTO.
A specific limitation of this study is the use of parental reports of children's heights and weights, although previous research has shown that they are reasonably reliable (36). This was confirmed in our sample on a subsample at 11 years of age: Home measurements of height and weight, taken by trained researchers, correlated 0.83 and 0.90, respectively, with parental report.
Results
Twin analyses indicate increasing heritability
The intraclass twin correlations for BMI are shown in 1 (correlations split by sex shown in Supplementary Table S1 online). At each age, MZ correlations exceeded DZ correlations, suggesting genetic influence. Estimating heritability by doubling the difference between the MZ and the DZ correlations indicated that the genetic influence on BMI increased progressively from 0.56 at age 4 and 0.64 at age 7, to 0.78 at age 10, and 0.74 at age 11. Results from standard twin model-fitting analyses (14,37) (Supplementary Table S3 online) were consistent with estimates from twin correlations—showing a significant linear trend in the heritability of BMI across early-to-middle childhood.
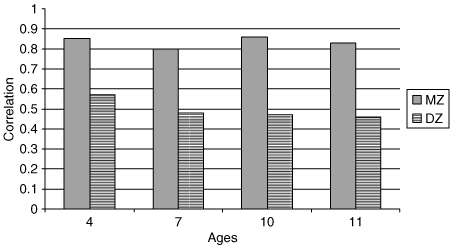
Intraclass twin correlations for BMI. All correlations significant at α level < 0.01. N values (number of twin pairs) were: 4 years: MZ = 1,422, DZ = 2,160; 7 years: MZ = 1,336, DZ = 1,812; 10 years: MZ = 1,483, DZ = 2,090; 11 years: MZ = 1,526, DZ = 2,725. DZ, all dizygotic twins; MZ, monozygotic twins.
Shared environmental influences on BMI in childhood decrease with age
The twin method produces estimates for two sources of environmental influence: shared environment (which makes twins growing up in the same family more similar) and nonshared environment (which do not contribute to familial resemblance). At age 4, the twin correlations indicated that the shared environment effect was moderate (29%), but it decreased to 16% at age 7 and 8% at both age 10 and age 11. The nonshared environment effect was modest (15%) at age 4 and showed no consistent age trend (20, 14, and 18%, respectively). Again, these results were confirmed by model fitting (Supplementary Table S3 online).
Genetic continuity for BMI from early-to-middle childhood
Genetic continuity for BMI was assessed to investigate whether the increases in heritability were due to new genetic influences. Longitudinal data were analyzed for BMI at ages 4, 7, 10, and 11 years. The phenotypic correlations between BMIs at different ages were generally lower between 4 years of age and the later assessments (from 4 to 7 years: r = 0.39; from 4 to 10 years: r = 0.36; from 4 to 11 years: r = 0.33; all significant at P < 0.01). Correlations were higher between BMI at 7 years of age and later assessments (from 7 to 10 years: r = 0.64; from 7 to 11 years: r = 0.60; both significant at P < 0.01) and between BMI at 10 and 11 years of age (r = 0.79, P < 0.01). This pattern confirms previous findings about the increasing stability of BMI in middle childhood (38).
Longitudinal model-fitting analyses were performed to assess developmental continuity and change for genetic and environmental influences on BMI. 2 presents the results from the correlated factors solution, which provides genetic, shared, and nonshared environmental correlations. The genetic correlation is independent of heritabilities at each age: The genetic correlation can be low even if the heritabilities are high and vice versa. It indicates the extent to which the same genes are operating at each age regardless of the magnitude of their effect on the phenotype. As shown in 2 (95% confidence intervals shown in Supplementary Table S4 online), the genetic correlations were moderate between age 4 and the later assessments (average = 0.51), and higher between the later ages (7, 10, and 11 year average = 0.80). Environmental influences largely contribute to change across time for BMI, with low-to-moderate correlations for both shared and nonshared environmental influences; the only exception being between the ages 10 and 11 years where the correlations are higher (0.99 for shared environment and 0.48 for nonshared environment) which can be attributed to the negligible influence of shared environment at those ages (6 and 5%, respectively).
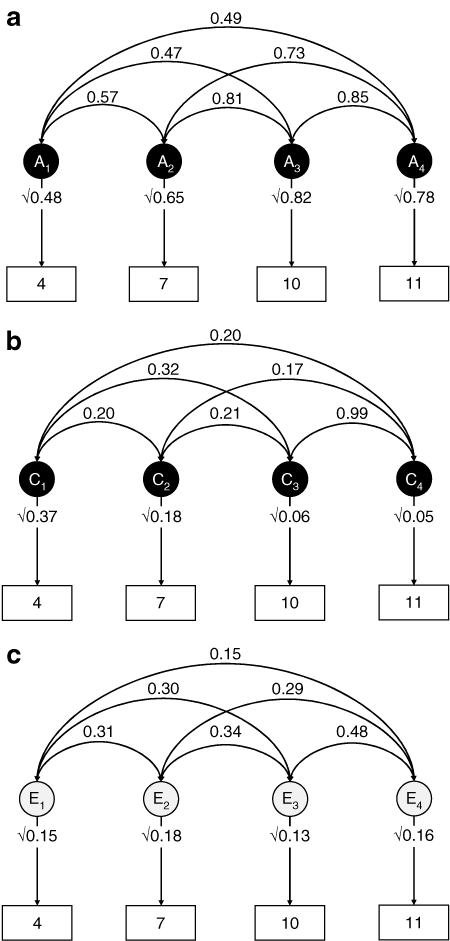
Longitudinal model-fitting analyses on BMI at 4, 7, 10, and 11 years. (a) Genetic influences, (b) shared environmental influences, and (c) nonshared environmental influences. In path diagrams the rectangular boxes refer to observed phenotypes (BMI s.d.), and the circles represent latent genetic and environmental factors; the single-headed arrows represent partial regressions of the variable on the latent factor (i.e., the relative influence of the latent variable, e.g., A, on the phenotype), and finally the curved connectors represent correlations between the connected factors. A, additive genetic influences; C, shared (common) environmental influences; E, nonshared environmental influences.
Of the phenotypic correlation between ages 4 and 7, 76% can be attributed to genetic influences. When the phenotypic correlations are higher between ages 7 and 10 years, and 10 and 11 years, 89 and 85%, respectively, can be attributed to genetic influences in common (95% confidence intervals shown in Supplementary Table S4 online).
Polymorphism in FTO gene progressively more strongly associated with BMI over childhood
Association analyses using both an additive model and allowing for nonadditive effects indicated significant associations between the FTO polymorphism and BMI at ages 7, 10, and 11 years, but not at age 4 years. Results from this analysis can be found in Table 1, which also includes mean BMI by genotype group, the P value of the additive model, the effect size (presented as R2) and the P value for the comparison between the additive and nonadditive models.
 |
At each age, the AA genotype has a higher BMI than the AT or TT genotypes. As shown in 3, the association across the three genotypes is perfectly additive at 7, 10, and 11 years. In line with the twin analyses, the effect size of the association between FTO and BMI increases with increasing age from a nonsignificant association at 4 years (0% of the variance) to significant associations at ages 7, 10, and 11 years, explaining 0.5, 0.9, and 0.8% of the variance, respectively, in BMI. The effect size at ages 10 and 11 years is equivalent to that in the original report (16). None of the associations differed significantly from an additive model (indicated by no significant P values in the comparison between the additive and nonadditive models shown in Table 1 and 3). At 7, 10, and 11 years, P values for the association under the additive model remained significant after correction for multiple testing (using a false discovery rate of 0.05 (ref. 39)). (Details regarding the association split by sex can be found in the Supplementary Note and Supplementary Table S5 online).
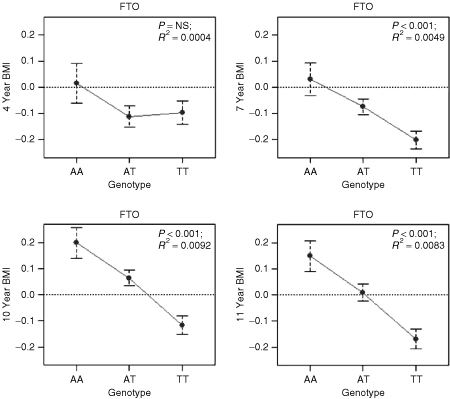
FTO association results—genotype by phenotype (BMI s.d.) at 4, 7, 10, and 11 years. FTO genotypes (AA, AT, and TT) plotted against BMI s.d. scores at ages 4, 7, 10, and 11 years. Error bars represent ± 1 s.e. In the analyses split by sex we do find a significant association at 4 years of age for girls only (see Supplementary Note and Supplementary Table S5 online) and significant associations for both boys and girls at the later ages.
FTO association parallels results from twin analyses
The genetic results from the twin analyses (using either twin correlations or model-fitting approaches) indicated increasing heritability for BMI from early-to-middle childhood; from 48% at 4 years of age to 78% at 11 years of age. Genetic correlations from the longitudinal analyses, which represent the probability of genes for one age being associated with BMI at other ages, were consistently high (average 0.65) and particularly high between the later ages (7, 10, and 11 years average = 0.80). This means that for a gene associated with BMI at 4 years there is a 50% chance that the same gene will be associated with BMI at 7, 10, and 11 years, whereas for an association at 7 years of age there is an 80% chance of finding it at 10 and 11 years of age.
The association analyses are consistent with these quantitative genetic predictions. At 4 years of age, when the heritability of BMI is lower, the association with FTO is nonsignificant, but as the heritability and genetic continuity of BMI increases at 7, 10, and 11 years, the association between FTO and BMI appears. The effect size of the association between FTO and BMI increases in parallel with the increasing heritability of BMI (see 4).
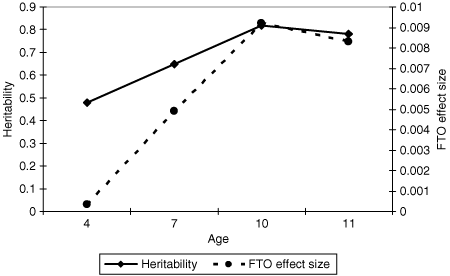
Heritability of BMI and FTO effect size. FTO association results parallel the results from the twin model-fitting analyses: As heritability of BMI increases, the effect size of the FTO association also increases.
Discussion
Using data from a large, population-based twin study, we conclude that heritable genetic influences on BMI become increasingly strong from birth to early adolescence. Results from the longitudinal analyses suggested that continuity in genetic influences largely accounted for the continuity of BMI across age. The finding of genetic continuity suggests that genes associated with BMI at one age will also be associated with BMI at other ages. This means that the increases in heritability are due to the same genes having stronger effects and not different genes acting at each age. Developmental association analyses for a polymorphism in the FTO gene directly paralleled the finding of increasing heritability, with the effect size of the association between BMI and FTO increasing with age.
Increasing heritability in childhood is seen in other traits (6). The well-documented increases in the heritability of intelligence (40) have been attributed to gene-environment correlation (6,41,42), the idea that individuals seek out environments that influence the same phenotype (e.g., bright children seek out the company of other bright children and the intellectual stimulation promotes further gains). Measures of heritability will inevitably include the variance due to genetically driven environmental exposures. In the case of obesity, it could also be argued that individuals with genetically determined tendencies to overeat might seek out situations in which there is more opportunity to overeat. However, it is also possible that gene expression changes developmentally (6), resulting in greater genetic influence on a trait. FTO is widely expressed; in a human tissue panel highest expression was in the brain and pancreatic islet (16), and future work should be able to elucidate whether gene expression changes across time, or whether the effects of expressed transcripts simply accumulate over time. In adults an inverse relationship between FTO gene expression and obesity has been found (43); however, results may be very different during adolescence, particularly during puberty. A recent animal study found that the expression of FTO in the arcuate nucleus varied as a function of nutritional status (feeding and fasting) (44). In the fasted mice, FTO mRNA levels were reduced by 60%. It would not be ethical to replicate the study in humans, but the animal findings provide support for gene-environment interplay in the association between FTO and obesity.
The high heritability of BMI and obesity (2) tells us that in the current environment some children are finding it more difficult to regulate their weight because of their genetic background. But genetic influences are only probabilistic risk factors, and must be considered alongside environmental influences. One likely possibility, mentioned earlier, is genotype-environment correlation. That is, genetic influences may be driving environmental exposure, so that throughout the life span individuals at risk for obesity find themselves in increasingly obesogenic environments as they seek out environments that are correlated with their genetic propensities.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http:www.nature.comoby
Acknowledgment
We gratefully acknowledge the ongoing contribution of the parents and children in the Twins Early Development Study (TEDS). TEDS is supported by a program grant (G0500079) from the UK Medical Research Council and the work on obesity in TEDS is supported in part by a grant from the Biotechnology and Biological Sciences Research Council (31/D19086). J.W., R.P., and S.C. conceived, designed, and funded the study. C.M.A.H. performed the statistical analyses and drafted the manuscript. E.L.M. performed the genotyping. O.S.P.D. developed the association package in R and assisted with statistical analyses. All authors contributed to the final critical revision of the manuscript.
Disclosure
The authors declared no conflict of interest.





