Postnatal Catch-up Growth After Fetal Protein Restriction Programs Proliferation of Rat Preadipocytes
Abstract
We studied the in vitro proliferation and differentiation of rat preadipocytes to investigate whether catch-up growth after prenatal protein restriction may program adipose precursor cells leading to development of increased adipose tissue mass. Pregnant rat dams were fed either an isocaloric low-protein diet (LP-8%) or control diet (C-20%). During lactation, in order to induce catch-up growth, dams from LP group were fed with the C diet and litter size was reduced to four pups instead of eight. Preadipocytes were isolated from weanling male pups (28 days of age). Differentiation and proliferation were assessed across time. At late stages of preadipocyte differentiation, no difference was observed in lipid accumulation of C or LP cultures but the mRNA expression of leptin was enhanced in LP cells. At early stages of culture, a higher DNA and protein content accompanied by a higher rate of proliferation was measured in adipocytes from LP cultures. Moreover, the mRNA expression of cyclin D1 was increased in these cells whereas the expression of peroxisome proliferators-activated receptor γ (PPARγ) and steroyl regulatory element binding protein (SREBP-1c) was significantly reduced during early stages. The results suggest that prenatal exposure to a LP followed by rapid catch-up growth is associated with a higher rate for proliferation in preadipocytes.
There is now increasing evidence that fetal environment may predispose to the development of human obesity (1). Indeed, epidemiological studies show a link between poor fetal growth and the development of impaired glucose tolerance and metabolic syndrome later in life (2,3). More direct evidence has been produced, mainly through animal models, indicating that maternal environment may influence the growth of the fetus that must adopt strategies to ensure survival, which will program its future health (4). Maternal protein restriction during gestation and lactation is a widely used model of fetal and early postnatal growth retardation. It has been shown to cause selective insulin resistance in adipocytes isolated from growth-restricted offspring indicating a programming of adipose tissue by low-protein diet (LP) (5). However, two independent laboratories realized in collaboration a study that showed no effect of protein malnutrition during gestation and/or lactation on the capacity of preadipocytes to divide or store fat in cells isolated from fetuses, neonates, and weanling offspring (6).
Nevertheless, we and others have suggested that protein malnutrition during gestation followed by nutritional excess and rapid growth during the immediate postnatal period may predispose rats and mice to develop increased adiposity in later life (7,8). Therefore, changes in fat tissue programming may be analyzed through preadipocyte cell culture. For that purpose, we studied the influence of postnatal catch-up growth in the suckling period after a fetal protein restriction on the proliferation and differentiation of preadipocytes isolated from stroma-vascular fraction of perigonadal adipose tissue of weaned male rats.
Materials and Methods
Pregnant rats were fed, throughout pregnancy, a diet containing 20% protein (control-C) or an isocaloric diet containing 8% protein (low protein-LP) purchased from Hope Farm (Woerden, Netherlands). The composition and source of diets were described elsewhere (6). To induce a forced catch-up growth, pups from LP litters were reduced to four (three males and one female) instead of eight (four of each sex) in C, and all dams were transferred to control diet (C). At day 21 of age, all pups were weaned onto a standard laboratory chow diet (Carfil Quality, Turnhout, Belgium). Male rats were killed by decapitation at 4 weeks of age. All animal procedures were performed according to the guidelines of the animal ethics committee of the Université catholique de Louvain, Belgium.
Preadipocytes were isolated from perigonadal adipose tissue of C and LP rats as described previously (6). The preadipocyte cells were cultured in 6- or 24-well plates at a density of 12 × 104 cells/cm2 in DMEM containing 4.5 g/l glucose, glutaMAX, and pyruvate (Gibco, Paisley, UK) supplemented with 10% (v:v) fetal bovine serum (Cambrex Bio Science, Verviers, Belgium) and antibiotics. The cells were maintained at 37 °C in a 5% CO2 atmosphere for 24 h. Then, the medium was changed to a differentiation medium containing DMEM with the same components as for seeding, and 14.5 nmol/l insulin and 1 nmol/l dexamethasone (Sigma, St Louis, MO) were added. The differentiation medium was changed every 48 h and preadipocytes were cultured for 9 days after inoculation.
The rate of differentiation was evaluated by scoring the lipid deposition across time with a specific fluorescent staining for intracellular lipid droplets, AdipoRed (Cambrex Bio Science, Walkersville, MD). After 4, 7, and 9 days of differentiation, cells from different wells were stained according to manufacturer's instructions for 15 min. Cells were fixed and mounted on glass slides with Vectashield (Vector Laboratories, Burlingame, CA) as mounting media containing DAPI (diamino-2-phenylindole) (Sigma, St Louis, MO) for the nuclei staining. A duplicate set of cells per individual experiment was used. Cells were examined with a Reichert Polyvar fluorescence microscope and images were captured with a Nikon DS-U1 camera. By counting a minimum of 100 cells/dish, the differentiation was assessed calculating the proportion of total cells and scaled stages of adipose conversion. Stage 0 represents nondifferentiated cells whereas stage 1 stands for cells that initiate adipogenesis. At stage 2, cells feature lipid droplets but their nucleus is not yet off-centered, a characteristic observed at stage 3.
The protein content was determined at day 4, 7, and 9 on harvested cells from independent wells using Bicinchoninic Acid Protein Assay (Pierce, Rockford, IL). DNA was measured by fluorimetry as described elsewhere (6). Cell proliferation rate was calculated by counting the DAPI-stained nuclei on microscope (Reichert Polyvar microscope) across time at day 1, 2, 3, and 4 of culture. The number of nuclei counted at day 2, 3, and 4 were expressed relative to the amount of cells counted on first day for the same cell culture.
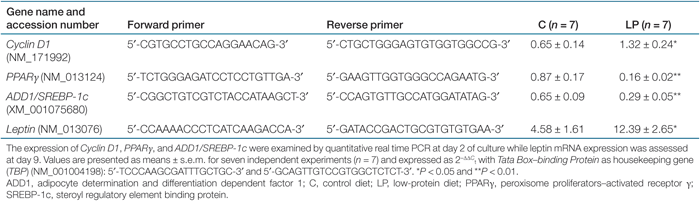
Total RNA was isolated using Tri Reagent (Sigma, St Louis, MO). One µg of total RNA was reverse transcribed in a volume of 20 µl with Super Script III First Strand Synthesis System for reverse transcriptase-PCR (Invitrogen, Carlsbad, CA). For each reverse transcriptase reaction, one sample was set up without reverse transcriptase enzyme to provide a negative control. Primers were designed using Primer Express software (Applied Biosystems, Foster City, CA) and were purchased from Sigma Genosys (Suffolk, UK) (see Table 1). Messenger RNA expression was quantified using quantitative PCR Plus for SYBR Green I (Eurogentec, Seraing, Belgium) and the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). The authenticity of the PCR products was verified by melting curve. Data were obtained as Ct values and normalized to the expression of Tata Box-binding Protein as the relative expression (ΔΔCt = ΔCt target gene - ΔCt housekeeping gene). The final values are expressed as 2−ΔΔCt for each sample.
 |
Eleven litters/group were used for a total of 11 cultures/group. Seven were used for protein and DNA contents as well as for Real-Time PCR experiments whereas four were used for cell counting at days 1–2–3 and 4. Experimental results are reported as means ± s.e.m. Data were analyzed by unpaired Student t-test using GraphPad Prism Software after testing data for normal distribution and homogeneity of variances. Differences with a P value <0.05 were considered significant. Comparison of proliferation rate has been done on areas under the curve as data are expressed as percentage.
Results
To determine whether perinatal malnutrition influenced the fate of adipose tissue precursor cells, preadipocytes isolated from perigonadal stroma-vascular fraction were cultured in differentiation conditions for 9 days. The differentiation rate was estimated across time (after 4, 7, and 9 days of culture) by rating the accumulation of triglyceride droplets in cells. As shown on 1, no significant differences were observed for C and LP differentiation rates at the three observed times and this was corroborated by the measurement of specific marker enzymes' activities (glycerol phosphate dehydrogenase and lipoprotein lipase), which did not differ between the two groups (data not shown).
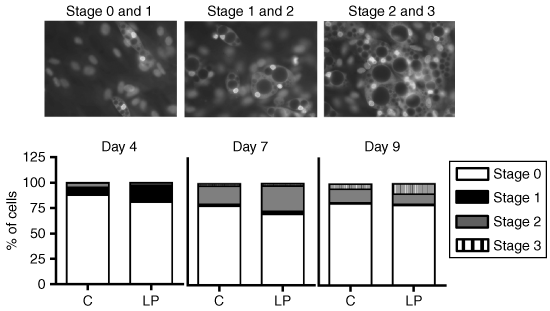
Effect of fetal protein restriction and postnatal catch-up growth on the differentiation of perigonadal stroma-vascular cells from weaned male offspring. Cell differentiation was estimated by grading the stages of lipid accumulation across time. Values are presented as percentage of the total number of cells. Data are means for seven independent cultures (n = 7) with at least two replicates per culture. C, control diet; LP, low-protein diet.
However, the analysis of leptin mRNA expression at day 9 showed a significantly enhanced level of expression in LP cells compared with C indicating that the influence of fetal growth retardation and subsequent catch-up growth may persist in LP preadipocytes even after 9 days of culture (Table 1).
The cell growth and expansion was estimated by measuring the protein and DNA content at discrete times (day 4, 7, and 9 of culture). The protein as well as DNA content was significantly higher in LP cultures at day 4 but no difference was observed for later days of culture (2). To explain this difference, the early proliferation rate was analyzed after 1, 2, 3, and 4 days after cell plating. The increase in the number of cells across time was higher for LP cultures and the area under the proliferation curve was significantly different from C (2). These results indicated an effect of the LP followed by catch-up growth on the proliferation of adipose precursor cells and this was supported by differences observed between LP and C at day 2, in the mRNA level of a regulator of cell cycle, Cyclin D1 (Table 1).
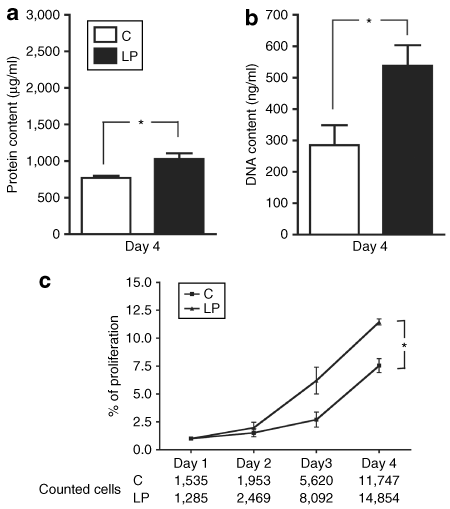
Effect of fetal protein restriction and postnatal catch-up growth on the proliferation of perigonadal stroma-vascular cells from weaned male offspring. (a) Protein and (b) DNA content were evaluated after 4 days of culture. Values are presented as means ± s.e.m. for seven independent cultures (n = 7) with at least two replicates per culture. *P < 0.05. (c) Preadipocyte proliferation rate was evaluated by counting cells across time. Values are presented as means ± s.e.m. for four independent experiments (n = 4) and expressed relatively to the cell content of each culture at day 1. Statistical significance was calculated for areas under the curve *P < 0.05. C, control diet; LP, low-protein diet.
As the LP preadipocytes showed a difference in cell growth during the early stages of culture, the levels of peroxisome proliferators-activated receptor γ (PPARγ) and adipocyte determination and differentiation dependent factor 1/steroyl regulatory element binding protein (SREBP-1c) mRNA were analyzed after 2 days of culture (Table 1). Both are important transcription factors involved in preadipocyte differentiation and expressed early during this process (9). Their expression was significantly downregulated in LP cultures.
Discussion
In this study, we looked at the mismatch of a postnatal catch-up growth after growth retardation on the in vitro differentiation and proliferation of preadipocytes isolated from perigonadal stroma-vascular cells of weaned offspring.
Our previous study showed that catch-up growth after fetal malnutrition favors the programming of overweight and adiposity in 38-week-old male rats. Moreover, the analysis of fat cell diameter in these rats showed, in parallel to hypertrophy, a second population of smaller adipocytes (10). Consistent with these data, we reported here that after lactation, preadipocytes from LP male rats presented higher early rate of proliferation marked by an increase in DNA and protein contents in LP cultures at day 4, as well as, an increase in cell number after 3 and 4 days of culture.
Similar findings were reported in a recent study where preadipocytes from fetal protein-restricted rats have an increased proliferation rate under standard culture conditions but showed reduced growth rate under poor serum conditions (11). However, no catch-up growth was induced after fetal malnutrition and the adipose precursor cells were studied at 130 days of age when rats presented visceral obesity and insulin resistance (12). In our study, we can yet observe modifications in the pool of fat precursor cells at an earlier age, showing that catch-up growth after fetal growth retardation rapidly influences preadipocytes by accelerating the proliferation rate. Furthermore, a previous report by Dugail and co-workers demonstrated an increased proliferation and differentiation in preadipocytes from normal birth weight rats that were overfed during the suckling period (13). Therefore, a mismatch of post- to prenatal growth, as well as catch-up growth of pups with low birth weight, can be deleterious for adiposity development.
Surprisingly, PPARγ and SREBP-1 mRNA levels were downregulated in LP cultures during early times compared to C. This can be explained by considering adipogenesis in a global way. Indeed, preadipocyte differentiation is a well-regulated process in which an appropriate hormonal stimulation leads to the progressive induction of morphological and physiological characteristics of adipocytes. This process, involves several key transcription factors such as adipocyte determination and differentiation dependent factor 1/SREBP-1c, CCAAT/enhancer binding proteinβ, and CCAAT/enhancer binding proteinδ, which in turn activate PPARγ and CCAAT/enhancer binding proteinα (9). Preadipocyte proliferation and differentiation are considered as mutually exclusive events. During adipogenesis, adipocyte precursors cells undergo clonal expansion, then stop proliferating, before starting the differentiation process (14). In our results, while PPARγ and SREBP-1c were decreased in preadipocytes from LP offspring, Cyclin D1 was upregulated. Cyclin D1 is an important indicator of proliferation rate, like other D-type cyclins. Together with their kinases, these proteins are necessary for entrance and progression through the G1 phase of the cell cycle (15).
Leptin mRNA analyzed at 9 days of culture presented a higher level of expression in LP cells. We and others have previously reported an increase in leptin plasma concentrations when animals were submitted to fetal malnutrition (10,16). It has also been shown that leptin mRNA level was induced by maternal protein malnutrition in offspring's adipose tissue (12). Here, the analysis of leptin mRNA level was performed after 9 days of culture and indicates a permanent influence of the fetal protein restriction and forced catch-up growth on the expression of that adipocyte-specific molecule. In addition, it has been shown that leptin could act as an autocrine factor with a proadipogenic effect as well as an increase of proliferation in rat preadipocytes (17,18). The involvement of such hormonal inducers in our animal model of catch-up growth is not known and a possible link between aberrant mitogenic factors and the subsequent increase in proliferation rate in vitro must be further investigated.
In conclusion, in this study we demonstrated that a fetal protein restriction followed by a catch-up growth induced an increase in perigonadal fat mass and programmed a higher rate for preadipocyte proliferation. These results support the adverse effect of a postnatal catch-up growth in the programming of obesity.
Acknowledgment
We thank Marie-Therèse Ahn for the technical assistance. This work was supported by the European Commission (FOOD-CT-2005-007036), the Partenon Trust (London, UK), the Belgian Fonds National de la Recherche Scientifique (FNRS), and the Belgian Fonds pour la Recherche dans l'Industrie et l'Agriculture (FRIA).
Disclosure
The authors declared no conflict of interest.





