Atherogenecity of LDL and Unfavorable Adipokine Profile in Metabolically Obese, Normal-weight Woman
Abstract
Objective: The relationship of visceral adiposity with adipocytokines and low-density lipoprotein (LDL) particle distribution and oxidation in Asian metabolically obese, normal-weight (MONW) individuals has not been evaluated. We aimed to investigate the association between visceral adiposity and adipocytokines and cardiovascular disease (CVD) risk factors in MONW Korean women with normal glucose tolerance.
Methods and Procedures: We examined the metabolic characteristics of 135 non-obese (BMI <25 kg/m2) women aged 25–64 years. Twenty-five women (BMI <25 kg/m2 and visceral fat adiposity (VFA) ≥100 cm2) were classified as MONW and 25 women (BMI <25 kg/m2 and VFA <100 cm2), pair-matched for age, weight, height, and menopausal status, as control group. Plasma lipid profiles and adipocytokines were evaluated in these two groups.
Results: MONW subjects had higher systolic (P < 0.05) and diastolic blood pressure (P < 0.005) and higher concentrations of triacylglycerol (TG) (P < 0.005), insulin (P < 0.01), and free fatty acid (FFA) (P < 0.05) than control subjects. There was no significant difference between two groups in LDL-cholesterol (LDL-C) concentrations; however, MONW subjects had smaller LDL particles (P < 0.01) and higher concentrations of oxidized LDL (ox-LDL) (P < 0.05) compared with controls. Moreover, MONW subjects had higher concentrations of tumor necrosis factor-α (TNF-α) (P < 0.05), interleukin-6 (IL-6) (P < 0.05) and leptin (P < 0.05), and lower plasma adiponectin concentrations (P < 0.05). Higher intake of saturated fat with lower ratio of polyunsaturated fatty acids (PUFAs) to saturated fatty acids (SFA) and lower fiber intake than normal subjects were found in MONW women.
Discussion: We found an unfavorable inflammatory profile and a more atherogenic LDL profile in MONW female subjects even in the absence of a known CVD risk factors. Moreover, MONW consumed more saturated fat and less fiber than the control group.
Introduction
In the 1980s, the metabolically obese, normal weight (MONW) concept was first proposed by Ruderman et al. (1,2) that individuals who have normal body weight or BMI, but who display characteristics of people with adult-onset obesity, are hyperinsulinemic, insulin-resistant, hypertriglyceridemic, and eventually predisposed to type 2 diabetes and cardiovascular disease (CVD). Since then, a considerable body of evidence suggests a high prevalence of MONW individuals in the general population (3,4). One possibility of MONW individuals showing a cluster of obesity-related metabolic abnormalities has been explained by an increase in adipocyte size in adult life (1,2). Enlarged fat cells can release more free fatty acids (FFAs) and other adipocytokines that could potentially contribute to insulin resistance (IR) (4,5). Indeed, results from computed tomography indicate that elevated abdominal visceral fat is a critical variable for this adverse metabolic profile (6,7,8).
The association between abdominal fat and increased CVD risk is supported by a number of population studies, primarily carried out in white individuals (8,9,10). Interestingly, the strength of this relation appears to be greater in women. However, much less is known for other ethnic groups. In this regard, both body composition and fat distribution are significantly different between Asian and white women, and the classification of visceral obesity has a lower threshold in the Asian region (11). Non-obese (BMI <25 kg/m2) individuals with high visceral fat area (≥100 cm2) have been categorized as MONW in the Japanese population (4,12,13,14,15). However, whereas the association between MONW and classical CVD risk factors has been well defined, the relation with other emerging risk factors such as adipocytokines and low-density lipoprotein (LDL) particles or LDL oxidation has not been evaluated. Our working hypothesis is that MONW individuals have a profile of adipocytokines, and LDL subfractions and oxidation that are consistent with an increased CVD risk compared with control women who lack the MONW phenotype.
Methods and procedures
Subjects
Healthy female subjects were recruited from a clinical study conducted by the National Research Laboratory of Clinical Nutrigenetics and Nutrigenomics (Program no. R0A-2005-000-10144-0) in Yonsei University. Among the eligible 135 non-obese (BMI <25 kg/m2) women aged between 25 and 64 years old, subjects who meet the following criteria were included in this study: (i) normal BMI (18–25 kg/m2); (ii) sedentary lifestyle (<2 h/week of structured exercise); (iii) nonsmokers; and (iv) none to low alcohol consumers. Subjects were also excluded from the study if they were observed with any history of clinical evidence of the followings: (i) CVD, peripheral vascular disease, or stroke; (ii) diabetes or impaired glucose tolerance (fasting serum glucose ≥100 mg/dl and 2-h serum glucose ≥200 mg/dl after a 75 g oral glucose tolerance test); (iii) orthopedic limitations; (iv) body weight fluctuation ±2 kg or more in the past 1 year; (v) thyroid or pituitary disease; (vi) infection by medical questionnaire examination and complete blood count; (vii) acute or chronic inflammatory disease; and (viii) medication that could affect cardiovascular function and/or metabolism. Written informed consent was obtained from all subjects, and the protocol was approved by the Institutional Review Board of Yonsei University.
Selection of MONW and control individuals
We selected individuals within the MONW group as those with BMI <25 kg/m2 and visceral fat adiposity (VFA) (≥100 cm2) according to the definition previously established by Katsuki et al. (7). The prevalence of MONW in our starting population of 135 non-obese women was 18.5%. MONW subjects (n = 25) were pair-matched to 25 controls defined as having BMI <25 kg/m2 and VFA <100 cm2. Pair-matching was based on similarities of age (within 1 year), body weight (within 2 kg), height (within 2 cm), and menopausal status (14 premenopausal and 11 postmenopausal in each group).
Anthropometric parameters, blood pressure measurements, and blood collection protocol
Body weight and height were measured in the morning, light clothed without shoes. Body weight was measured using a TBF-105 body fat analyzer (Tanita, Tokyo, Japan) and standing height was measured using a wall stadiometer. The BMI was calculated as body weight in kilograms divided by height in square meters (kg/m2). Body fat percentages were measured with a TBF-105 body fat analyzer (Tanita). Waist circumference was measured at the umbilical level with the subjects standing after normal expiration, and the hip girth was measured at the widest part of the hip, and then the waist-to-hip ratio was calculated. Blood pressure was read from the left arm of seated patients with an automatic blood pressure monitor (TM-2654; A&D, Tokyo, Japan) after 20 min of rest. The average of three measurements was recorded for each subject.
Venous blood specimens were collected in EDTA-treated and plain tubes after a 12-h fast. The tubes were immediately covered with aluminum foil and placed on ice until they arrived at the laboratory room (within 1–3 h) and were stored at −70 °C until analysis.
Assessment of abdominal fat distribution using computed tomography of the fat areas at the L1 and L4 vertebrae
Abdominal fat areas were measured by computed tomography scanning using a General Electric High Speed Advantage 9800 scanner (Milwaukee, WI). Two cross-sectional images were made for each subject; abdomen at the levels of 1st lumbar (L1) vertebra and 4th lumbar (L4) vertebra. Each computed tomography slice was analyzed for the cross-sectional area of fat using a density control program available in the standard general electric computer software. Parameters for total abdominal fat density at the levels of L1 and L4 were selected between the range of −150 and −50 Hounsfield Units (16). Total abdominal fat area was divided into visceral and subcutaneous fat areas to calculate specific fat areas.
Serum lipid profile
Fasting serum total-cholesterol and triacylglycerol (TG) were measured using commercially available kits on a Hitachi 7150 Autoanalyzer (Hitachi, Tokyo, Japan). After precipitation of serum chylomicron, LDL, and very low-density lipoprotein with dextran sulfate-magnesium, high-density lipoprotein cholesterol (HDL-C) left in the supernatant was measured by an enzymatic method. LDL cholesterol (LDL-C) was estimated indirectly using the Friedewald formula for subjects with serum TG concentrations <4.52 mol/l (400 mg/ml). Serum apolipoprotein AI and B were determined by turbidometry at 340 nm using a specific antiserum (Roche, Basel, Switzerland).
Plasma LDL particle size
Particle size distribution of LDL (d1.019–1.063 g/ml) isolated by sequential flotation ultracentrifugation was examined by a pore-gradient lipoprotein system (CBS Scientific, CA) using commercially available non-denaturing polyacrylamide slab gels containing a linear gradient of 2–16% acrylamide (Alamo Gels, San Antonio, TX). Standards of latex beads (34 nm), thyroglobulin (17 nm), apoferritin (12.2 nm), and catalase (10.4 nm) were used to estimate the relative migration rates of each band. The gels were scanned by GS-800 Calibrated Imaging Densitometer (Bio-Rad Laboratories, Graz, Austria). LDL particle size was calculated with reference to the relative migration value of the standards.
Glucose, insulin and FFAs, homeostasis model assessment–IR, and oral glucose tolerance test
Fasting glucose was measured by a glucose oxidase method using the Beckman Glucose Analyzer (Beckman Instruments, Irvine, CA). Insulin was measured by radioimmuno-assays with commercial kits from Immuno Nucleo (Stillwater, MN). FFAs were analyzed with a Hitachi 7150 autoanalyzer (Hitachi, Tokyo, Japan). IR was calculated with the homeostasis model assessment using the following equation:
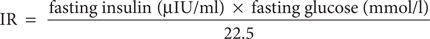
For the oral glucose tolerance test, all subjects ingested a 75-g glucose solution after an overnight fast. Serum samples were collected before and 30, 60, 90, and 120 min after the glucose load. The criteria of diagnosis and classification of diabetes mellitus developed by the American Diabetes Association (17) was used to determine whether subjects had diabetes or impaired glucose tolerance.
Definition of metabolic triad
Lamarche et al. (18) proposed that combined features of hyperinsulinemia, elevated apolipoprotein B levels, and small, dense LDL size (named “atherogenic metabolic triad”) could predict more powerfully the CVD risk beyond the traditional lipid triad. In order to assess the heightened CVD risk of MONW individuals, we compared the frequencies of traditional lipid triad (elevated TG, LDL-C, and reduced HDL-C) with atherogenic metabolic triad (elevated fasting insulin, elevated apo B, and small LDL size) between normal and MONW group. The median of each parameter in non-obese subjects (n = 35) who were not diagnosed with metabolic syndrome used as cutoff points to characterize those triads. The traditional lipid triad was defined as subjects who have TG ≥99 mg/dl, LDL-C ≥121.2 mg/dl, and HDL-C ≤54 mg/dl and atherogenic metabolic triad, subjects who have insulin ≥6.80 mU/l, apo B ≥82 mg/dl, and LDL size ≤26.3 nm.
CRP, IL-6, TNF-α, and adiponectin concentrations
Serum high-sensitive C-reactive protein (CRP)was measured with an Express+ autoanalyzer (Chiron Diagnostics, Walpole, MA) using a high-sensitivity CRP-Latex (II) X2 kit (Seiken Laboratories, Tokyo, Japan) that allowed detection of CRP levels as low as 0.001 mg/dl and as high as 32 mg/dl. Serum concentrations of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were measured, in duplicate, using an enzyme immunoassay (R&D systems, MN) according to manufacturers instructions. Plasma adiponectin concentration was measured using an enzyme immunoassay (Human Adiponectin ELISA kit, B-Bridge International, CA). All these assays were read using a Victor2 (Perkin Elmer life sciences, Turku, Finland) at 450 nm and wavelength correction was set to 540 nm.
Plasma ox-LDL and serum leptin
Plasma ox-LDL was measured using an enzyme immunoassay (Mercodia, Uppsala, Sweden) using similar conditions to those described above for adipokines. Serum leptin was measured using Packard Cobra II 5005 R-Counter with the human leptin RIA kit from Linco.
Assessment of food intake and physical activity level
Information about habitual food intake was obtained using both a 24-h recall method and a semiquantitative food frequency questionnaire. We used the former to carry out analyses and the latter to check whether the data collected by 24-h recall methods was representative of the usual dietary pattern. Nutrient intake data were calculated as mean values from a 3-day food record (2 week days and 1 weekend) through the 24-h recall method using the database of the computerized Korean food-code, based on food composition tables by National Rural Living Science Institute (7th edn, 2000) in Korea. Total energy expenditure (TEE) (kcal/day) was calculated from activity patterns, including basal metabolic rate, physical activity for 24 h (19), and specific dynamic action of food. Basal metabolic rate for each subject was calculated with the Harris–Benedict equation (20).
Statistical analysis
Statistical analyses were performed with SPSS version 12.0 for Windows (Statistical Package for the Social Science, SPSS, Chicago, IL). An independent t-test was used to compare the differences between normal controls and MONW individuals. Frequency distributions were tested by χ2 test between groups. It was examined whether each variable presented normal distribution before statistical testing, and then logarithmic transformation was performed on the skewed variables. For descriptive purposes, mean values are presented using untransformed and unadjusted values. Results are expressed as mean ± s.e. A two-tailed value of P < 0.05 was considered statistically significant.
Results
Clinical characteristics and body fat distribution of the study groups
Table 1 shows age, BMI, body weight, and height for both controls and MONW individuals. By design, these variables were identical for these two groups of women. Likewise, both groups were comparable for body fat percent, waist and hip circumferences and waist-to-hip ratio. There were no between-group differences in subcutaneous fat areas at L1 and L4. However, visceral fat areas at L1 (P < 0.001) and L4 (P < 0.001), as well as systolic (P < 0.05) and diastolic (P < 0.005) blood pressure were significantly higher in MONW individuals compared with controls.
| Normal subjects (n = 25) | MONW subjects (n = 25) | |
|---|---|---|
| Age (years) | 49.0 ± 1.56 | 49.2 ± 1.73 |
| Height (cm) | 158.6 ± 0.84 | 158.8 ± 0.96 |
| Weight (kg) | 61.0 ± 0.70 | 61.8 ± 0.77 |
| BMI (kg/m2) | 24.2 ± 0.13 | 24.5 ± 0.12 |
| Fat (%) | 31.5 ± 0.65 | 33.1 ± 0.82 |
| Waist (cm) | 85.0 ± 1.07 | 87.7 ± 1.11 |
| Hip (cm) | 95.8 ± 0.64 | 96.7 ± 0.63 |
| Waist hip ratio | 0.89 ± 0.01 | 0.91 ± 0.01 |
| Metabolic syndromea (%) | 24 | 34 |
| 1st lumbar vertebra | ||
| Visceral fat (cm2) | 77.8 ± 4.97 | 107.1 ± 5.34*** |
| Subcutaneous fat (cm2) | 118.8 ± 5.50 | 131.0 ± 4.76 |
| Visceral/subcutaneous fat ratio | 0.66 ± 0.04 | 0.83 ± 0.04** |
| 4th lumbar vertebra | ||
| Visceral fat (cm2) | 64.8 ± 2.67 | 107.6 ± 2.89*** |
| Subcutaneous fat (cm2) | 174.9 ± 7.73 | 189.7 ± 6.13 |
| Visceral/subcutaneous fat ratio | 0.39 ± 0.02 | 0.58 ± 0.03*** |
| Blood pressure (BP) | ||
| Systolic BP (mm Hg) | 112.3 ± 3.06 | 121.4 ± 3.27* |
| Diastolic BP (mm Hg) | 70.4 ± 2.72 | 81.6 ± 2.65** |
- Mean ± s.e.
- MONW, metabolically obese, normal weight.
- a Defined by guidelines of the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) criteria
- * P < 0.05
- ** P < 0.01
- *** P < 0.001 compared with normal subject group.
Metabolic characteristics and adipocytokines levels
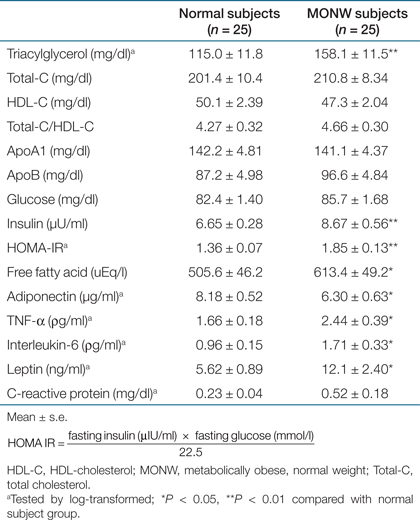
Metabolic variables, including inflammatory biomakers and other adipocytokines are presented in Table 2. MONW individuals had higher concentrations of TG (P < 0.005), insulin (P < 0.01), homeostasis model assessment–IR (P < 0.01), and FFA (P < 0.05). However, no significant differences between groups were noted for total-cholesterol, LDL-C and HDL-C, apo A1, apo B, as well as glucose plasma concentrations. In regard to LDL profile, there was no difference between groups for LDL-C concentrations (128.3 ± 9.99 mg/dl vs. 131.9 ± 7.44, P = 0.77); however, MONW subjects showed smaller LDL particles (26.4 ± 0.18 nm vs. 25.6 ± 0.22, P < 0.01) and higher concentrations of ox-LDL (31.8±3.67 U/l vs. 46.3 ± 5.56, P < 0.05) compared with normal controls (1). Atherogenic metabolic triad of elevated fasting insulin, apo B, and small, dense LDL size were 3.3-fold more prevalent (40%) in the MONW group than in normal subjects (12%, P < 0.01), whereas it did not change the proportion of traditional lipid triad of increased TG, LDL-C, and reduced HDL-C levels between normal and MONW group (24% vs. 36%). MONW subjects showed higher concentrations of TNF-α (P < 0.05), IL-6 (P < 0.05), leptin (P < 0.05) and CRP (nonsignificant trend) but lower plasma concentrations of adiponectin (P < 0.05) compared with normal controls.
 |
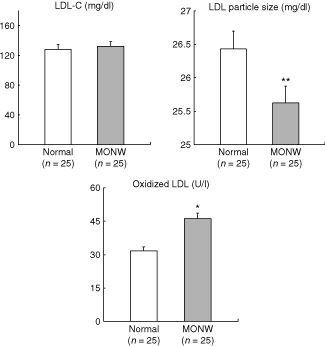
: LDL-cholesterol, LDL particle size, and oxidized LDL in normal and MONW female subjects. Data are presented as mean ± s.e. *P < 0.01 compared with normal subjects.
Habitual total calorie and nutrient intakes
There was no significant difference between groups in total calorie intake and the proportion of energy intake derived from macronutrients. However, MONW individuals showed higher intake of saturated fat with lower ratio of polyunsaturated fatty acids (PUFA) to saturated fatty acids (SFA) and lower fiber intake than control subjects (Table 3). In addition, SFA intake was positively associated with VFA (r = 0.383, P < 0.05) and IL-6 concentration (r = 0.421, P < 0.05).
| p subjects (n = 25) | MONW subjects (n = 25) | |
|---|---|---|
| Total calorie intake (kcal) | 2003 ± 26 | 2034 ± 28 |
| Total energy expenditure (kcal) | 1978 ± 32 | 2007 ± 44 |
| % of carbohydrates | 62.5 ± 0.95 | 60.9 ± 1.24 |
| % of protein | 16.3 ± 0.66 | 16.9 ± 0.57 |
| % of fat | 21.4 ± 0.63 | 22.2 ± 1.02 |
| SFA (g) | 11.6 ± 1.36 | 16.7 ± 2.20* |
| MUFA (g) | 13.9 ± 1.08 | 11.5 ± 1.27 |
| PUFA (g) | 12.6 ± 0.82 | 10.3 ± 0.95 |
| PUFA/SFA | 1.38 ± 0.18 | 0.76 ± 0.13* |
| Fiber (g) | 16.5 ± 1.01 | 12.2 ± 1.08** |
- Mean ± s.e.
- MONW, metabolically obese, normal weight; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; SFA, saturated fatty acids.
- * P < 0.05
- ** P < 0.01 compared with normal subject group.
Discussion
In this study, MONW women, characterized by a non-obese BMI (<25 kg/m2) but a high VFA (≥100 cm2), showed a known metabolic disturbances such as higher blood pressure and higher concentrations of FFA, TG, and insulin compared with control women (BMI <25 kg/m2 and VFA <100 cm2). Furthermore, the MONW phenotype was associated with a more atherogenic LDL profile and an unfavorable pattern of adipocytokines. This cluster of obesity-related abnormalities revealed in MONW women may be driven by high visceral fat accumulation rather than total adiposity or simple central fat, because age, BMI, and menopausal status matched normal female subjects showing no significant differences in body fat percent, waist circumference and waist-to-hip ratio from MONW female subjects. Interestingly, MONW women displayed a dietary pattern consistent with higher consumption of saturated fat, lower ratio of PUFA to SFA, and lower fiber intake compared with control women.
In an extensive review of MONW individuals, Ruderman et al. (4) have noted that most MONW individuals have some increase in adipose tissue mass compared with normoinsulinemic individuals of similar height and weight, possibly due to weight gain in adult life. It has become clear that elevated abdominal visceral fat can be an intriguing factor contributing to metabolic obesity in normal-weight individuals (4,10,21). However, it is not fully understood how much increases in adipose mass related to metabolic derangements of the IR. Katsuki et al. (7) clearly showed visceral fat accumulation as a major contributor to the development of IR in Japanese MONW subjects with normal glucose tolerance. An expanded visceral adipose depot, due to its particular metabolic characteristics has been associated with impaired FFA metabolism, including high circulating FFA concentrations. The flux of FFA toward the liver favors an increased TG secretion and reduced hepatic degradation of insulin, leading to hypertriglyceridemia and hyperinsulinemia (22).
Hypertriglyceridemia, resulting from abnormalities in TG and hepatic lipase metabolism or high-carbohydrate intake is associated with the presence of small LDL particles (23). In our study, the presence of smaller LDL particles in MONW women compared with controls is unlikely to be due to differences in carbohydrate intake, as this was not different between groups. Moreover, the difference in LDL particles distribution was present even though LDL-C concentrations were similar for both groups. This is consistent with a previous report in viscerally obese patients (24).
As mere simple measurement of the cholesterol content of a lipoprotein particle such as total-cholesterol, LDL-C, and HDL-C failed to detect individuals at high risk for coronary artery disease (18), additional metabolic features to predict coronary heart disease risk has been investigated. The results of the Quebec Cardiovascular study have shown that simultaneous measurement of insulinemia, apolipoprotein B, and LDL particle size (called, “atherogenic metabolic triad”) could predict more powerfully the coronary heart disease risk beyond the traditional lipid triad of TG, LDL-C, and HDL-C (18). There is a metabolic rationale linking the expanded abdominal visceral depot to liver IR, hyperinsulinemia, increased secretion of TG-rich lipoproteins, reduced hepatic degradation of apolipoprotein B, and to the formation of small LDL and HDL particles (24). In this study, MONW subjects who have a high abdominal visceral fat showed 3.3-fold higher prevalence of atherogenic metabolic triad than normal subjects, whereas no difference was observed in the frequency of traditional lipid triad between groups. This result indicated that increased visceral adipose fat can be predictive of an increased risk of coronary heart disease beyond the presence of traditional risk factors, suggesting that the atherogenic metabolic triad provides a better approach to identify MONW individuals. However, further prospective studies in large-scale populations are needed to confirm it.
Other studies have shown that levels of circulating ox-LDL may predict future cardiovascular events and suggested that high ox-LDL is more likely a cause rather than a consequence of CVD (25,26,27). A multiethnic study (28) has found that dyslipidemia, inflammation, gender, ethnicity, and current smoking are the strongest correlates of circulating ox-LDL. Thus, our data suggest that higher concentrations of ox-LDL might be partly related to increased inflammation in MONW individuals, since most of those factors (same gender, homogeneous ethnicity, and absence of smoking) were fixed in both groups. In fact, previous studies have shown the association of ox-LDL levels with circulating concentrations of IL-6 (29), CRP (30), and fibrinogen (28). Leptin, another protein secreted by adipocytes, was also found to have a relation with circulating ox-LDL (31).
Higher concentrations of inflammation biomarkers, TNF-α and IL-6, in the MONW group could be the result of the larger visceral fat areas in this group, as other anthropometric characteristics were not significantly different between groups. This is consistent with the current knowledge supporting that visceral rather than subcutaneous adipose tissue represents the main source of adipocytokines, thus supporting the correlation between visceral fat and both IR and CVD (32,33). The moderately high concentrations of inflammatory markers observed in our MONW group are consistent with a state of chronic low-grade inflammation similar to that observed in more apparently obese subjects. This is also consistent with the reduced plasma concentration in the MONW group of an anti-inflammatory factor such as adiponectin.
Adipocytokine secretion may be regulated by the nutritional state (34). Thus, stearic acid intake was associated with increased fibrinogen concentrations (35), and the ratios of PUFA to SFA in plasma phospholipids and cholesterol esters showed an inverse association with IL-6 (36). In addition, a dietary pattern high in processed meats and refined grains and low in cruciferous and yellow vegetables correlated positively with inflammatory markers, including IL-6 or TNF-α (37). Consistent with this notion, MONW women in our study showed higher consumption of saturated fat with lower ratio of PUFA to SFA (1.38 vs. 0.76) and lower fiber intake (17 g/day vs. 12 g/day) compared with control women, despite similar percent energy intakes of proteins, fats, and carbohydrates.
Our results indicate the associations between dietary habits and inflammatory markers, despite the much lower percent of energy intake from fat in our study subjects and in the Korean population at large (38), compared with those reported in white populations. Korean adults have increased the percent of calories from fat in diets from 6% in 1969 to 20% in 2005 (38), and the mortality rate (per 100,000 of the population) as a result of ischemic heart disease has rapidly increased form 6.8% in 1988 to 27.5% in 2005 (39). The increase in CVD rates is partially related to the rapid increase in the number of elderly subjects; however, the increase of fat intake and abdominal fat accumulation in Korean parallel also the disease trends. The observation that the increases in disease rates take place in the context of dietary habits considered healthy in white populations suggest that the Korean population may be genetically predisposed to be negatively affected by adverse dietary changes at a lower threshold than whites.
In conclusion, we observed the presence of unfavorable inflammatory profile and a more atherogenic LDL in MONW female subjects, even in the absence of known CVD risk factors such as elevated concentrations of total-cholesterol and LDL-C, decreased concentrations of HDL-C, and smoking. Interestingly, MONW women consumed more animal fats and less vegetables and whole grains than control women (data not shown), suggesting the change of dietary composition as a potential therapeutic approach to treating MONW individual. However, our study has some limitations. Because of its cross-sectional design, we cannot establish the directionality of the associations. For example, whereas the general assumption is that inflammation is consequent to increased visceral fat accumulation (32,33), it has been suggested that obesity is a result of inflammatory disease (40). Despite this limitation, the atherogenecity of LDL and unfavorable inflammatory status in MONW subjects with altered metabolic profile could be a prognostic indicator of the risk of CVD and type 2 diabetes. Therefore, it is important to identify and treat these individuals before these diseases become overt. Lifestyle interventions, including diet combined with exercise therapy can easily reduce visceral fat and CVD risk factors, which in turn prevent CVD and type 2 diabetes, especially before they have begun to develop.
Disclosure
The authors declared no conflict of interest.
Acknowledgments
We sincerely thank research subjects who participated in the studies described in this report. We also thank the support of Korea Science and Engineering Foundation (KOSEF), the Korea government Ministry of Science and Technology (M10642120002-06N4212-00210), National Research Laboratory project no. R0A-2005-000-10144-0, Ministry of Science and Technology, Korea Health 21 R&D Projects, Ministry of Health & Welfare (A000385, A020593, A050376), Korea Research Foundation Grant funded by Korea Government (MOEHRD, Basic Research Promotion Fund) (KRF-2006-311-C00640), Brain Korea 21 Project, Yonsei University College of Human Ecology, Yonsei University, and Contracts 53-K06-5-10 and 58-1950-9-001 from the US Department of Agriculture Research Service on this study.





