Differential Regulation of Adiponectin Receptor Gene Expression by Adiponectin and Leptin in Myotubes Derived from Obese and Diabetic Individuals
Abstract
Objective: This study aimed to investigate the regulation of adiponectin receptors 1 (AdipoR1) and 2 (AdipoR2) gene expression in primary skeletal muscle myotubes, derived from human donors, after exposure to globular adiponectin (gAd) and leptin.
Research Methods and Procedures: Four distinct primary cell culture groups were established [Lean, Obese, Diabetic, Weight Loss (Wt Loss); n = 7 in each] from rectus abdominus muscle biopsies obtained from surgical patients. Differentiated myotube cultures were exposed to gAd (0.1 μg/mL) or leptin (2.5 μg/mL) for 6 hours. AdipoR1 and AdipoR2 gene expression was measured by real-time polymerase chain reaction analysis.
Results: AdipoR1 mRNA expression in skeletal muscle myotubes derived from Lean subjects (p < 0.05) was stimulated 1.8-fold and 2.5-fold with gAd and leptin, respectively. No increase in AdipoR1 gene expression was measured in myotubes derived from Obese, Diabetic, or Wt Loss subjects. AdipoR2 mRNA expression was unaltered after gAd and leptin exposure in all myotube groups.
Discussion: Adiponectin and leptin are rapid and potent stimulators of AdipoR1 in myotubes derived from lean healthy individuals. This effect was abolished in myotubes derived from obese, obese diabetic subjects, and obese-prone individuals who had lost significant weight after bariatric surgery. The incapacity of skeletal muscle of obese and diabetic individuals to respond to exogenous adiponectin and leptin may be further suppressed as a result of impaired regulation of the AdipoR1 gene.
Introduction
The adipokines adiponectin and leptin contribute to whole-body substrate use and appetite control (1, 2) and are both potential mediators of skeletal muscle metabolic activity. Adiponectin, also known as Acrp30, adipoQ, apM-1, and GBP28 [reviewed by Kadowaki and Yamauchi (3)], is a 30-kDa protein that is secreted exclusively in differentiated adipocytes. Adiponectin increases fatty acid transport and β-oxidation in skeletal muscle (4, 5). These actions require the phosphorylation and activation of adenosine monophosphate-activated protein kinase (AMPK)1 (6, 7), although adiponectin-mediated increase in peroxisome proliferator-activated receptor α transcriptional activity may also be involved (4). Unlike the majority of cytokines, adiponectin is reduced in insulin-resistant states, including obesity, type 2 diabetes, and coronary artery disease (8, 9, 10, 11, 12). In addition to the suppressed circulating plasma adiponectin evident in these disease states, we have recently demonstrated reduced activation of AMPK and fatty acid β-oxidation in response to exogenous globular adiponectin in cultured primary myotubes derived from obese and type 2 diabetic subjects, when compared with myotubes derived from lean subjects (5). These data indicate that, in addition to reduced circulating adiponectin, there is also defective adiponectin signaling in the skeletal muscle of obese and diabetic individuals.
Adiponectin receptor binding is mediated by two distinct receptors, adiponectin receptors 1 (AdipoR1) and 2 (AdipoR2). AdipoR1 is expressed preferentially in skeletal muscle and demonstrates higher affinity for globular adiponectin (gAd) (4). Conflicting data exist on the expression of AdipoR1 and AdipoR2 in the skeletal muscle of obese or type 2 diabetic subjects (13, 14). Other reports demonstrate a significant negative correlation with insulin sensitivity for either AdipoR1 alone (15) or both AdipoR1 and AdipoR2 (16). The gene expression of both receptor isoforms is increased by fasting and reduced by re-feeding, providing further evidence of physiological regulation (17). However, the expression of AdipoR1 and AdipoR2 is not regulated by insulin exposure, fibrates, or thiazolidinediones in primary cultured myotubes derived from human subjects (15, 18, 19). The possibility that adiponectin itself is a regulator of AdipoR1 and AdipoR2 expression has yet to be determined. We, therefore, examined the expression of AdipoR1 and AdipoR2 in the primary cultured myotubes derived from a cohort of individuals who were lean, obese, type 2 diabetic, or had lost a significant amount of weight after weight loss surgery. Furthermore, the actions of gAd on the expression of the AdipoR1 and AdipoR2 genes in these myotubes were measured.
Like adiponectin, leptin also activates AMPK and up-regulates skeletal muscle β-oxidation (20). These actions of leptin seem synergistic to that of adiponectin, because combined administration of adiponectin and leptin normalizes insulin sensitivity in lipoatrophic mice, whereas this is only partially achieved by either adiponectin or leptin alone (21). There is also cross-talk at the transcriptional level, with leptin treatment in ob/ob mice and in adipocyte cell cultures directly increasing adiponectin mRNA expression and secretion (22). For this reason, we have tested the actions of leptin on the expression of the AdipoR1 and AdipoR2 genes in primary cultured myotubes.
Research Methods and Procedures
Source of Human Skeletal Muscle
Individuals undergoing routine surgical treatment were recruited to participate in this study. People with diabetes (Diabetic) and obese patients (Obese) were recruited during lap-banding surgery. Individuals who were undergoing surgical treatment for replacement or re-adjustment of the Lap-Band (Inamed Health-Australia, Gordon, New South Wales, Australia) after weight loss following the initial lap-banding surgery (Wt loss) were also recruited. In addition, lean individuals who were undergoing routine gastric surgery (Lean) were recruited. Subject characteristics are detailed in Tables 1and 2. Written informed consent was obtained from all subjects. This study was approved by the Human Ethics Research Committee of Deakin University, The Avenue Hospital, and Cabrini Hospital, Melbourne, Victoria, Australia. A muscle sample was obtained from the rectus abdominus muscle, after a fast (8 to 18 hours) and after general anesthesia was induced with a short-acting propofol and maintained by a fentanyl, rocuronium, and volatile anesthesia mixture.
| Group | ||||
|---|---|---|---|---|
| Lean | Obese | Diabetic | Wt Loss | |
| Age (years) | 44.1 ± 3.8 | 36.2 ± 4.1‡ | 50.1 ± 1.9† | 34.8 ± 3.0‡ |
| Sex | 2 Male | 2 Male | 3 Male | 2 Male |
| 5 Female | 5 Female | 4 Female | 5 Female | |
| Weight (kg) | 62.8 ± 3.7†‡ | 136.5 ± 18.1* | 113.3 ± 3.9* | 91.7 ± 7.5† |
| BMI (kg/m2) | 21.7 ± 0.7†‡ | 47.3 ± 4.5* | 38.8 ± 1.8* | 31.7 ± 1.4*† |
| Fasting blood glucose (mM) | 5.2 ± 0.1‡ | 5.0 ± 0.1‡ | 8.3 ± 1.0*† | 4.7 ± 0.1‡ |
| Fasting blood insulin (μU/liter) | 4.2 ± 1.2†‡ | 15.7 ± 2.6*‡ | 22.9 ± 2.0*† | 8.7 ± 1.0‡ |
- Donors derived from Lean, Obese, Diabetic, and individuals who lost a significant amount of weight (Wt Loss) groups. Data are mean ± standard error.
- * Different from lean group (p < 0.05).
- † Different from obese group (p < 0.05).
- ‡ Different from diabetic group (p < 0.05).
| Fasting blood | Fasting blood | ||||
|---|---|---|---|---|---|
| Group | Age (years) | Weight (kg) | BMI (kg/m2) | glucose (mM) | insulin (μU/liter) |
| Wt loss group presurgery | 29.8 ± 2.9 | 141.3 ± 12.3 | 49.3 ± 4.3 | 5.1 ± 0.2 | 21.9 ± 7.8 |
| Wt loss group at time of collection | 34.8 ± 3.0* | 91.7 ± 7.5* | 31.7 ± 1.4* | 4.7 ± 0.1 | 8.7 ± 1.0 |
- Data presented as mean ± standard error.
- * Different from pre-surgery (p < 0.05).
Primary Skeletal Muscle Cell Culture
Primary skeletal muscle cell culture was established according to the method described by Blau and Webster (23) and modified by Gaster et al. (24). All cell culture reagents were purchased from Gibco (distributed by Invitrogen, Mount Waverley, Victoria, Australia) unless otherwise stated. In brief, muscle samples, approximately 50 to 100 mg, were washed in serum-free α-minimal essential medium (α-MEM), minced, and enzymatically dissociated by a series of incubations in 0.05% trypsin/EDTA. Fetal bovine serum was added to the supernatant collected before filtration through a 100-μm cell filter (BD Biosciences, Melbourne, Victoria, Australia). Cells were then collected by centrifugation and re-suspended in growth medium. Growth medium contained α-MEM supplemented with 10% fetal bovine serum (vol/vol), 0.5% penicillin (vol/vol), and 0.5% amphotericin B (Fungizone; Invitrogen) (vol/vol). Cells were cultured on an uncoated flask for 30 minutes before the cell media were transferred to a flask coated with essential coating medium (ECM) (Sigma, Sydney, New South Wales, Australia). Growth medium was changed every other day until cells reached ∼70% confluency. Cells were then passaged once more before storage in liquid nitrogen.
Subsequently, the cells were thawed and seeded onto an ECM-coated flask and grown until ∼70% confluency before trypsinization and seeding onto ECM-coated 6-well plates (Greiner; distributed by Interpath Services, West Heidelburg, Victoria, Australia) for experimental treatment. All cells were treated during Passage 4. Before experimental treatment, the cells were grown to confluency before differentiation for 4 days in α-MEM, 2% horse serum (vol/vol), 0.5% penicillin (vol/vol), and 0.5% Fungizone (vol/vol). The evening before experimental treatment, the cells were serum-starved overnight in α-MEM. The cells were exposed to gAd (0.1 μg/mL), leptin (2.5 μg/mL), or 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) (2 mM) for 6 hours. Recombinant gAd was produced as a C-terminal glutathione-S-transferase fusion protein by the method previously reported (5). The selected doses of gAd and AICAR were chosen on the basis of previously published data on maximal intracellular activation of AMPK phosphorylation (6). Similarly, the leptin dose was also chosen on the basis of maximal activation of AMPK in cultured human muscle cells (unpublished data).
Real-Time Polymerase Chain Reaction
After 6 hours of exposure to gAd, leptin, or control (α-MEM), the medium was removed and cells were rinsed with 1× phosphate-buffered saline. RNABee (Tel-Test, Friendswood, TX) was added to the cells to lyse the cells. The RNABee homogenate was then stored at −80 °C before total cellular RNA extraction.
First-strand cDNA was generated from 0.5 μg RNA using an AMV RT kit (Promega, Madison, WI). Real-time polymerase chain reaction was performed using the ABI PRISM 5700 sequence detection system (Applied Biosystems, Foster City, CA). Polymerase chain reactions were performed using SYBR Green I chemistry (Applied Biosystems). Oligonucleotide primers were: AdipoR1: CGCCATGGAGAAGATGGAA, TCATATGGGATGACCCTCCAA; AdipoR2: GGATCCCCGAACGCTTTTT, TGAGACACCATGGAAGTGAACAA; cyclophilin: CATCTGCACTGCCAAGACTGA, TTCATGCCTTCTTTCACTTTGC, forward and reverse primers, respectively) designed using Primer Express software package, version 1.0 (Applied Biosystems) from GenBank sequences (AdipoR1: NM-015999; AdipoR2: NM-024551; cyclophilin: X52851). Fluorescence emission data were captured, and gene expression was determined using the critical threshold (CT) values for each gene, with all data expressed relative to the housekeeping gene (cyclophilin) using the 2−ΔCT equation, as arbitrary units (25).
Statistical Analysis
All data are presented as means ± standard error of the mean. Paired Student's t tests were performed on the samples to compare treatment vs. control. Analyses of differences between subject characteristics were conducted by means of a one-way ANOVA with Tukey post hoc tests. Significance was accepted when p < 0.05.
Results
Subject Characteristics
The characteristics of the subject groups are shown in Table 1. The Diabetic group was significantly older than the Obese and Wt Loss groups (p < 0.05). The Lean group had a lower BMI than the Wt Loss group (p < 0.05), but both of these groups had a lower weight and BMI than either the Obese or Diabetic groups (p < 0.05). The Diabetic group had elevated fasting blood glucose and insulin compared with all other groups (p < 0.05). The Obese group also had elevated insulin levels compared with the Lean group (p < 0.05).
The results in Table 2 demonstrate that the Wt Loss group, on average, had undergone the original Lap-Band surgery 5 years earlier and had lost an average of nearly 50 kg during this time.
Adiponectin Receptor mRNA Analysis
There were no significant differences in baseline mRNA from untreated myotubes for AdipoR1 (Lean: 1.0 ± 0.17; Obese: 2.0 ± 0.41; Diabetic: 1.2 ± 0.23; Wt Loss: 1.6 ± 0.41, in arbitrary units) or AdipoR2 (Lean: 0.25 ± 0.09; Obese: 0.49 ± 0.16; Diabetic: 0.23 ± 0.04; Wt Loss: 0.50 ± 0.15, in arbitrary units). Stimulation of skeletal muscle myotubes with gAd resulted in a 1.8-fold increase in AdipoR1 mRNA expression in the myotubes derived from Lean individuals alone (p < 0.05) (Figure 1). In contrast, there was no increase in AdipoR1 mRNA in response to gAd treatment in the Obese, Diabetic, and Wt Loss subject groups. AdipoR2 mRNA expression was not significantly different in response to gAd in myotubes derived from all subject groups (Figure 1). There was a tendency for adiponectin to reduce AdipoR2 mRNA in the non-lean groups, but this was not statistically significant.
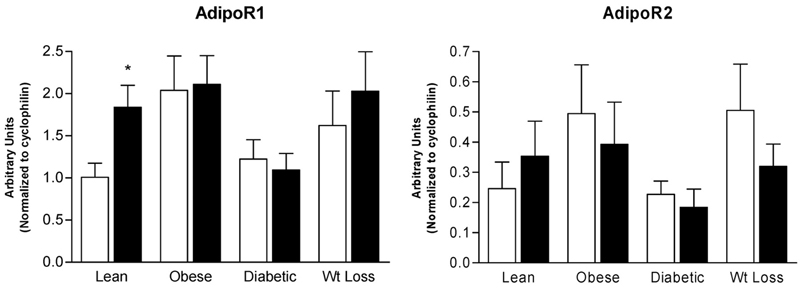
mRNA expression of adiponectin receptors AdipoR1 and AdipoR2 after treatment with gAd (closed bars, 0.1 μg/mL) for 6 hours in cultured skeletal muscle myotubes derived from Lean, Obese, Diabetic, and Wt Loss subjects. Open bars, control samples. Data are shown as means ± standard error of the mean (n = 7) and are expressed in arbitrary units. * significantly different from control sample (p < 0.05).
Treatment of skeletal muscle myotubes with leptin resulted in nearly a 2.5-fold increase in AdipoR1 mRNA expression in the myotubes derived from Lean individuals (p < 0.05) (Figure 2) but not in the other groups. No significant effects of leptin on AdipoR2 mRNA expression were detected in myotubes derived from any of the subject groups; there was, however, a tendency (p = 0.068) for leptin to decrease AdipoR2 mRNA in the Wt Loss group (Figure 2), as was observed for adiponectin (Figure 1).
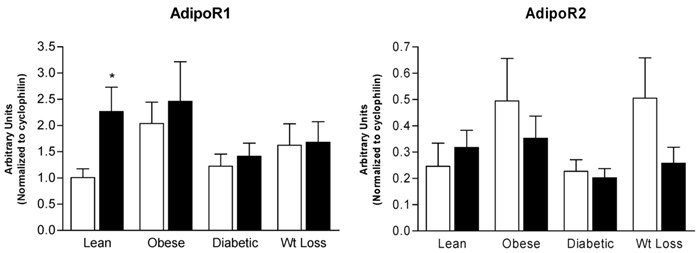
mRNA expression of adiponectin receptors AdipoR1 and AdipoR2 after treatment with leptin (closed bars, 2.5 μg/mL) for 6 hours in cultured skeletal muscle myotubes derived from Lean, Obese, Diabetic, and Wt Loss subjects. Open bars, control samples. Data are shown as means ± standard error of the mean (n = 7) and are expressed in arbitrary units. * significantly different from control sample (p < 0.05).
AICAR treatment of the myotubes failed to alter the gene expression of either AdipoR1 or AdipoR2 in all donor groups (data not shown).
Discussion
The adipokines adiponectin and leptin are important regulators of skeletal muscle oxidative metabolism (2, 3). The actions of adiponectin in skeletal muscle are mediated by two adiponectin receptor isoforms, AdipoR1 and AdipoR2. A 45% decrease in AdipoR1 gene transcription has been reported for transformed lymphocytes from African-American individuals with diabetes, but there was no difference observed between white controls and diabetics (13). Differences in AdipoR1 single nucleotide polymorphism allele expression have been observed, but these have not been related to diabetic condition (13). The present study, consistent with that of Debard et al. (14), did not detect differences in the expression of either AdipoR1 or AdipoR2 in primary cultured myotubes from obese, diabetic, and formerly obese subjects. However, there appeared to be a tendency for greater expression of both receptor isoforms in the myotubes cultured from obese donors, which may partially explain the lack of responsiveness in these samples after stimulation with gAd or leptin. The key finding from the present study was the rapid and marked activation of AdipoR1 gene expression in cultured myotubes from lean, but not obese, diabetic, or even weight-loss individuals after both gAd and leptin treatment. No actions of either gAd or leptin were observed on AdipoR2. These data indicated a novel positive regulation of AdipoR1 to both gAd and leptin in myotubes derived from healthy individuals and demonstrated that this positive regulation is abolished in insulin-resistant states. Thus, this defective gene regulation may contribute to the blunted activation of β-oxidation and AMPK phosphorylation that we observed after gAd treatment in human primary cultured myotubes from obese and type 2 diabetic individuals, which was overcome by pharmacological activation of AMPK (5).
AdipoR1 and AdipoR2 are distinct receptors encoded on separate genes, with a 76% homology in the mouse genome (4). Both receptors are widely expressed, although AdipoR1 is most predominant in skeletal muscle (4). In the present study, the expression of AdipoR1 was approximately 4-fold greater than that of AdipoR2, similar to previous data from L6 rat skeletal muscle cells (26), although considerably less than that previously reported in muscle biopsy samples taken from the vastus lateralis muscle in healthy subjects, in which expression of AdipoR1 was 25- to 70-fold greater than that of AdipoR2 (27). The myotubes used in the present study were passaged for a total of four times, eliminating environmental influences on the muscle (such as circulating hormones), which may still exist at earlier passages (28). Yet, despite the loss of environmental influence, the myotubes still, in part, reflect the metabolic physiology of the muscle donor, such as insulin resistance (29, 30) and AMPK signaling (5), which allows the investigation of many metabolic abnormalities that exist in vivo. Although failing to reach significance, the propensity for greater expression of both AdipoR1 and AdipoR2 in myotubes derived from obese individuals might also suggest persistent dysregulation of the basal expression of these genes. The heightened basal expression may act to limit the capacity for further increases in gene expression after adipokine stimulation. Interestingly, the maximal gene expression for AdipoR1 after either adiponectin or leptin treatment in the lean subjects was equivalent to the basal rates of expression present in the myotubes derived from obese individuals. For reasons that remain unclear, the present data demonstrate that the acute responsiveness of genes to the adipokines adiponectin and leptin may also represent a persistent alteration in cellular regulation in obesity and diabetes.
AdipoR1 binds predominantly to gAd, in contrast to AdipoR2, which preferentially binds to full-length adiponectin (4). Thus, the chosen treatment of gAd may have a greater influence on the binding and, hence, receptor activity of the AdipoR1 forms. Currently, little is known of the downstream signaling events initiated by adiponectin that may influence transcriptional events. AMPK is thought to have nuclear functions (31), although increased peroxisome proliferator-activated receptor α activity may also be involved (4). Interestingly, hepatic over-expression of gAd, by adenoviral infection, failed to impact on the expression of AdipoR1 in rodent skeletal muscle (32). Thus, it remains unclear, from the present study, how gAd and leptin treatment is able to activate the expression of AdipoR1 in myocytes cultured from lean individuals only. These data also fail to describe the mechanisms behind the loss of sensitivity to both gAd and leptin in myocytes derived from obese, diabetic, and formerly obese individuals. To partially examine possible mechanisms, we examined the response to the selective activation of AMPK using AICAR. This treatment failed to elicit any alteration in AdipoR1 gene expression, suggesting that AMPK phosphorylation fails to mediate this action. More detailed analysis of the upstream promoter regions of AdipoR1 is required to gain a greater insight into the transcription factors capable of interacting with and regulating the expression of this gene.
To the best of our knowledge, this is the first study to demonstrate that leptin impacts on AdipoR1 gene expression. Functional leptin receptors and leptin expression have recently been identified in skeletal muscle myotubes (33). Leptin and other adipokines have previously been demonstrated to up-regulate adiponectin mRNA expression (22, 34). Interestingly, combined doses of adiponectin and leptin are required to completely reverse insulin resistance in lipoatrophic mice, an effect only partially observed with administration of each adipokine individually (21). It can be speculated that there are complementary actions of adiponectin and leptin on the expression of selected skeletal muscle genes, providing a mechanism of “cross-talk” to potentiate the cellular actions of adiponectin.
In conclusion, skeletal muscle myotubes developed from severely obese and diabetic subjects demonstrated an impaired ability to increase the expression of AdipoR1 after gAd and leptin exposure. This adipokine insensitivity persisted even after nearly 50 kg of weight loss in formerly severely obese individuals. These data indicate that a cellular defect in the intracellular signaling after adipokine exposure in obesity-prone and diabetic human myocytes prevents adaptive up-regulation in the expression of the AdipoR1 mRNA. This may be a contributing factor to the “adiponectin resistance” previously observed in the skeletal muscle of obese and insulin-resistant individuals. Future studies are required to further elucidate the extent and mechanisms of the diminished activation of AdipoR1 by adiponectin and leptin seen in myotubes derived from obese, diabetic, and obesity-prone individuals.
Acknowledgments
We gratefully acknowledge the invaluable contributions of the surgeons, Simon Woods, Monash University Academic Surgery Unit, Cabrini Hospital, Malvern, Australia, and Mark Lawrence, Obstetrics and Gynaecology, Bayside Health. This study was supported by the National Health and Medical Research Council (D.C.-S. and B.E.K.) and Australian Research Council (B.E.K.). G.R.S. is a Canadian Institutes of Health Research “Target Obesity” Research Fellow, and B.E.K. is an Australian Research Council Federation fellow.





