CD137 differentially regulates innate and adaptive immunity against Mycobacterium tuberculosis
Abstract
Protective immunity against Mycobacterium tuberculosis is primarily mediated by the interaction of antigen-specific T cells and antigen presenting cells, which often depends on the interplay of cytokines produced by these cells. Costimulatory signals represent a complex network of receptor–ligand interactions that qualitatively and quantitatively influence immune responses. Thus, here we investigated the function of CD137 and CD137L, molecules known to have a central role in immune regulation, during human tuberculosis (TB). We demonstrated that M. tuberculosis antigen stimulation increased both CD137 and CD137L expression on monocytes and NK cells from TB patients and healthy donors, but only up-regulated CD137 on T lymphocytes. Blockage of the CD137 pathway enhanced the levels of interferon (IFN)-γ and tumor necrosis factor (TNF)-α produced by monocytes and NK against M. tuberculosis. In contrast, CD137 blockage significantly decreased the specific degranulation of CD8+ T cells and the percentage of specific IFN-γ and TNF-α producing lymphocytes against the pathogen. Furthermore, inhibition of the CD137 pathway markedly increased T-cell apoptosis. Taken together, our results demonstrate that CD137:CD137L interactions regulate the innate and adaptive immune response of the host against M. tuberculosis.
Tuberculosis (TB) remains an enormous global health problem despite current drug treatment. The disease causes nearly 9 million new cases and 2 million deaths annually worldwide, and is among the most common causes of morbidity and mortality in patients with HIV infection. Bacillus Calmette-Guérin, the only available vaccine, is of variable efficacy, especially in TB-endemic regions, such as Argentina. Development of a more effective vaccine depends on a better understanding of the human immune response to this pathogen. Although protective immunological mechanisms against Mycobacterium tuberculosis are not fully understood, resistance to mycobacterial infections is primarily mediated by the interaction of antigen-specific T cells and macrophages.1,2 This interaction is often dependent on the interplay of cytokines produced by these cells. Even though a broad spectrum of cytokines may contribute to protection, a type 1 response, dominated by interferon (IFN)-γ secretion, is considered a principal mediator of protective immunity against M. tuberculosis.2,3 Besides, CD8+ T lymphocytes also contribute to protection by lysing infected cells.4 In addition, innate immune response-related pro-inflammatory cytokines, interleukin-6, interleukin-12, tumor necrosis factor (TNF)-α, initiate events that limit mycobacterial growth by recruiting monocytes into the lesions and activating them.5 In particular, TNF-α, mainly secreted by activated macrophages, contributes to anti-tubercular action and limits disease pathology.1 Therefore, it is considered of vast interest to investigate the regulation of cytokine responses during active TB.
Several signaling proteins contribute to the active up- or down-regulation during the priming of a T cell,6 modulating the level and pattern of cytokines produced by those cells upon antigen stimulation. For example, during the immune response of the host against M. tuberculosis, we demonstrated that SLAM (signaling lymphocytic activation molecule) and ICOS (inducible costimulator) enhanced IFN-γ secretion,7,8 whereas the SAP (SLAM-associated protein), CD31 and PD-1 (programmed cell death 1) interfered with Th1 responses.7,9,10 Another signaling molecule that participates in the regulation of T-cell effector functions is the receptor CD137 (4-1BB), a member of the TNF receptor (TNFR) superfamily that can have a costimulatory role for T-cell immunity upon binding with its ligand CD137L.11,12 In particular, it was reported that CD137:CD137L interactions boost CD8 T-cell responses, although the expression profile of CD137 is now known to be quite broad, being present or induced on various types of immune cells, and not solely restricted within T-lineage cells.13,14 Moreover, during persistent infection, CD137 expression can be prolonged.14 In addition, CD137 may be an effective costimulator for enhancing T-cell responses in chronically infected individuals,14 promoting Th1 cytokine responses.14 Therefore, our specific aim was to elucidate whether CD137 and its ligand participated in the regulation of cell-mediated immune responses to M. tuberculosis.
Results
M. tuberculosis increased the expression of CD137 and CD137L on cells from the innate and adaptive immune response
The timing of CD137/CD137L interactions and their effects on T cells may depend on the availability and induction of the ligand and the receptor during a particular immune response.14
Therefore, we considered important to analyze the expression profile of CD137 and CD137L on cells from the innate and adaptive immune response during active TB. We first investigated CD137 and CD137L expression on monocytes and NK cells from TB patients and healthy donors (HD) upon M. tuberculosis antigen (M.tb Ag) stimulation. Although, it is controversial whether CD137 is expressed on human monocytes15,16 ON (overnight) M.tb Ag stimulation induced CD137 expression on CD14+ cells from TB patients and HD (Figure 1a, left panel). Besides, CD137L expression was significantly augmented on monocytes after M.tb Ag stimulation (Figure 1a, right panel). Moreover, significant percentages of CD137 and CD137L were also detected on CD56bright NK cells of both groups of individuals (Figure 1b).
We then analyzed the levels of CD137 and CD137L on lymphocytes. Minimal levels of CD137 were detected on resting T cells from TB patients (data not shown), as previously reported in human T cells.17 However, 5 days of M.tb Ag stimulation significantly increased CD137 expression, with higher levels of the receptor on CD3+ T cells from TB patients as compared with HD (Figure 1c, left panel). In contrast, no detectable levels of CD137L could be detected on T lymphocytes (Figure 1c, right panel).
CD137:CD137L pathway modulated the cytokine microenvironment during human TB
Cytokines display a crucial role during the immune response of the host against M. tuberculosis. Thus, we investigated the potential function of CD137:CD137L interactions on the modulation of cytokine production by cells from the innate and adaptive immune response. To do this, we analyzed two central cytokines in TB: TNF-α and IFN-γ. Our results demonstrated that blockage of CD137 pathway significantly augmented both TNF-α and IFN-γ production by TB patients and HD after 16 h (ON) of M.tb Ag stimulation (Figure 2). In contrast, CD137 or CD137L blockade after PMA stimulation inhibited IFN-γ and TNF-α secretion by both groups of individuals (data not shown). Interestingly, after 2 and 5 days of M.tb Ag stimulation, blockage of CD137 increased TNF-α production but decreased IFN-γ secretion (Figure 2). Similar results were obtained when CD137L was blocked (data not shown).
Our results indicate that CD137:CD137L interactions might induce distinct effects on cytokine secretion, probably by different cell types during the initial and later phases of the immune response. To further investigate this possibility, we analyzed the production of cytokines at the single-cell level. As shown in Figures 3a and b, ON stimulation with M.tb Ag significantly increased CD14+TNF-α+ and CD14+IFN-γ+ cells. Interestingly, CD137 blockade strikingly augmented the percentage of specific CD14+TNF-α+ and CD14+IFN-γ+ producing cells in both groups of individuals (Figures 3a and b). Furthermore, blockage of CD137:CD137L pathway also augmented CD3−CD56bright TNF-α producing NK cells against M.tb Ag (Figure 3c). Remarkably, IFN-γ production by NK cells was also augmented after blocking CD137 (Figure 3d). Thus, CD137:CD137L pathway operates diminishing the secretion of TNF-α and IFN-γ by monocytes and NK cells. We then hypothesized that early during M. tuberculosis infection, interactions between CD137 and CD137L on cells from the innate immune response would regulate the secretion of TNF-α and IFN-γ, likely by preventing cytokine over production that might cause tissue damage, but allowing the presence of enough levels of cytokines to control the infection.
We also investigated whether CD137:CD137L interactions regulated the percentage of IFN-γ and/or TNF-α producing lymphocytes. Thus, peripheral blood mononuclear cells (PBMC) from TB patients and control individuals were stimulated with M.tb Ag±anti-CD137 monoclonal antibody (mAb) and the percentage of IFN-γ and TNF-α producing cells were determined. As shown in Figure 4, TB patients and HD produced significant numbers of CD3+IFN-γ+ and CD3+TNF-α+ T cells against M.tb Ag, and CD137 blockade strikingly diminished the percentage of specific IFN-γ/TNF-α producing lymphocytes. Considering that the production of IFN-γ by T lymphocytes is crucial to control M. tuberculosis infection, we also investigated the effect of blocking CD137 on IFN-γ secretion by CD4+ and CD8+ T cells. Our data demonstrated that CD137:CD137L interactions enhance IFN-γ production from both CD4+ and CD8+ T cells (Figure 4c). Together, our results demonstrated that the CD137:CD137L pathway might differently regulate cytokine production during innate and adaptive immune response against M. tuberculosis.
CD137:CD137L interactions regulated the survival and lytic degranulation of T lymphocytes in TB
We next evaluated whether signaling through CD137 might modulate other effector functions of T cells in TB. Then, we first analyzed the role of CD137:CD137L interactions on CD8+ T-cell-specific lytic degranulation against M.tb Ag. We observed that blockage of CD137 significantly decreased the percentage of CD3+CD8+ T cells from TB patients expressing CD107a/b (Figures 5a and b). CD8+ T cells from HD showed a weak lytic degranulation against M.tb Ag, with no significant variations after the CD137:CD137L pathway blockade (Figure 5a). Therefore, our data indicate that CD137 signaling up-regulates the cell degranulation of CD3+CD8+ lymphocytes after M.tb Ag stimulation.
Since its discovery, the studies that followed established CD137 as a potent costimulatory molecule. Thus, signaling through CD137 was demonstrated to induce T-cell expansion, up-regulation of anti-apoptotic genes and prevention of activation-induced cell death.17 So, we next studied the role of CD137 signaling on cell proliferation. As shown in Figure 6a, PBMC from HD displayed higher proliferation against M.tb Ag as compared with TB patients. Moreover, the blockage of CD137 or CD137L significantly decreased the specific proliferation against M.tb Ag in both group of individuals (Figure 6a), clearly demonstrating that CD137:CD137L interactions induced M.tb Ag-specific cell expansion. Then, we studied the potential role of the CD137:CD137L pathway on T-cell apoptosis. Our results confirmed that M.tb Ag stimulation led to apoptosis of T lymphocytes from TB patients (Figures 6b and c), as measured by Annexin V/PI (propidium iodide) staining, in line with previous reports.18 Furthermore, blockage of CD137 (or CD137L) significantly augmented the percentage of apoptotic M.tb Ag-stimulated CD3+ T cells (Figures 6b and c). Moreover, as described in Figure 6c, the CD137:CD137L pathway inhibited apoptosis of both CD4+ and CD8+ cells in comparable way, indicating that signaling through CD137 prevents programmed cell death of specific T cells during active disease. Since TNF-α is a pro-apoptotic soluble factor, these results are in correlation with our data demonstrating that CD137 pathway diminished TNF-α secretion.
Discussion
Costimulatory signals do not only provide an ‘on or off’ switch but rather represent a complex network of receptor–ligand interactions that qualitatively and quantitatively influence immune responses and affect all its major participants including effector lymphocytes and dendritic cells.19 Among TNFR superfamily members, CD137 has been shown to display diverse roles in adaptive and innate immune responses, but little is known about its function during the regulatory pathways that contribute to persistence of intracellular bacteria.20 Therefore, in this work we investigated the role of the CD137:CD137L pathway during chronic human TB.
CD137 participates at different stages and influences distinct aspects of the immune response, likely due to the differential expression of receptor and ligand relative to other costimulatory molecules.14 With non-replicating Ag, CD137 is expressed only transiently on T cells in vivo.21 However, when Ag persists, CD137 expression can be prolonged.22,23,24 Thus, the effects of CD137 pathway on immune cells may depend in part on its expression pattern in the particular model studied. Our results showed that stimulation with M.tb Ag markedly increased the levels of CD137 and CD137L on monocytes. Accordingly, it was reported that various stimuli, including innate and adaptive signals, can promote the expression of CD137L or other TNFR ligands on antigen presenting cells.25 Besides, although the expression of CD137 on activated human NK cells has been recently described in patients with acute myeloid leukemia,26 our data demonstrated that M.tb Ag increases both CD137 and CD137L expression on CD56bright NK cells. These data are in line with our previous results on the expression and role of PD-1 on NK cells in TB.27 Moreover, after 5 days of M.tb Ag stimulation, a significant augment of CD137 on T lymphocytes was also detected.
We showed that M.tb Ag induced differential expression of CD137 and CD137L on cells from the innate and adaptive immune response. Thus, we hypothesized that CD137 and CD137L might participate in the modulation of the host immunity against this pathogen. When we blocked the CD137 pathway, we observed a significant increase in the number of CD14+ and CD3−CD56+bright TNF-α and IFN-γ-secreting cells, demonstrating that the CD137 pathway inhibits cytokine secretion from cells of the innate immunity. In agreement with our findings, it was recently published that the consequences of CD137–CD137L interaction between human NK cells and acute myeloid leukemia blasts reduced NK-cell activation and IFN-γ production.28 On the other hand, we demonstrated that CD137:CD137L interactions significantly augmented CD3+TNF-α and IFN-γ producing lymphocytes against M.tb Ag, denoting that CD137 signaling promotes T-cell effector functions. Therefore, our findings show opposite effects of the CD137/CD137L pathway on different cells of the host immune response. Accordingly, it has been demonstrated that whereas CD137 activation enhances T-cell responses,14 it inhibits human NK-cell effector functions.28
Traditionally, CD137 was reported to be expressed and to induce responses on CD8 T cells preferentially.14 In fact, in different models of acute virus infection, the effect of CD137L appears to be limited to CD8+ T lymphocytes.29,30 However, the CD137 costimulatory pathway was also shown to affect CD4+ T-cell responses to viruses that cause latent infections.23,31 In line with these reports, we have demonstrated that, during the immune response to M. tuberculosis, the blockade of CD137 enhances apoptosis and diminishes the percentage of CD4+ and CD8+ IFN-γ producing T cells.
CD137 has been established as a potent costimulatory molecule.12,32 Accordingly, we observed that signaling through CD137 regulated apoptosis and proliferation of immune cells and promoted specific lytic degranulation in TB patients. In line with our data, it was shown that CD137L-deficient mice displayed a loss of CTL-effector function during latent murine gammaherpesvirus-68 infection.31 Thus, in contrast to previous reports showing that signaling through CD137 did not contribute to Mycobacterium avium immunity,33 here we demonstrate that the CD137 pathway participates in the immunity against mycobacterial infection.
In the present study, we showed that CD137:CD137L interactions may work differently on different cell populations, inhibiting or increasing a particular cytokine secreted by a particular cell type. Whereas the protective role of IFN-γ in TB is well established,2 TNF-α exhibits a very complex network of interactions and many of its activities are still not fully understood.34 However, it is known that TNF-α displays a main function in controlling M. tuberculosis infection, activating macrophages early during the immune response and participating in granuloma formation.35,36 Conversely, excessive levels of TNF-α may cause tissue damage in vivo,34 including hyperinflammation, caseous necrosis and cachexia.37 Moreover, it has been reported that a correlation exists between TNF-α levels and clinical severity of human TB.38 Furthermore, the function of TNF-α in humans is of clinical interest due to the association of anti-inflammatory TNF-blocking drugs with reactivation of latent TB.39,40
We demonstrated that CD137 signaling diminished TNF-α production by monocytes (and NK cells) at early time points of M.tb Ag stimulation. Therefore, since monocytes are the main source of TNF-α, the CD137 pathway would be inhibiting TNF-α production by these cells, thus preventing the down-regulation of the required IFN-γ produced by T cells.35,36 In fact, later during the immune response and through the interaction between T cells and antigen presenting cells, CD137:CD137L augmented IFN-γ levels and T-cell effector functions. Those levels of specific IFN-γ against M.tb Ag would be also regulated by SLAM, ICOS and PD-17,8,10 to reach the necessary amounts of the cytokine in the microenvironment.
Contradictory information regarding the role of the CD137:CD137L pathway on cytokine production during different bacterial infections has been published.20,41 For example, it was reported that CD137−/− and CD137L−/− mice were resistant to septic shock induced by lipopolysaccharide (LPS).42 In contrast, stimulation through CD137 was demonstrated to protect mice from Listeria monocytogenes sepsis-induced death.20 Moreover, it has been proposed that CD137 would have opposite functions in Gram-negative and Gram-positive bacterial infections.42 Therefore, CD137 would have different roles depending on the infecting bacterial species. Accordingly, here we demonstrated that CD137/CD137L signaling displayed a specific role against M. tuberculosis infection. In particular, we propose that CD137/CD137L signaling on innate cells would operate as a negative feedback regulatory mechanism to avoid an exacerbated inflammatory response and to limit collateral damage during the immune response to M. tuberculosis, but allowing the presence of enough levels of cytokines to control the infection. However, later during the immune response, CD137 signaling would stimulate the production of IFN-γ by T cells to reach the required protective levels to fight M. tuberculosis. Therefore, our results suggest that the CD137 pathway regulates the levels of IFN-γ and TNF-α required by the host to combat M. tuberculosis at different steps of the immune response. Therefore, the present findings support a dual role of the CD137/CD137L pathway in mediating both stimulatory and inhibitory signaling activities during human infection.
Anti-CD137 antibodies are powerful immune modulators and have shown promise in therapeutic mouse models of cancer, viral disease and autoimmunity. Moreover, phase I and II clinical trials using anti-CD137 therapy for advanced cancers are underway.43 By using blocking mAbs, we demonstrated that CD137/CD137L pathway regulates the immune response during active TB. However, caution must be used in designing ways to manipulate CD137 in human therapy, given that agonistic anti-CD137 antibodies can cause severe immune system abnormalities13,43 and considering that the CD137:CD137L pathway may operate differently on distinct cells from the innate and adaptive immune response.
Methods
Study subjects
Healthy adults (n=40) with no history of TB participated in the study. All participants had received Bacillus Calmette-Guérin vaccination at birth. A previous established M. tuberculosis-specific IFN-γ assay (Quantiferon TB Gold test, Cellestis Inc., Valencia, CA, USA) was used to differentiate individuals with LTBl (latent TB infection) from true HD without LTBI. Subjects with LTBI were excluded from the study. HIV-uninfected patients (n=40) with active TB were evaluated at the Hospital Muñiz (Buenos Aires, Argentina). The diagnosis of TB was established based on clinical and radiological data together with the identification of acid-fast bacilli (AFB) in sputum (+/−): 36/4. The ages of each group (median (interquartile range) were HD, 34.3 (22–58); and TB, 32.2 (18–63). The male:female ratio of each group was HD, 28:12; TB, 26:14.
All participating patients had received <1 week of anti-TB therapy. Peripheral blood samples were collected in heparinized tubes from all individuals after receiving informed consent. The local ethical committee approved all the studies performed.
Antigen
In vitro stimulation of cells throughout the present study was performed with a cell lysate from the virulent M. tuberculosis H37Rv strain, prepared by probe sonication. In brief, the reagent was obtained generously through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: M. tuberculosis, Strain H37Rv, whole-cell lysate, NR-14822. Afterwards, probe sonication was applied to the cell lysate following the standard methods. The preparation was finally suspended in free pyrogen phosphate-buffered saline and adjusted to a concentration of 1 × 108 bacteria ml–1 (optical density (OD)600 nm=1). The antigen preparation is indicated as ‘M. tuberculosis antigen (M.tb Ag)’ throughout the manuscript.
Culture conditions
PBMC were isolated by density gradient centrifugation on Ficoll-Paque (Amersham Biosciences, Buckingham, UK), resuspended in supplemented RPMI1640 (Gibco, Carlsbad, CA, USA) and cultured (1 × 106 cells ml–1) in flat-bottom 24-welll or 96-well plates (Greiner Bio-One, Frickenhausen, Germany). In different experiments, cells were incubated in the presence/absence of M.tb Ag (10 μg ml–1). At different times, CD137 and CD137L expression was determined by flow cytometry (see below). For blocking experiments, cells were incubated 30 min with blocking mAbs (BD, Bergen County, NJ, USA) against CD137 (5 μg ml–1, 4B4-1), CD137L (5 μg ml–1, C65–485), or isotype control. Then, cells were stimulated with or without M.tb Ag. After 16 h, 4 or 5 days, the percentage of IFN-γ or TNF-α-secreting cells, lytic degranulation and apoptosis were determined by flow cytometry (see below). Cells cultured with M.tb Ag±blocking mAbs against CD137 or CD137L for 5 days were also pulsed with [3H]TdR (1 μCi per well), harvested 16 h later and [3H]TdR incorporation was measured in a liquid scintillation counter. In separated experiments, mAbs anti-CD137 or anti-CD137L were added to cells with or without the specific Ag. After 16, 48 h or 5 days, IFN-γ and TNF-α production was evaluated by ELISA (enzyme-linked immunosorbent assay) following the manufacturer's instructions (eBioscience, San Diego, CA, USA).
Flow cytometry
In different experiments, PBMC were cultured with M.tb. Ag±CD137 or CD137L blocking mAbs and stained for CD3, CD4, CD8, CD56, CD14, CD137 (0.5 μg ml–1, 4B4-1) and CD137L (0.5 μg ml–1, C65-485) expression using specific mAbs (BD). Intracellular cytokine staining was also performed to determine IFN-γ and TNF-α (eBioscience) production at the single-cell level as reported.44 Negative control samples were incubated with irrelevant, isotype-matched mAbs in parallel with experimental samples. Samples were analyzed on a FACS ARIA II Cell sorter (BD).
Lytic degranulation
CD107a/b lysosome-associated membrane protein-1/2 expression was used to measure CD8+ T lymphocyte degranulation, as previously described.45 Negative control samples were incubated with irrelevant, isotype-matched mAbs in parallel with all experimental samples.
Annexin V and PI staining
PBMC were cultured with M.tb. Ag±CD137/CD137L blocking mAbs. After 5 days, the percentage of apoptotic/necrotic CD3+, CD3+CD4+ or CD3+CD8+ cells was determined using the Annexin V-FITC Apoptosis Detection Kit I (BD) following the instructions of the manufacturer.
Statistical analysis
Statistical analysis was performed using the non-parametric Wilcoxon rank sum test for paired samples and the Mann–Whitney U-test for non-paired samples. Values of P<0.05 were considered significant.
ACKNOWLEDGEMENTS
This investigation received financial support from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT 01384 VEG, PAE-PICT 02332 VEG) and the University of Buenos Aires (UBACyT X087 VEG); National Institutes of Health (Grant RAI07900A-01A1, VEG). DFDP was a fellowship from ANPCyT, Argentina. JOJ and IBA are fellows of CONICET, Argentina. VEG and VP are members of the Researcher Career of CONICET, Argentina. We thank Dr Peter Barnes for many insightful discussions. We also thank Catalina Feledi for technical assistance.
Conflict of interest
The authors declare no conflict of interest.
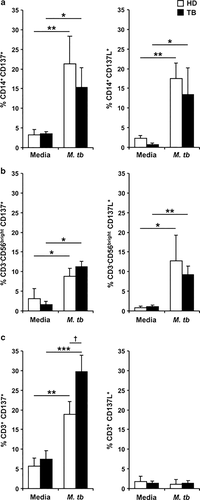
M. tuberculosis increased the expression of CD137 and CD137L on cells from the innate and adaptive immune response. PBMC from TB patients and HD were stimulated±M.tb Ag for 16 h (a), 24 h (b) or 5 days (c). CD137 (left panel) and CD137L (right panel) expression was determined by flow cytometry. The cytometric analysis was performed first gating on monocytes by light scatter and then by gating on CD14+ cells (a), or first gating on lymphocytes by light scatter, and then on CD3−CD56bright for NK cells (b) or on CD3+ for T lymphocytes (c). Each bar represents the mean±s.e.m. from each group (seven individuals per group). ∗∗∗P<0.001; ∗∗P<0.01; ∗P<0.05 Wilcoxon rank sum test. †P<0.05 Mann–Whitney U-test.
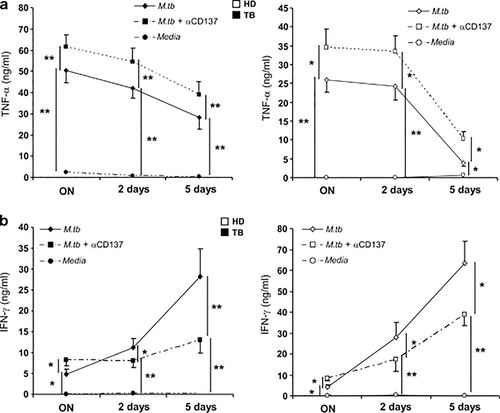
Role of the CD137:CD137L pathway on the cytokine microenvironment during human TB. PBMC from TB patients and HD were stimulated with M.tb Ag, in the presence or absence of CD137 blocking mAb. After 16 h (ON), 2 or 5 days cell-free supernatants were collected and assayed for TNF-α (a) and IFN-γ (b) production by ELISA. The mean±s.e.m. (15 individuals per group) of IFN-γ and TNF-α secretion levels is shown for each time. ∗∗P<0.01; ∗P<0.05 Wilcoxon rank sum test.
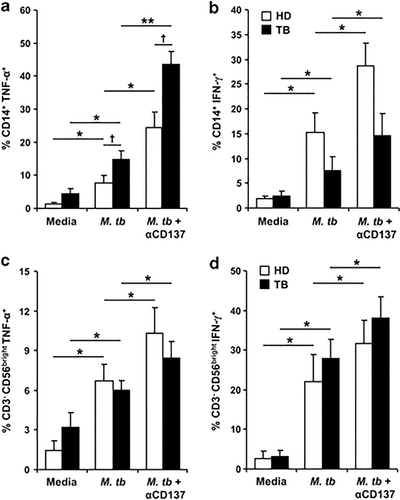
Effect of CD137:CD137L pathway on the production of cytokines by monocytes and NK cells. (a, b) PBMC from TB patients and HD were stimulated with M.tb Ag in the presence or absence of blocking anti-CD137mAb. After 16 h, the intracellular expression of TNF-α (a) and IFN-γ (b) was determined by flow cytometry, first gating on monocytes by light scatter, then by gating on CD14+ cells. Each bar represents the mean±s.e.m. of the percentage of CD14+cytokine+ cells for each group (11 individuals per group). (c, d); PBMC from TB patients and HD were stimulated with M.tb Ag for 24 h in the presence or absence of blocking anti-CD137mAb and intracellular TNF-α (c) and IFN-γ (d) expression on CD56bright NK cells were determined by flow cytometry by first gating on lymphocytes by light scatter, then by gating on CD3− cells and finally on CD56bright NK cells. Each bar represents the mean±s.e.m. (10 individuals). ∗∗P<0.01; ∗P<0.05 Wilcoxon rank sum test. †P<0.05 Mann–Whitney U-test.
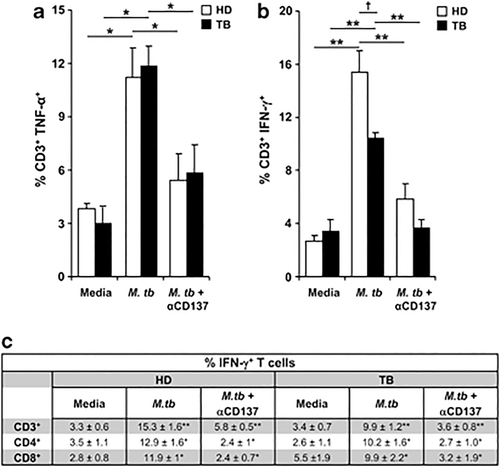
Effect of CD137:CD137L pathway on the production of cytokines by T lymphocytes. PBMC from TB patients and HD were stimulated with M.tb Ag for 4 days in the presence or absence of anti-CD137 blocking mAb. (a, b) Intracellular TNF-α (a) and IFN-γ (b) expression were determined by flow cytometry on T cells. Each bar represents the mean±s.e.m. (17 individuals per group). ∗∗P<0.005; ∗P<0.05 Wilcoxon rank sum test. †P<0.05 Mann–Whitney U-test. (c) The number of IFN-γ producing cells was also determined on CD3+CD4+ and CD3+CD8+ cells. Lymphocytes were gated based on their light scatter profile and gated further based on CD3+ and CD4+ or CD8+ expression. Data of nine independent experiments are shown (mean±s.e.m.). P-values were calculated using the Wilcoxon rank sum test by comparing the mean of IFN-γ producing T cells cultured with media versus M.tb Ag and cells cultured with M.tb Ag versus M.tb Ag + α-CD137.
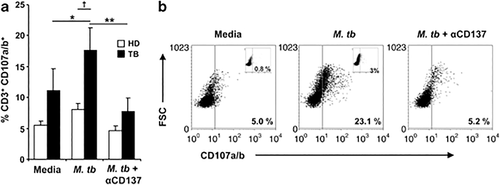
Role of CD137 pathway on lytic degranulation against M. tuberculosis. PBMC from TB patients and HD were stimulated with M.tb Ag in the presence or absence of blocking anti-CD137mAb. After 5 days, the expression of CD107a/b was determined on CD3+CD8+ T cells by flow cytometry. (a) Each bar represents the mean±s.e.m. of the percentage of CD3+CD8+CD107a/b+ cells (seven individuals per group). (b) A representative TB patient out of seven is shown. Isotype controls (inset) are also shown. ∗∗P<0.01; ∗P<0.05 Wilcoxon rank sum test. †P<0.05 Mann–Whitney U-test.
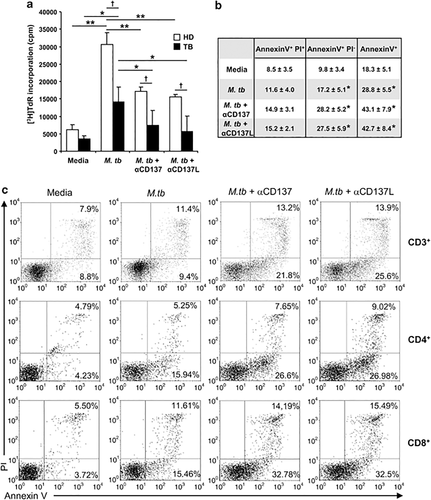
Effect of CD137:CD137L pathway on proliferation and T-cell apoptosis against M. tuberculosis. (a) PBMC from TB patients and HD were cultured in the presence of M.tb Ag ±CD137 or CD137L blocking mAbs. After 5 days, cells were pulsed with TdR (1 μCi per well), harvested 16 h later, and [3H]TdR incorporation was measured in a liquid scintillation counter. Each bar represents the mean±s.e.m. from each group (nine individuals per group). (b, c) PBMC from TB patients were cultured in the presence of M.tb ±CD137 or CD137L blocking mAbs. After 5 days, the cells were stained with Annexin V and PI together with CD3. The percentage of apoptotic/necrotic cells was determined by flow cytometry by gating on CD3+ T cells. (b) Data of seven independent experiments are shown (mean±s.e.m.). P-values were calculated using the Wilcoxon rank sum test by comparing the mean of the percentage of Annexin V+ or Annexin V+IP− T cells cultured with M.tb versus media, M.tb+anti-CD137 or M.tb + anti-CD137L. (c) The number of apoptotic/necrotic cells was also determined on CD3+CD4+ and CD3+CD8+ cells. A representative dot plot out of seven TB patients analyzed is shown. ∗∗P<0.01; ∗P<0.05 Wilcoxon rank sum test. †P<0.05 Mann–Whitney U-test.





