Biological implications of glycosaminoglycan interactions with haemopoietic cytokines
Abstract
Heparan sulphate (HS) glycosaminoglycans (GAGs) are an integral part of the signalling complex of fibroblast derived growth factor (FGF) family members, HS being regarded as a coreceptor. FGFs are also retained in the tissues by binding to HS structures. Early studies on the contribution of the bone marrow stroma to haemopoiesis suggested that cytokines with a role in haemopoiesis were similarly retained in the stroma through interactions with HS. However, the functional outcomes of these cytokines binding HS were poorly understood. Here the GAG-binding properties of cytokines of the four α-helical bundle family and the biological consequences of such binding are reviewed. From this analysis it is apparent that although many of these cytokines do bind GAGs, GAG binding is not a consistent feature, nor is the site of GAG binding conserved among these cytokines. The biological outcome of GAG binding depends, in part, on the location of the GAG-binding site on the cytokine. In some cases GAG binding appears to block signalling, whereas in others signalling is likely to be facilitated by binding. It is postulated that the interactions of these cytokines with their receptor complexes evolved independently of GAG binding, with GAG binding being an additional feature for a subset of this cytokine family. Nevertheless, because GAG binding localizes cytokines to sites within tissues, these interactions are likely to be critically important for the biology of these cytokines.
The functions of haemopoietic cytokines were considered to be due solely to their interactions with specific receptor complexes. However, it is becoming clear that to describe the full biological activities of these cytokines the microenvironmental context in which the cytokines are presented to their receptors must be considered. In many cases, the interactions of the cytokine with components of the extracellular matrices (ECM) of the tissue environment can have a profound effect on the activity of the cytokines. Of particular importance in this regard are the interactions of cytokines with the glycosaminoglycan (GAG) chains of proteoglycans of the ECM. Moreover, as other proteoglycans are integral membrane components of cell surfaces, the cell surface microenvironment will also contribute to the functional outcome of cytokine–receptor interactions. In some situations, the interaction of a cytokine with GAGs will have a positive effect on cytokine signalling through its specific cell surface receptor complex, and on other occasions, this effect will be a negative one. Key determining factors as to the functional outcomes are first, the location of the GAG-binding site on the cytokine, and second, the kinetics of GAG–cytokine interactions; both of which are, in turn, determined by the structures of the cytokine and the interacting GAG chain. This means that the biological consequences of cytokine–GAG interactions must be determined independently for each cytokine.
Structure of heparin and heparan sulphates
Proteoglycans are a diverse group of macromolecules, but all consist of a protein core to which is attached at least one anionic GAG chain. Three structurally determined types of GAG chains can be distinguished in proteoglycans: heparan sulphate (HS)/heparin, chondroitin/dermatan sulphate and keratan sulphate, the best studied and probably the most important of these for binding cytokines in vivo are the HSs. Heparin is structurally similar to HS and is often used experimentally as a ‘model’ for HSs. Both are highly heterogeneous, linear polysaccharides and both have a polysaccharide backbone of alternating disaccharide units comprising a uronic acid and a glucosamine. The uronic acid is either a glucuronic or iduronic acid and both can occur as unsulphated residues or as 2-O-sulphated residues. The glucosamines are variously N-sulphated or N-acetylated or, rarely may exist as a free amine. The N-sulphated glucosamines may carry no O-sulphates or they may carry O-sulphates at the C3 (rarely) or C6 positions, or both C3 and C6 positions, whereas the N-acetylated glucosamines may be unsulphated or O-sulphated at C6.1 The potential for structural diversity when these units are arranged as disaccharides and the disaccharides are combined to form chains is enormous. However, the full potential diversity is not realized because of constraints of the biosynthesis process. What are found are HS chains where highly sulphated regions (S-domains) are interspersed with regions that are predominantly glucuronic acid and low in sulphates. Mixed sequences that contain glucuronic acid and N-sulphated glucosamines are also found. Heparin is more highly sulphated than HS having a higher ratio of iduronic acid to glucuronic acid and more N-sulphated glucosamines with the result that stretches of a trisulphated disaccharide structure are common. Heparin chains are of variable length with a molecular weight range of 5–40 kDa (average 12 kDa). HSs isolated from tissues are also polydisperse with a molecular weight range of 5–50 kDa and an average weight of approximately 30 kDa,2 although this can vary depending on the tissues from which they are derived.
Specificity of heparin/HS–protein interactions
There has been much discussion as to the specific activity of heparin and HSs and whether defined heparin/HS sequences interact with specific proteins. The fact that defined sequences exist has been demonstrated by the pentasaccharide sequence that is required for binding to antithrombin III. This sequence binds its protein ligand with high affinity, and this has been exploited in the development of the synthetic antithrombin III binding pentasaccharide as an anticoagulant drug (Arixtra). Another uncommon HS structure was found to bind the Herpes simplex gD protein to cell surfaces during the process of virus infection. Both of these unusual sequences contain a 3-O-sulphated N-unsubstituted glucosamine.1, 2 Other sequences have been found to bind growth factors of the FGF family with a degree of specificity. Thus, in nature different HS structures can bind to the same growth factor but with varying affinities and conversely, it seems that particular heparin/HS sequences that uniquely bind only one protein are lacking.3 Nevertheless, antibody staining studies revealed that particular HS epitopes are expressed in defined locations within selected tissues. These data suggest that HS biosynthesis is regulated by the various cell types within the tissues resulting in the creation of localized extracellular microenvironments. Other studies with various FGFs and their receptors indicate that these proteins preferentially bind certain HS structures that are spatially and temporally regulated in the tissues during embryo development.4, 5
Although an exquisite binding specificity similar to that seen with antibody–antigen interactions is probably not the nature of most HS–heparin-growth factor interactions, in vivo the varying patterns of HS structures expressed in the tissues are sufficient to cause different growth factors to localize to specific tissue sites. Whether this is due to the specific expression of short tailored saccharide sequences or whether the organization of the highly sulphated, non-sulphated and mixed regions along the HS chain produces a pattern that is recognized by the growth factors, is not yet clear. It is most likely that the tissue-binding patterns displayed by various FGF/FGF-receptor complexes4, 5 are determined by a combination of uncommon and common HS sequences together with a requirement for certain spacing of the sulphated/mixed regions along the saccharide chain.6 When the three-dimensional structure of HSs rather than the sequence of monosaccharides is considered the lack of relationship between monosaccharide sequence and binding specificity becomes clearer. An analysis performed by Mulloy and Forster7 on the crystal structure of FGF-1 complexed with a heparin oligosaccharide (2axm.pdb) revealed that a number of different heparin pentasaccharide sequences would contain a motif that binds FGF-1. The critical factor for binding is the spatial arrangement of a cluster of three sulphates, part of a second sulphate cluster and the carboxylate that occurs between the two clusters of sulphates; the nature of the underlying saccharide backbone is less important. Moreover, additional substitutions with more sulphate groups do not increase the affinity of binding and the conformation of heparin in solution is such that this motif can appear on either side of the polysaccharide chain. When all factors are taken into account, 31 different pentasaccharide sequences will contain a single FGF-1-binding motif.7 Clearly, specificity issues of heparin/HS motifs that bind proteins should be discussed in terms of the three-dimensional pattern of the motif rather than the sequence of saccharides that make up this pattern.
Interactions of FGFs, FGF-receptors and GAGs
Members of the FGF family are considered as ‘prototypical heparin-binding cytokines’8 possibly more because these were the first heparin-binding cytokines discovered and are the best studied, rather than the characteristics and the biological outcomes of this binding being typical of heparin-binding cytokines. It is now well appreciated that for the signalling outcomes of FGF receptor activation by its FGF ligand to be fully realized, the presence of a HS chain is obligatory. Although the FGFs can bind their receptors in the absence of a HS chain, the GAG chain increases the affinity of the interaction by more than an order of magnitude. The molecular mechanism by which this occurs has been extensively examined by X-ray crystallographic, solution NMR and biochemical studies and has been shown to involve the GAG chain binding not only the FGF but also its receptor.9, 10, 11, 12, 13, 14 These studies have provided evidence for two different heparin/FGF/FGF-receptor complexes, in one the stoichiometry of the FGF-1/FGFR2/heparin complex is 2:2:1, whereas in the other the stoichiometry of the FGF-2/FGFR1/heparin complex is 2:2:2. It is beyond the scope of this review to discuss the evidence surrounding each of these complexes and the reader is referred to recent reviews on this topic.15, 16 Nevertheless, it is clear that heparin or HS binding has a positive effect on receptor signalling and that it stabilizes and/or facilitates the formation of a signalling complex on the cell surface that involves two growth factor molecules and two receptors.
Heparin/HS-binding cytokines involved in haemopoiesis generally signal through heterodimeric receptors and so a direct analogy with members of the FGF family is not always possible. Indeed, as Mulloy and Rider8 point out, it is not known to what extent each HS–cytokine interaction has evolved independently, and is therefore functionally and structurally distinct.
GAGs and haemopoiesis
The possibility that the interaction of immunologically relevant cytokines with GAGs could positively contribute to cytokine activities was first realized in the late 1980s as a result of studies with long-term bone marrow cultures on the contribution of the bone marrow stroma to haemopoiesis. At that time, haemopoiesis was usually studied using soft gel culture systems with added cytokines sustaining haemopoiesis for only a few days. Dexter and Spooncer17 discovered that haemopoiesis can be maintained in stromal cell cocultures for several months in the absence of added exogenous growth factors, and growth factors are not released into the fluid phase of such cultures. They argued that growth factors being synthesized by the stromal cells must be retained in the stromal cell layer. They went on to provide evidence that biologically active interleukin-3 (IL-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF) were retained by binding HS on stromal cell surfaces18 and suggested that this was a mechanistic explanation for the dependence of haemopoietic cells on intimate stromal cell contact for their long-term proliferation and survival. The localization of GM-CSF to cell surfaces was confirmed by antibody staining; anti-GM-CSF antibody stained the surface of fibroblasts and sites suggestive of deposited ECM in bone marrow cultures.19
These data and observations that separate colonies of differentiating haemopoietic cells form on bone marrow stroma, led to the idea that discrete niches specific to particular haemopoietic cell lineages and to stem cells orchestrate the regulated growth and differentiation of haemopoietic cells. As intracellular signals induced by stem or progenitor cell adhesion to stroma probably act with cytokine-induced signals to regulate the proliferation and differentiation of haemopoietic stem cells,20 this means cytokines, stromal cell surface molecules and the glycoproteins of the stromal ECM all contribute to maintaining the microenvironmental niches. Components critical for defining these niches appear to be the combination of cytokines synthesized and the particular HS structures that retain these cytokines in discrete locations in the cell layer.21, 22 The evidence supporting this came from experiments using long-term culture-initiating cells from human bone marrow cultures. These experiments demonstrated that the addition of HS isolated from a stromal cell line that supports haemopoiesis, to a cocktail of picogram concentrations of cytokines found in stromal cultures, promoted the continued proliferation of long-term culture-initiating cells in the absence of a stromal layer. The HSs effective in this assay had particular structural features. HSs from supportive stromal cultures contained structures rich in 6-O-sulphated glucosamines, whereas other HSs isolated from non-supportive stroma have less of these structures and are inactive.22 This O-sulphated HS was found to selectively bind FGF-2, IL-3, the chemokines, macrophage inflammatory protein-1α and platelet factor-4 (PF-4) and the matrix protein thrombospondin, whereas HS from non-supportive stroma bound only FGF-2 and PF-4. These effects could be mimicked using a commercial preparation of selectively O-sulphated heparin (prepared by N-desulphation and N-reacetylation of heparin), which, when immobilized, also supported the adhesion of CD34+ progenitors. These data led Gupta et al.22 to propose that HS proteoglycans are responsible for the juxtaposition of haemopoietic progenitor cells, stromal cells, cytokines and ECM proteins and are therefore central components of microenvironmental niches in the bone marrow stroma. The study of Bruno et al.21 similarly supported the conclusion that HS proteoglycans isolated from a human bone marrow stromal cell line interacts with cytokines (IL-3 and stem cell factor/Kit ligand) and the ECM protein thrombospondin to augment progenitor cell localization within the haemopoietic microenvironment.
The HS rich in 6-O-sulphated glucosamine isolated from stromal cells and the commercial preparation of selectively O-sulphated heparin were described as representing an ‘average structure’ and sulphation pattern, required for binding multiple cytokines and matrix proteins.23 However, these GAGs are not a single entity but a heterogeneous mix of a multitude of structures. Thus, whether discrete entities within this mix are binding the various cytokines or whether one entity is interacting with multiple cytokines is impossible to determine. Given that generally more than one cytokine is involved in the development of a particular haemopoietic cell lineage, it may be expected that in vivo the bone marrow microenvironmental niches are defined so that a number of structurally related cytokines are colocalized. Whether these niches contain an ‘average’ HS structure that binds a number of related cytokines, or numerous HS structures so that multiple cytokines are bound is biologically irrelevant, as the outcome is the same.
From this discussion it is apparent that haemopoietic cytokines are preferentially localizing to particular sites within tissues probably by binding to HSs in much the same way as described for the FGF family. The interaction of at least some of these cytokines with HSs in the tissues appears to enhance cytokine activity. However, for the most part detailed structure–function studies, similar to those that have been extremely informative as to the mechanism of HS augmentation of FGF signalling are lacking for the cytokines of the immune system. The studies that are available are largely binding studies with little detail as to the effect HS binding has on the cytokines' interactions with their receptors.
Short-chain four α-helical bundle cytokines
Most information on heparin/HS interactions with immunologically relevant cytokines concerns cytokines of the four α-helical bundle family. This family has been divided into the short-chain four α-helical bundle family and the long-chain four α-helical bundle family. Cytokines of the short-chain four α-helical bundle family are primarily involved in immunohaemopoietic regulation. Mulloy and Forster7 have used molecular modelling in an attempt to predict whether or not cytokines of this family are likely to bind heparin. Two conformations of a heparin pentasaccharide were modelled: one with the two iduronic acids in the 1C4 chair conformation and the other where the iduronic acids are in the 2S0 skew-boat conformation, coordinates being derived from the NMR structure for the predominant repeating trisulphated disaccharide (1hpn.pdb). Both pentasaccharides had glucosamine at the reducing and non-reducing termini and all exocyclic bonds were regarded as rotatable with the exception of the glycosidic linkages. Heparin–protein complexes calculated to have intermolecular interaction energies of approximately −1000 kcal mol−1 or less were taken as indicative of a capacity to bind heparin.7 Of the cytokines examined the following were predicted to bind heparin: IL-2, IL-3, IL-4, IL-5, IL-13, whereas GM-CSF, Flt3 ligand and stem cell factor, although having energies of less than zero, could not be confidently predicted as possessing an ability to interact with heparin. Interestingly, the energies for both heparin conformations were similar for all the cytokines tested. Such modelling data are not definitive on their own and should be supported by biological data to validate them.
The haemopoietic cytokines that share the β-common (βc) chain as part of their receptors, GM-CSF, IL-3 and IL-5, will be discussed first. Each of these cytokines signals through a receptor complex consisting of their own specific receptor-α chain complexed with βc. The stoichiometry of the active receptor complexes is unclear but for GM-CSF the most recent data suggests that the GM-CSF/GM-CSF receptor-α (GM-CSFRα)/βc complex forms in the ratio 1:1:2.24
GM-CSF
An explanation for the conflicting modelling data and the experimental findings already reported for GM-CSF in terms of its capacity to bind heparin and HS lies in the fact that the modelling has not taken into account the nature of the bone marrow microenvironment. In the bone marrow haemopoietic progenitor cells are localized in poorly oxygenated niches25 and poorly oxygenated tissues are associated with local acidosis. Furthermore, a study of myeloid progenitor cells proliferating in contact with bone marrow-derived stroma revealed that the interaction of the haemopoietic cells with the stroma caused an accumulation of sialylated glycoconjugates and proteoglycans at the junctions of the two cell types, leading to a high local concentration of negatively charged molecules.26 This would be expected to cause further local acidification.
The local pH can have profound effects on the ability of some proteins to bind heparin or HS, particularly so when this binding site involves histidines. We have recently demonstrated this with the cell adhesion molecule PECAM-127 and GM-CSF seems to behave similarly. GM-CSF seems to undergo a conformational change at low pH that exposes hydrophobic pockets in the protein, permitting heparin binding. It has been reported that high molecular weight complexes, probably involving various molecules of heparin interacting simultaneously with several GM-CSF molecules, form at low pH.28 The heparin-binding site on GM-CSF was described as involving the C-helix, and in particular the region around H83, K85 and H87 was indicated, with the data also supporting a role for K72 and K74 located at the N terminus of the C-helix. In addition, replacement of H15 in the A-helix, with an alanine residue significantly reduced the affinity of GM-CSF for heparin.29 Examination of the surface electrostatic potential of human GM-CSF at acidic and neutral pH indicated that when the three histidines (H15, H83 and H87) are protonated a large continuous positive surface is created on the face of the protein where H83, H87 and K85 are exposed.29 In our modelling H15 is largely buried in the space-filled model (Figure 1, 1csg.pbd), but when protonated it may cause a realignment of the A-helix, which favours heparin binding. This heparin-binding site is somewhat removed from the key amino acids involved in binding the GM-CSF receptor subunits. In helix-A, E21, and in helix-D, E108 and D112, have been shown to be important for interacting with the βc chain and the GM-CSFRα-chain of the receptor complex, respectively.30 Indeed, the residues E108 and D112 are on a different face of the protein from the proposed GAG-binding site (Figure 1). Thus, from this analysis it is expected that heparin/HS binding will not interfere with the biological activity of GM-CSF.
A study examining the ability of human GM-CSF to stimulate the mitogenic activity of human osteoblastic cells indicated that not only does GM-CSF bind to HS on the surface of osteoblasts but binding was also important for the full mitogenic activity of this cytokine. The requirement for HS binding was demonstrated using assays where the osteoblastic cell cultures were treated with either heparinase III or sodium chlorate,31 treatments that respectively remove the HS chains or disrupt the sulfation of the HS chains during biosynthesis. Most interestingly, GM-CSF was found to bind to the HS chains of the proteoglycan syndecan-2. Syndecan-2 anti-sense constructs not only decreased the mitogenic activity of GM-CSF, an effect that was not rescued by heparin, but they also reduced the levels of activated extracellular signal-regulated kinase-1, a downstream effect of GM-CSF receptor signalling.32 Syndecan-2 was found to coimmunoprecipitate with the α-chain of the GM-CSF receptor, suggesting a complex of cytokine, GM-CSFRα and syndecan-2 may exist on the cell surface. Although it is likely that the HS chains of syndecan-2 contribute to receptor complex formation, the data suggest that the core protein also has a role in modulating GM-CSF signalling. Whether this effect will also be seen with other syndecans and other cell types, for example, syndecan-4, which is expressed on bone marrow stromal cells, is unknown. Nor is it known whether the GAG chains on syndecan-2 interact directly with GM-CSFRα. Nevertheless, these data indicate that the GM-CSF/GAG/receptor complexes are not analogous to the growth factor/GAG/receptor complexes seen with FGF family members.
IL-3
There are few biochemical or structural data on whether or not heparin/HS binds IL-3. The binding data to indicate that human IL-3 interacts with HS are confined to an affinity coelectrophoresis experiment reported by Gupta et al.22showing that radiolabelled HS is retarded when electrophoresed through a gel containing IL-3. Further affinity coelectrophoresis experiments indicated that human IL-3 interacted with a sub-population of structures contained in a commercial preparation of selectively O-sulphated heparin.23 Unfortunately, the modelling analysis described by Mulloy and Forster7 is hampered by the pdb IL-3 structure that is available. The NMR structure (1JLI) used for modelling analysis is of an IL-3 variant that, as well as having truncations at both the N and C termini, also has 14 residue changes within the remaining protein.33 This variant is said to be fully active in cell proliferation and receptor-binding assays,33 which is surprising given that two residues claimed to be important for interacting with the IL-3 receptor34 are changed. Importantly, one of the amino acids (R29), identified as being critical for heparin binding from the modelling study using this structure, is a glutamine in the wild type. Such a change may be expected to significantly alter the ability of the identified site to bind heparin. However, H26 is adjacent to the suggested binding site, and when protonated, may provide sufficient additional basic charge to the cluster of K28, R108 and R109 to facilitate binding of a heparin pentasaccharide. Potentially, there is an alternative heparin-binding site on the B–C face of the protein, which may bind heparin fragments longer than the pentasaccharide. This possible site involves R63 and K66 on the B-helix, K79 on the C-helix and H98 and K100 in the C–D loop (Figure 2, 1jli.pdb), but as with GM-CSF it is probable that greater binding will be achieved at low pH.
The possible heparin-binding site on the B–C face is well removed from the receptor-binding region on the A–D face of the molecule;34 however, the site predicted from modelling in part overlaps with the receptor-binding region, residue K116 belonging to both binding sites.7, 34 If the heparin and the receptor-binding sites overlap it is expected that heparin would inhibit IL-3 activity. Various functional studies have indicated that the interaction of murine IL-3 with heparin/HSs presents IL-3 in an active form.35, 36 However, to our knowledge, the only studies involving human IL-3 and HS are those reported above from Verfaillie's laboratory, but in this study the functional analyses of the effects of HS were generally performed with a cocktail of cytokines and chemokines.22 However, they did report that selectively O-sulphated heparin in combination with human IL-3 and either macrophage inflammatory protein-1α (MIP-1α) or PF-4 could support long-term culture-initiating cell proliferation for up to 5 weeks.23 In these assays IL-3 is viewed as a growth promoting cytokine whereas MIP-1α and PF-4 are growth inhibitory. Thus, it is reasonable to assume that the interaction of human IL-3 with heparin and HS does not inhibit IL-3 functional activity. This suggests that a heparin-binding site on the B–C face, removed from the site that interacts with the IL-3 receptor complex, is most likely.
IL-5
IL-5 is different from the other cytokines of this group in that it is produced as a disulphide-linked homodimer. The dimer is an elongated disc made up of two domains and each domain is made up of three helices of one monomer and one helix from the other monomer. An IL-5 monomer has no biological activity, nor can it inhibit the receptor-binding activity of the homodimer. Thus, dimer conformation is necessary for presentation of the receptor-binding sites, and because it is a homodimer there are two receptor-binding sites per each biologically active IL-5 homodimer. The receptor-binding sites have been investigated by site-directed mutagenesis and these studies have indicated that a region in the C–D loop centred around R91 and a region around the C terminus of the D-helix, and in particular E110, are important for binding the α-chain of the receptor.37, 38 The βc chain of the receptor seems to require E13 of helix-A in its binding motif.39 IL-5 is unique among this group of cytokines in relation to the specificity of its biological activity; it acts to promote the differentiation and proliferation of eosinophils, and in humans its receptor is confined to eosinophils and their precursors, and basophils.40
The site identified by Mulloy and Forster7 as the potential heparin-binding site on IL-5 stretches along the C-helix and onto the C–D loop. This means that there are potentially two heparin-binding sites per functional IL-5 molecule and they may overlap with the IL-5-receptor-α- (IL-5Rα) binding site. There has been one publication reporting that human IL-5 binds GAGs.41 A heparin-enzyme-linked immunosorbent assay (ELISA) of the following format was used to monitor heparin-cytokine binding: IL-5 was immobilized and reacted with a ‘model proteoglycan’ comprising biotin-labelled albumin conjugated with heparin chains, with binding being detected by conjugated streptavidin. These experiments revealed that cations, and in particular zinc ions, were required for IL-5 binding to heparin. Heparin binding was blocked by the H30 anti-IL-5 monoclonal antibody and heparin-like HS structures, digestible by heparinase I, were favoured over other HS structures for blocking the binding of the heparin conjugate to IL-5. Murine IL-5 was also found to bind heparin but less well than human IL-5.
Most interesting were the biological implications of HS binding. HS was found to augment the IL-5 proliferative activity of the human IL-5 responsive cells, Baf-IL-5, and this augmentation was inhibited by the H30 antibody.41 These data were curious given that the epitope of the H30 antibody was mapped to the beginning of β-pleated sheet region comprising the C–D loop of one monomer and the A–B loop of the other monomer and more particularly, R90, R91 and R92;42 this overlaps with the region predicted to bind heparin from the modelling analysis and is very close to the region required for IL-5Rα binding. Notably, there was a small concentration range of both HS and IL-5 over which the augmentation of proliferation was observed; at higher HS concentrations not balanced by a higher IL-5 concentration the effect was inhibition. We have shown that at certain 3T3 cell concentrations HS on 3T3 cell surfaces enhanced IL-5 activity, an effect that was blocked by chlorate treatment, or the addition of heparin. Heparin alone was found to inhibit IL-5 activity and when heparin was added to the chlorate-treated 3T3 cell layer the effect was inhibition of IL-5 activity, very similar to that seen in the absence of the 3T3 cell monolayer (Figure 3). In these experiments SWISS embryonic 3T3 cells, a cell line known to support haemopoiesis, were allowed to form a monolayer, and IL-5 was preincubated with the monolayer before the addition of Baf-IL-5 cells with or without heparin. Baf-IL-5 proliferation was used as a measure of IL-5 activity.
We believe that the explanation for these data lies in the manner IL-5 binds its receptor and in the fact that IL-5 possesses two heparin-binding sites and two receptor-binding sites per homodimer, and both types of binding site overlap. Although there are two receptor-binding sites per IL-5 homodimer, one homodimer engages with one receptor complex.43 The determination of the crystal structure of the human βc chain revealed that it is a stable, intertwined homodimer and this homodimer is likely to be the functional form of the receptor.44 To date there are no data to suggest that GAGs bind either IL-5Rα or βc. These factors and the fact that IL-5 acts efficiently in the absence of GAG binding, as demonstrated by IL-5-induced proliferation of Baf-IL-5 cells that do not have HS on their surfaces,14, 41 indicate the modulation of IL-5 activity is likely to occur through a different mechanism to that seen with the FGF family.
Specifically, the IL-5 receptor is not preformed on the cell surface; rather βc is recruited into the receptor complex through contacts with the IL-5/IL-5Rα complex, which associates before βc recruitment. The complexing of βc causes an affinity conversion of approximately twofold. The data suggest that the IL-5/IL-5Rα/βc complex is relatively short-lived.45 It is likely that the interaction of IL-5 with a limiting amount of HS present on the 3T3 cell surface is such that only one GAG-binding site is occupied. This could serve to align/concentrate IL-5 molecules in the vicinity of components of the receptor complex on the Baf-IL-5 cells, thereby shifting the equilibrium towards more stable receptor complex formation and signalling. IL-5 binding to soluble heparin is unlikely to position the cytokine in a way that allows stabilization of the receptor complex. Rather, if the heparin concentration is sufficient it will bind to both GAG-binding sites on the molecule and so mask both IL-5Rα- binding sites with the result that IL-5 activity is inhibited.
IL-4 and IL-13
These two cytokines are closely related to four-helix bundle short-chain cytokines. Although IL-13 shares 25% homology with IL-4 at the amino-acid level, the amino acids contributing to the hydrophobic structural core of IL-4 are conserved in IL-13.46 This means that the three-dimensional structures of these proteins are similar and they share receptors. However, because mature IL-13 comprises 113 amino acids and IL-4 a total of 129 amino acids there are structural differences. There are variations in the lengths of the helices, and in particular, in the B- and C-helices, which in IL-13 are about half the length of those in IL-4.47 On haemopoietic cells IL-4 binds a receptor consisting of IL-4Rα and the common γ-chain (γc). The γc is also part of the receptor complexes recognized by IL-2, IL-7, IL-9, IL-15 and IL-21,48 whereas on cells of non-haemopoietic origin both IL-4 and IL-13 use a receptor comprising IL-4Rα and IL-13Rα1.49 IL-4 binds to IL-4Rα with very high affinity and the recruitment of γc or IL-13Rα1 into the receptor complex adds very little additional affinity.50 By comparison, IL-13 initially binds IL-13Rα1 with a relatively slow on-rate but an overall moderately strong affinity, and the recruitment of IL-4Rα markedly increases the affinity of IL-13 binding.51
The possibility that IL-4 may bind GAGs was first investigated as a way of explaining the findings of an immunohistochemical study that showed IL-4 localized to the human amnion epithelium, in an extracellular position.52 These researchers found IL-4 was retained on a heparin-sepharose column eluting with 0.5 M NaCl and heparin and IL-4 interactions with HS or dermatan sulphate inhibited one function attributed to IL-4 but had no affect on another. A few years later Lortat-Jacob et al.53 also demonstrated that an ECM could retain IL-4, for their experiments they used the commercial preparation Matrigel. In other experiments radiolabelled HS was found to bind to IL-4-coated plates and this binding was best inhibited by heparin and N-desulphated re-N-acetylated heparin, HS, dermatan sulphate. Chondroitin-6-sulphate also inhibited but a higher concentration of GAG was required, whereas chondroitin-4-sulphate was a very poor inhibitor. Cleavage of HS with either nitrous acid or heparinase III revealed that the N-sulphated domains of HS are required to inhibit radiolabelled HS from binding to immobilized IL-4.53 No functional studies were performed.
In another study a positive effect of GAGs on IL-4 activities was reported. Here IL-4-mediated differentiation of dendritic cells was investigated. IL-4 increased the expression of dendritic cell maturation markers on human monocytes cultured in GM-CSF and varying concentrations of IL-4.54 As these cells expressed HS and dermatan sulphate (chondroitin-sulphate B) on their surfaces, the possibility that these GAGs were contributing to cell differentiation was examined by treating the cells with chlorate to disrupt GAG sulphation. Chlorate treatment reduced expression of the markers, and it also slightly decreased the ability of these cells to stimulate peripheral blood leukocyte proliferation (a marker of dendritic cell functionality). Interestingly, treatment of the cells with chondroitinase ABC significantly reduced the phosphorylation of STAT6, an effect that is most likely attributable to signalling through the IL-4Rα/IL-13Rα1 receptor complex in maturing dendritic cells.55 An ELISA-type GAG-binding assay suggested that IL-4 bound heparin most strongly, and the other GAGs, including HS and dermatan sulphate, very poorly. Binding to various structurally modified heparins indicated a role for N-sulphates and 2-O- and 6-O-sulphates in the binding motif and binding to highly sulphated dermatan sulphates obtained from invertebrates was significantly higher than that seen with the more usual dermatan sulphate structure. The authors concluded that dermatan sulphate on dendritic cell surfaces facilitated IL-4 signalling.54
These data are curious in the light of the heparin-binding site on IL-4 identified by Mulloy and Forster.7 Their data suggest that heparin binds a site that overlaps with the site recognized by IL-4Rα;50 heparin binding may therefore be expected to inhibit IL-4 activities. We have also examined IL-4 interactions with heparin in some depth and our studies concur with the modelling analysis (Coombe DR et al., unpublished data). In addition, our biological analyses clearly indicate that heparin binding inhibits IL-4-mediated cell proliferation stimulated by signalling through the IL-4Rα/IL-13Rα1 receptor complex. This is as expected from the GAG-binding site identified from the modelling.7 Thus, possibly what den Dekker et al. are observing54 has more to do with GM-CSF interactions with cell surface HSs/dermatan sulphates than a direct IL-4 effect. Clearly, further analyses are required to determine whether dermatan sulphate binds to IL-4 in the same manner as heparin.
As yet there are no published reports of IL-13 binding to GAGs, although our preliminary data suggest that the prediction from the modelling is correct (Coombe DR et al., unpublished data). Further analyses are required to determine whether GAG binding to IL-13 modulates its interactions with its receptor.
IL-2
A study reported in 1992 was the first indication that IL-2 may bind GAGs.56 These data were confirmed in a later study where an ELISA approach of the following format was used to examine GAG–cytokine interactions: heparin-conjugated bovine serum albumin was immobilized, then reacted with the cytokine with binding being detected by a cytokine-specific antibody.57 IL-2 was found to bind heparin-conjugated bovine serum albumin with low affinity (estimated Kd ∼0.5 μm) and interactions with a highly sulphated heparin-like structure were favoured over other types of GAG structures. The only HS to inhibit IL-2 binding to the heparin conjugate was an HS that had a heparin-like composition, that is, high sulphation and a high proportion of uronic acids epimerized to iduronate. Interestingly, there was no evidence that when added separately to IL-2, the IL-2 receptor chains IL-2Rα, IL-2Rβ, or γc inhibited the association of IL-2 with heparin-conjugated bovine serum albumin.58 In an attempt to determine the GAG-binding site on IL-2, four single amino-acid mutants were tested for heparin binding. This analysis was complicated by the detection monoclonal antibodies used in the ELISA binding the various mutants to differing extents; nevertheless it appeared that T51P and Q126D failed to support binding, whereas R38A bound strongly.58 From these data and because heparin binding did not alter the biological activity of IL-2 it was concluded that the heparin-binding site was most likely to be in the cluster of basic amino acids comprising K32, K76, R81 and R83, a site overlapping that proposed by Mulloy and Forster7 from their modelling analysis.
To appreciate the implications of a heparin-binding site at this location an understanding of IL-2 interactions with its receptor chains is required. The IL-2Rβ and γc chains are both necessary and sufficient for signalling. However, neither chain alone has sufficient affinity for IL-2 to initiate a stable complex. An intermediate affinity receptor is formed from the complex of IL-2Rβ/γc and this complex is capable of signalling in in vitro systems when concentrations of IL-2 are high. IL-2Rα alone can bind IL-2 with low affinity having a Kd in the 10–20 nm range, but it is the trimeric complex comprising IL-2Rα/IL-2Rβ/γc that is the high-affinity IL-2 receptor capable of efficient signalling.59 Interestingly, fluorescence resonance energy transfer experiments revealed that IL-2 is not required for these three receptor chains to interact, but it is required for an interaction that is sufficiently stable for coprecipitation of the IL-2R subunits.
Molecular modelling analyses indicated that the primary energetic determinant in the IL-2-binding epitope for interactions with IL-2Rα is a hydrophobic cluster around F42 and L72 on the IL-2 A–B loop and helix-B′, respectively; this region inserts into a recessed pocket on IL-2Rα.60 Other amino acids relevant to the heparin-binding site on IL-2 that appear to have a peripheral involvement in contacts with IL-2Rα are R38 and possibly K35.60 The γc chain interactions with IL-2 centre around Q126 on helix-D of IL-2 with residues S127 and S130 also contributing; however, the γc chain interface with IL-2 is quite small as the interaction of γc with IL-2Rβ assists in stabilizing the quaternary complex.61 There are two IL-2 residues shown to be critical for IL-2Rβ binding; these are D20 and N88 and both are involved in hydrogen bond networks with water molecules and the side chains of IL-2Rβ. Both are well away from the proposed GAG-binding site. There are extensive interactions between IL-2Rβ and γc, indicative of the role of receptor–receptor interactions in assembling the quaternary complex, and although these two chains have no measurable affinity towards one another this receptor–receptor contact could be an important energetic determinant for complex assembly.61 It is believed IL-2 serves to stabilize complex formation by guiding the alignment of the numerous hydrogen bonds and van der Waals contacts that mediate the association of IL-2Rβ and γc61 and we believe the immobilization of IL-2 by HS on cell surfaces assists in this process.
Given the sites on IL-2 required for receptor binding, and given that IL-2 interactions with soluble heparin do not diminish its biological activity, it is reasonable to predict that the location of the heparin-binding site is at one end of IL-2 comprising the basic cluster of R38, K35, K32, Q74, K76, R81 and R83 in the A–A′ loop and B–C loops (Figure 4). The exact involvement of all these amino acids, and particularly R38 given its contribution to IL-2Rα binding, awaits further analysis. It is tempting to speculate that a biological outcome of the association of IL-2 with tissue/cell-associated HS is to retain IL-2 in a configuration that assists in formation of the quaternary signalling complex. The location of the heparin-binding site on IL-2 is consistent with such a role. The idea that in vivo IL-2–GAG interactions serve to concentrate or sequester the cytokine to locations where it is required has been proposed previously58 and is supported by the finding that in the spleen IL-2 colocalizes with particular HS structures confined to the red pulp and marginal zones.62 The marginal zones are populated by lymphocytes and macrophages and this fits with the functions of IL-2. IL-2 is primarily secreted by T cells in response to antigen-induced activation and it provides growth and survival signals to naïve and newly activated T cells and regulates T cell expansion by triggering homeostatic apoptotic signals in antigen-activated T cells.59 IL-2 can also support B-cell growth and induce immunoglobulin secretion. If our speculation is correct immobilized HS that binds IL-2 should enhance IL-2 signalling. To our knowledge these experiments have not yet been performed.
Long-chain four α-helical bundle cytokines
Mulloy and Forster7 have also performed docking studies on members of the long-chain four α-helical bundle cytokines to obtain clues as to whether these cytokines may bind heparin. They found out of a total of nine cytokines four cytokines were strongly predicted to bind heparin. Of these, only one, IL-6, has been examined in biological assays for its heparin binding capability and another, G-CSF, was described as heparin binding from biological experiments but was not predicted to bind from the modelling.
IL-6
Both lymphoid and non-lymphoid cells produce IL-6; numerous stimuli induce IL-6, and it is multifunctional. Its functions include the proliferation of haemopoietic progenitor cells, proliferation and differentiation of T and B lymphocytes, support of megakaryopoiesis, regulation of the biosynthesis of acute-phase proteins and stimulation of osteoclast development.63 Two chains comprise the IL-6 receptor, IL-6Rα and gp130. IL-6 first binds IL-6Rα and then gp130 is recruited, the final stoichiometry of the complex being 2:2:2. Dimerization of gp130 is required for signalling.
IL-6 was shown to be a heparin-binding cytokine using the ELISA approach where heparin-conjugated bovine serum albumin was immobilized and the binding of IL-6 was detected using specific antibodies.64 The binding was dose-dependent and inhibited by added heparin, partially inhibited by HS, but not by chondroitin sulphates A and C or dermatan sulphate. A series of chemically modified heparins were markedly less efficient inhibitors than the parent GAG, although 2-O-desulphated heparin inhibited binding it was less efficient than unmodified heparin. An ELISA system using soluble recombinant receptor proteins was used to examine if heparin binding to IL-6 interfered with the formation of the receptor complex.64 When immobilized gp130 is used to capture IL-6/IL-6Rα; the inclusion of IL-6 in a complex with gp130 is completely inhibited by heparin. As heparin is able to capture IL-6Rα only when it has previously been incubated with IL-6, this suggests that heparin is stopping the IL-6/IL-6Rα complex from binding to gp130. Immobilized gp130 alone was unable to capture IL-6 or IL-6Rα. These data suggest that the heparin-binding site on IL-6 overlaps with the site that interacts with gp130. Other data indicate that heparin can also inhibit the formation of the IL-6/srIL-6Rα complex, presumably by binding to site(s) on IL-6 that overlap with the site required for IL-6Rα binding.
The modelling predicts a heparin-binding site on IL-6 that overlaps with sites required for receptor binding. An examination of 1p9m.pdb, the crystal structure of the hexameric complex of IL-6 with its receptor chains, and the report by Boulanger et al.,65 revealed that R179 and K171 are involved in binding to IL-6Rα, and K27 and R30 are involved in binding gp130. These four basic amino acids are part of the GAG-binding site on helices-A and -D proposed from the modelling analysis.7 The involvement of R40 and K41 suggested from the biochemical study seems unlikely as these amino acids are removed from sites involved in receptor binding. The biological effects of HS binding to IL-6 in vivo are unknown; clearly, an understanding of the kinetics and relative affinities of each of the interacting components of the complex is required before any speculations are possible.
G-CSF
Neutrophils are critical cells for protection against bacterial infections and as they have a short lifespan, in vivo constant renewal of neutrophil numbers is necessary. G-CSF is the key cytokine regulating neutrophil production, differentiation and activation. G-CSF influences the activities of mature neutrophils, stimulating (a) phagocytic and chemotatic activity, (b) production of reactive oxygen intermediates in response to bacterial peptides and (c) antibody-mediated cellular cytotoxicity. It was approved for use as a drug in 1991 for the treatment of neutropenia, a condition frequently associated with anticancer treatments and immunosuppression.66 In contrast to the other cytokine receptors discussed the G-CSF receptor is composed of two identical chains. The signalling unit is a 2:2 complex between human G-CSF and human G-CSFR and is quite different from the murine heterogeneous receptor complex. It appears as the association of two 1:1 complexes caused by the crossover interaction of the immunoglobulin-like domain of the receptor chain and the adjacent cytokine.67 This conformation is described as resembling the arrangement seen between IL-6 and gp130.67
The interaction of G-CSF with heparin was described from capillary zone electrophoresis studies, most being performed at low ionic strength in 50 or 25 mm phosphate buffer (pH∼7).68 In these non-physiological conditions the binding constants are low, in the order of 40–50 μm. It was reported that heparin oligosaccharides, of 1000–8000 Da, prepared by heparinase digestion could bind in these conditions.69 The possibility that carrageenans and dextran sulphates may bind G-CSF was also investigated and it was concluded that best binding occurred with the most highly sulphated carrageenan and dextran sulphate of size 40 kDa and above. Binding affinities were in the low μm range. Other studies on the biological effects of carrageenan and dextran sulphates suggested that the highly sulphated polysaccharides may block G-CSF-mediated cell growth and alter the expression of differentiation markers on the promylocytic G-CSF-dependent NFS-60 cells. However, as there was no control to confirm that the polysaccharides were binding G-CSF in these conditions, and not directly affecting the cells, these data are difficult to interpret.70, 71
We have also examined whether hG-CSF binds heparin using our published BIAcore methodology,72 but were unable to measure any convincing interactions between the fluid phase cytokine and immobilized heparin regardless of the immobilization method used. Various buffer conditions were examined: differing pH, going as low as 5.0 and low ionic strength to mimic the capillary zone electrophoresis, but no binding was detected (Coombe DR et al., unpublished data). Thus, our data agree with the modelling analysis and suggest that the interaction is of such low affinity that it is difficult to measure. Nevertheless, an examination of the structure of G-CSF suggests that the most likely heparin-binding site would involve basic residues on helices A and D and these sites are involved in interactions with the G-CSFR.67 Given the similarities between G-CSF interactions with its receptor and IL-6 interactions with its receptor chain gp130, possibly this is not surprising.
Conclusions
From the information available it seems that many of the four α-helical bundle cytokines do bind GAGs, but this is not a consistent feature of this group, nor is the site on the cytokine where the GAG binds conserved. Some of these cytokines do not bind GAGs and some bind with an affinity so low as to be not readily detected. Possibly, it is more informative to think about the function of the cytokine and whether GAG binding is appropriate for its functions. It could be argued that the role of G-CSF in the differentiation and proliferation of neutrophil precursors in the bone marrow indicates that an ability to bind GAGs, and so be retained in the microenvironment of the bone marrow, would be advantageous. However, for GAG binding to facilitate G-CSF–receptor interactions a binding site distinct from that of the receptor-binding site is required. This is not likely without substantial amino acid changes to G-CSF. Thus, it appears that an ability to bind HS in the tissues is likely to have evolved after the cytokine–receptor complexes evolved. It is likely the cytokine structures and the receptor complexes coevolved and the ability to bind HS is an additional feature that can modulate cytokine–receptor interactions in some instances. This contrasts to the situation with the FGF family where a heparin/HS binding capability is integral to the function of the growth factor. The focus of this analysis was the contribution of GAG binding to cytokine signalling through their cell surface receptors. Information on whether GAG binding inhibits the enzymatic degradation of the four α-helical bundle cytokines, as is the case with the FGF family members is missing. To our knowledge there is no such information for these cytokines.
An understanding of the kinetics of GAG–cytokine interactions is a key factor in appreciating how GAGs may contribute to either facilitating, or interfering with the formation of the receptor complexes through which the four α-helical bundle cytokines signal. It appears that knowledge of the affinity with which GAG structures bind a cytokine is not sufficient to predict the relative biological activities of these GAGs. Gupta et al.23 have shown that heparin, which binds PF-4 and thrombospondin with four- to fivefold greater affinity than the selectively O-sulphated heparin (prepared by N-desulfation and N-reacetylation of heparin) does not support long-term culture-initiating cell maintenance in the absence of a stromal layer, whereas the O-sulphated heparin has this activity. They suggest that the kinetics of binding is important. A slow association rate and a slow disassociation rate, as seen with the O-sulphated heparin, means that once bound, the proteins do not rapidly disassociate despite an overall lower affinity. This example illustrates that because a GAG chain binds a cytokine, it does not automatically mean that it can modulate cytokine signalling.
To begin to understand the biological implications of GAG–cytokine interactions, binding kinetic studies, functional studies, modelling of GAG–cytokine interactions and NMR or crystal structures of the complex of GAG chain/cytokine/cytokine–receptor are all required. Presently, much of this information is lacking for the four α-helical bundle cytokines. For some of these cytokines the true role of HS binding may be to retain the cytokine in a particular location, not to modulate signalling. However, other cytokines of this group show no evidence of HS binding, whereas for others HS binding may both localize the cytokine and facilitate signalling. A further complication is that different HS structures could evoke different biological outcomes. Thus, each heparin/HS–cytokine interaction must be examined individually because predicting the biological consequences of GAGs interacting with cytokines of this group is hazardous.
ACKNOWLEDGEMENTS
This study was in part supported by a grant to DRC from Glycan Biosciences Pty Ltd. We thank Beverley Kinnear for allowing her unpublished data to be used in this review. We also thank Sandra Stevenson, Beverley Kinnear, Warren Kett and Keith Williams for their helpful comments on the manuscript.
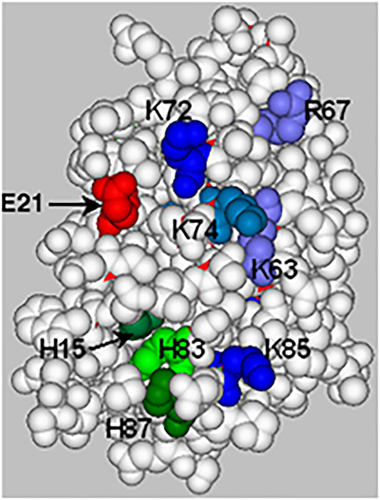
The B–C face of human granulocyte-macrophage colony-stimulating factor (GM-CSF) (1csg.pdb): a space-filled representation of the structure in which the amino acids likely to be involved in glycosaminoglycan chain binding are shown. The basic residues, lysine and arginine, are shown in various shades of blue and histidine residues in shades of green. The glutamic acid E21, shown in red, is involved in receptor binding.
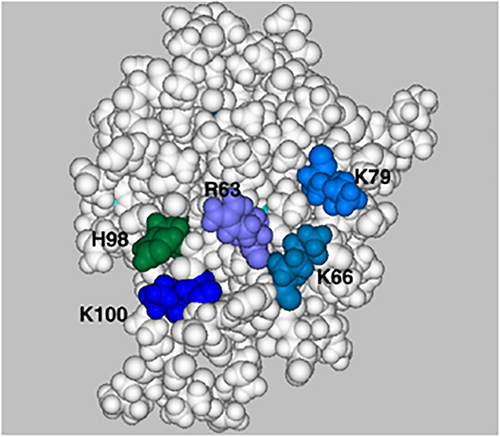
The B–C face of human interleukin (IL)-3 (1jli.pdb): a space-filled representation of the structure in which the amino acids likely to be involved in glycosaminoglycan chain binding are shown. The basic residues, lysine and arginine, are shown in various shades of blue and histidine residues in shades of green. This structure is of a truncated form of IL-3 with 14 altered amino acids, but all the highlighted amino acids are present in wild-type IL-3.
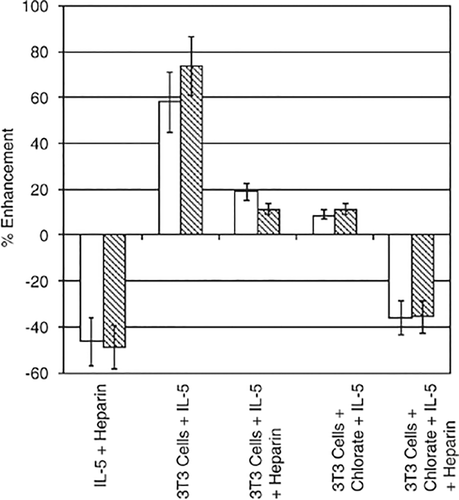
Heparan sulphate on a stromal cell layer can enhance the proliferative activity of IL-5. SWISS embryonic 3T3 cells created the stromal layer. These cells were cultured for 3 days in the presence or absence of 30 mM sodium chlorate before being treated with mitomycin-C to halt their proliferation. Human interleukin (IL)-5 (2 ng ml−1) was preincubated with the 3T3 cell layer 1 h before (open bars) the addition of Baf-IL-5 cells with or without 10 μg ml−1 of heparin, or (shaded bars) human IL-5 (2 ng ml−1) was preincubated for 1 h with the 3T3 cells, 10 μg ml−1 of heparin, or no heparin, being added to the 3T3 cells 1 h before the addition of Baf-IL-5 cells. Concentrations of IL-5 and heparin are final concentrations in the tissue culture well. The proliferation of Baf-IL-5 cells was measured after 48 h. The effect of heparin on IL-5-mediated proliferation of Baf-IL-5 cells in the absence of a stromal layer is shown. Data are presented as % enhancement calculated relative to the proliferation of Baf-IL-5 cells stimulated by 2 ng ml−1 IL-5 in the absence of heparin or a stromal layer.
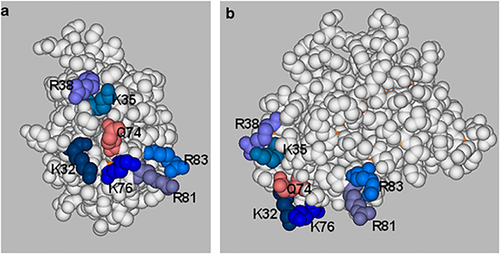
Space-filled representations of the structure of human interleukin (IL)-2 (3ink.pdb) in which the amino acids described as being involved in glycosaminoglycan (GAG) binding as shown. The basic residues, lysine and arginine, are shown in various shades of blue and a glutamine is shown in pink. Two views of the proposed GAG-binding site are given: (a) the end of the molecule showing the A–A′ loop and the B–C loop, and (b) the B–C face, which shows the GAG-binding site at one end of the molecule.





