Polyomavirus Infection and Acute Vascular Rejection in a Kidney Allograft: Coincidence or Mimicry?
Abstract
During the past decade, polyoma virus (PV) infection has emerged as an important cause of graft dysfunction and failure in kidney transplant recipients. Establishing the correct diagnosis can be difficult, however, because the histologic appearance of PV infection can resemble acute cellular rejection. We report a kidney-pancreas transplant recipient with PV infection, in whom both vascular and cellular rejection were dominant histologic features in a renal biopsy specimen. The patient was successfully managed by tapering immunosuppressive therapy, and continues to have good graft function 3 years after diagnosis of PV infection. This case highlights the spectrum of inflammatory changes associated with PV infection, and the need for caution when such changes are attributed to rejection.
Introduction
Polyomavirus (PV) infection is increasingly recognized as a cause of allograft dysfunction and graft loss in renal transplant recipients, with a reported prevalence of 1.9–4.5% (1–5). More potent immunosuppressive regimens with drugs such as mycophenolate mofetil and tacrolimus are thought to be a major factor in the apparent increase in incidence (4–6).
The diagnosis of PV infection is based primarily on histologic appearance, accompanied by electron microscopic and immunohistochemical evidence of PV in urine, tissue, or blood in patients with graft dysfunction. Establishing the diagnosis of PV infection definitively can, however, be challenging because not only does the histologic picture of PV resemble acute cellular rejection, but both processes may be present concurrently. PV infection is usually treated by tapering immunosuppression (4,7,8). Patients mistakenly diagnosed with rejection and treated with antirejection therapy often have accelerated declines in graft function with graft loss (4).
In this report, we present a case that highlights that acute vascular rejection can also be part of the histologic spectrum of PV infection in renal allografts.
Case Report
A 34-year-female with type 1 diabetes and end-stage diabetic nephropathy received a living-donor kidney transplant in 1995. The graft failed 1 week after transplantation due to vascular thrombosis. In May 1997, she underwent simultaneous kidney/pancreas transplantation and was immunosuppressed with antithymocyte immunoglobulin for 7 days, prednisone, azathioprine and cyclosporine. Serum creatinine on discharge was 80–90 μmol/L. Three months after transplantation, serum creatinine increased to over 140 μmol/L (Figure 1). A renal biopsy demonstrated acute vascular rejection, Banff 1997 grade IIB. She was treated with three doses of intravenous methylprednisolone (500 mg/day); azathioprine was discontinued and mycophenolate mofetil was begun. Serum creatinine returned to baseline. One year after transplantation, tacrolimus was substituted for cyclosporine because of gingival hyperplasia.
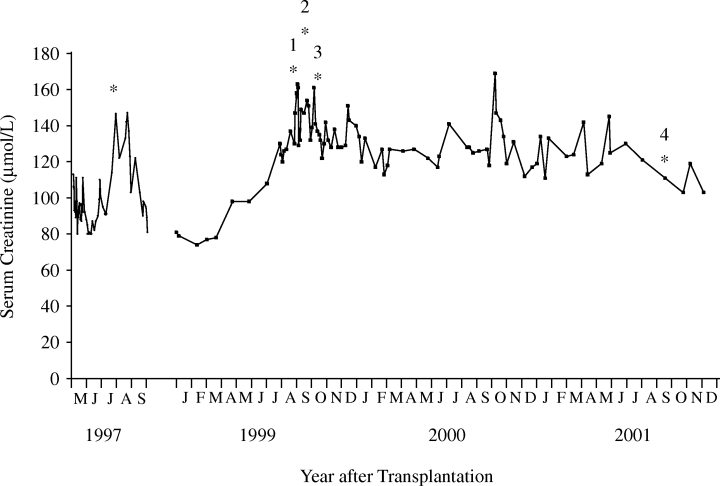
Serum creatinine after transplantation. Asterisks (*) indicate time of kidney biopsies and numbers correspond to the kidney biopsy specimens.
Two years after transplantation, serum creatinine increased to 140–150 μmol/L (Figure 1). A kidney biopsy was performed (August 1999), which revealed scattered inflammatory infiltrates, focal mild to moderate tubulitis, and viral inclusions in tubular epithelial cells (Figure 2). The presumptive diagnosis of cytomegalovirus infection was made, and treatment with intravenous ganciclovir was begun; subsequent immunohistochemical staining for cytomegalovirus was negative and a cytomegalovirus antigenemia assay was negative. The serum creatinine failed to improve and a renal biopsy was repeated (September 1999). This showed severe interstitial inflammation and numerous nuclear viral inclusion bodies in tubular epithelial cells (Figure 3A,B). Although most of the inflammatory changes were centered around cells with viral inclusion bodies, there were several areas of lymphocytic tubulitis in the absence of inclusion bodies (Figure 3D), which were consistent with acute cellular rejection. Electron microscopy of the biopsy specimen (Figure 3C) and urinary sediment demonstrated 45-nm diameter viral particles characteristic of PV. Treatment consisted of stopping mycophenolate mofetil, and lowering the dosage of prednisone and tacrolimus. A renal biopsy performed 1 month later (October 1999) because of persistently elevated creatinine showed severe tubulitis (Figure 4A), no viral inclusions, and mild intimal arteritis (Figure 4B); the biopsy was given a Banff 1997 score of grade IIa (mild vascular rejection).
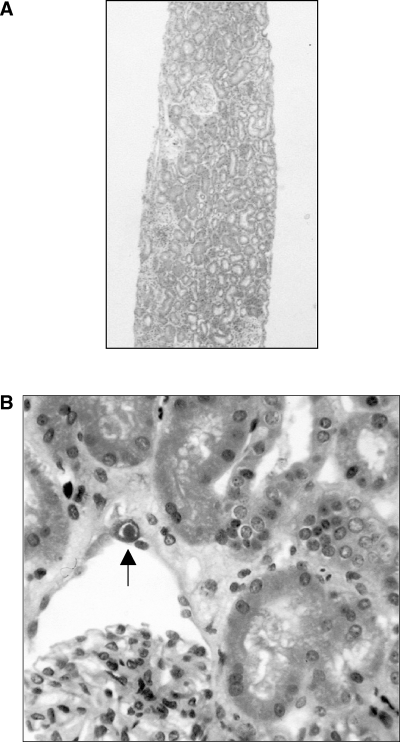
Renal biopsy 1. (A) A scattered inflammatory infiltrate can be appreciated at low power. H&E. Original magnification, × 30. (B) High power, HPS stain. Scattered cells in glomerular parietal epithelium (illustrated here) and tubular epithelium show karyomegaly, with purple intranuclear inclusions surrounded by a halo (arrow). The tubular epithelium also shows acute tubular degenerative changes. Hematoxylin-phloxine-saffron (HPS). Original magnification, × 200.
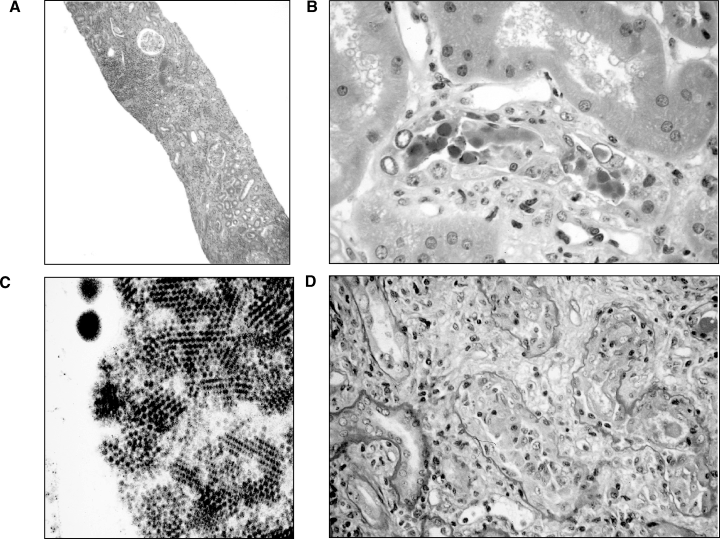
Renal biopsy 2. (A) Diffuse but focally more intense areas of inflammation are seen as compared to Figure 2(A). H&E. Original magnification, ×30. (B) Typical polyomavirus inclusions, with sloughing of epithelium into the tubules. Acute tubular degenerative changes are seen. H&E. Original magnification, ×325. (C) Electron microscopy. 45 nm viral particles characteristic of polyomavirus. (D) Severe tubulitis with lymphocytic infiltration in an area devoid of viral inclusions. Periodic-acid Schiff reaction (PAS). Original magnification, ×200.
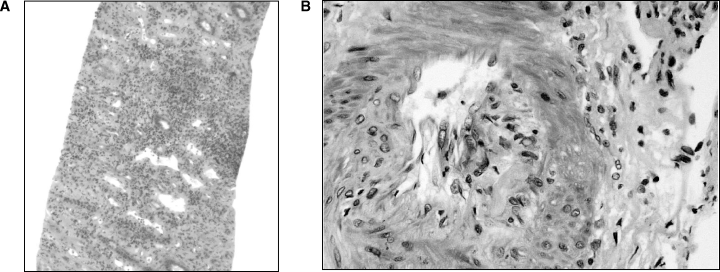
Renal biopsy 3. (A) Intense interstitial inflammation with lymphocytic infiltration and tubulitis. There were no clear viral inclusions. H&E. Original magnification, ×30. (B) In this somewhat tangential cut, subendothelial lymphocytic infiltration classic of vascular rejection is seen. Graded as acute vascular rejection, Banff 1997, type IIA. HPS stain. Original magnification, ×250.
No adjustments to the immunosuppressive regimen were made despite the pathological evidence of vascular rejection. Over the next few months, serum creatinine gradually improved (Figure 1) and has remained stable over the last 3 years. A renal biopsy obtained in October 2001 (Figure 5) showed foci of chronic inflammation (lymphoid follicles) in the outer medulla and at the cortico-medullary junction, minimal interstitial fibrosis, and no nuclear inclusions.
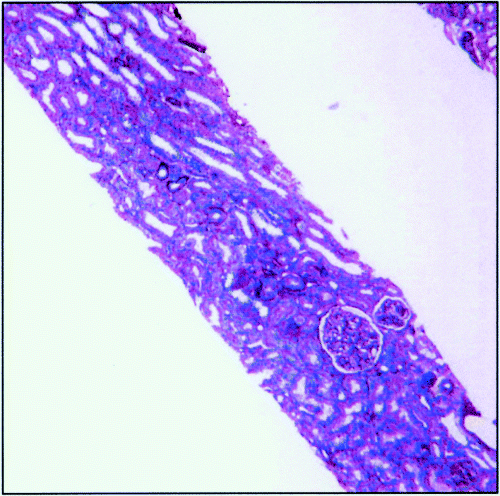
Renal biopsy 4. This biopsy shows minimal interstitial fibrosis, with no significant cortical changes. There is evidence of focal chronic inflammation (lymphoid follicles) in the outer medulla and at the cortico-medullary junction but not elsewhere in the biopsy. PAS. Original magnification, ×30.
Discussion
The case presented here illustrates the complexity of managing a patient with polyomavirus infection. First, there was difficulty in establishing the correct diagnosis. We initially suspected that cytomegalovirus was the likely cause of the viral inclusion bodies seen in the first biopsy obtained in 1999, because there was minimal associated inflammation. In retrospect, the large number of inclusions, particularly in distal tubules and collecting ducts, was an important clue. Second, histologic evidence of PV infection on the second biopsy was accompanied by areas consistent with the diagnosis of acute cellular rejection. Finally, the third biopsy showed features of acute cellular and vascular rejection in the absence of viral inclusion bodies.
Although it is generally accepted that PV infection may mimic acute cellular rejection, reports of other studies have suggested that acute cellular rejection may occur simultaneously with PV infection (5). Indeed, some patients have been successfully managed by treatment of both conditions (e.g. bolus steroid therapy followed by reduction of maintenance immunosuppression) (5,7). The relevance and treatment of ‘acute cellular rejection’ in the setting of PV infection, however, remains controversial, since the methods available to discriminate these entities are usually not definitive (9,10). Judicious reduction of immunosuppression with vigilant monitoring for rejection has been the most successful strategy for patients with PV infection (8).
The presence of vascular rejection in the third biopsy from our patient created a clinical dilemma. After choosing to ignore the ‘acute rejection’ seen on the previous biopsy, we were now faced with a biopsy that showed ‘worse rejection’. Interestingly, graft function as defined by serum creatinine had not deteriorated further during that time period, and was an important factor in our decision to not augment immunosuppressive therapy.
In summary, histologic features of vascular rejection may be seen in renal transplant recipients with PV infection. A recent report of a PV mutant causing diffuse and ultimately fatal vasculopathy underscores the range of presentations possible with this infection (11). This case also suggests that once the diagnosis of PV infection has been established, the pathologist and clinician should be very cautious in attributing inflammatory changes to rejection.




