The Addition of Sirolimus to Cyclosporine and Steroids Inhibits the Anti-Equine Antibody Response in Renal Transplant Recipients Treated with Antithymocyte Globulin
Abstract
Polyclonal antibodies, such as equine antithymocyte globulin (ATGAM®), are known to induce antibody formation. This study evaluated the in vivo effect of sirolimus on antibody formation associated with the use of equine antithymocyte globulin in renal transplant recipients. Recipients of either a living-related donor or cadaveric renal allograft received azathioprine (AZA) (n = 15), mycophenolate mofetil (MMF) (n = 12), or sirolimus (n = 15) in addition to baseline immunosuppression with corticosteroids, cyclosporine, and equine antithymocyte globulin. Immediately before transplantation and weekly for at least 1 month, sequential serum specimens were tested for the presence of human anti-equine antibody using an enzyme-linked immunosorbent assay (ELISA). Anti-equine antibody formation was significantly different among the three treatment groups. Fewer patients receiving MMF (17%, p = 0.007 vs. AZA) and sirolimus (13%, p = 0.003 vs. AZA) developed anti-equine antibody compared with AZA (66%). There was no significant difference (p = 0.81) in the sensitization to equine antithymocyte globulin when comparing the patients receiving MMF or sirolimus. In the sensitized patients, high anti-equine antibody titers (>1 : 500) were more common in those receiving AZA (n = 3) than MMF (n = 0) or sirolimus (n = 1). Compared to AZA, sirolimus, when given in combination with cyclosporine A, significantly reduced anti-equine antibody formation to a degree similar to MMF.
Introduction
Polyclonal antibodies have been utilized in organ transplantation for both prophylaxis and treatment of acute rejection (1). In renal transplantation, prophylactic therapy with polyclonal or monoclonal antibodies has been associated with a significant increase in graft survival and a decreased risk of rejection (1,2). Xenogeneic antibodies, however, can induce antibodies directed to the horse, mouse or rabbit immunoglobulin in these preparations. The antibodies produced may be of the IgG, IgM, or IgA isotype (3–6).
Antibody formation may result in immune-complex-mediated reactions as well as serum sickness (3–5,7). Serum sickness, which includes skin rash, arthritis, nephritis, and vasculitis (4), has been implicated in the development of renal complications (8) including graft failure due to immune complex deposition (4). Antibody formation to anti-lymphocyte globulin has been associated with a significant decrease in renal allograft survival (9). Serum sickness and antibody formation, however, may not be related to the occurrence of rejection episodes (3).
Sirolimus (Rapamune®), a macrocyclic lactone produced by Streptomycin hygroscopes, is a potent immunosuppressive agent (10). Sirolimus derives its immunosuppressive activity from inhibition of both T- and B cells (11). As reviewed by Sehgal, sirolimus inhibits interleukin-2 (IL-2)-dependent and -independent proliferation of stimulated human B-lymphocytes. It also precludes IL-2- and IL-6-dependent differentiation into antibody-producing cells; consequently, sirolimus decreases IgM, IgG, and IgA production. In rat models, it inhibits an allogeneic antibody response (12,13). The efficacy of sirolimus in preventing acute rejection after renal transplantation has been evaluated in a large multicenter trial (14), which demonstrated that sirolimus at either 2 or 5 mg/day, in combination with cyclosporine and corticosteroids, reduced the incidence of acute rejection.
The effect of concomitant immunosuppression on the in vivo humoral response to polyclonal antibodies has not been extensively evaluated. We have previously shown in renal transplant recipients that the incidence of antibody
formation to equine antithymocyte globulin (ATGAM®) was significantly lower with mycophenolate mofetil (MMF) than azathioprine (15). Antibodies developed with a peak at 2–4 weeks. This outcome was anticipated, since mycophenolate mofetil is known to inhibit B-cell proliferation and antibody production in vitro in humans and in vivo in animal models (16–18).
The influence of sirolimus on in vivo human antibody responses has not been assessed. The present study evaluates the in vivo effect of sirolimus on antibody formation associated with equine antithymocyte globulin administration in de novo renal transplant recipients.
Materials and Methods
Patients
Serum samples were collected from patients participating in one of three different Institutional Review Board-approved renal transplantation studies. Although the studies were not related and were performed at different times, they were otherwise of similar design and included recipients of a living-related donor or cadaveric renal allograft. Patients were more than age 18 and had not previously been exposed to equine-derived anti-lymphocyte preparations. Informed consent was obtained prior to enrollment in the three transplantation studies; however, the serum samples tested for anti-ATGAM antibodies were obtained and assayed as part of routine clinical practice. The analysis of the serum samples as part of this report was approved by the Institutional Review Board as an exempt study. Furthermore, the analysis was not part of any of the three clinical trials and was not supported by the sponsors of the clinical trials.
Immunosuppressant protocols
All patients received baseline immunosuppression consisting of corticosteroids, cyclosporine, and equine antithymocyte globulin. Corticosteroid administration included 1 g of methylprednisolone intravenously at the time of transplantation, 500 mg of methylprednisolone on day 1 (prior to first dose of equine antithymocyte globulin), and an oral steroid taper. Cyclosporine was given orally at a dose of 6–8 mg/kg/day. Target trough levels for cyclosporine in all arms were the same for the first month. Equine antithymocyte globulin was administered, beginning on day 1 at a dose of 15 mg/kg/day. In addition to the baseline immunosuppression, patients received one of the following additional immunosuppressive drugs within 24 h of transplantation: AZA (1–2 mg/kg/day), MMF (1 g b.i.d.), or sirolimus (9 mg load and then 2 mg/day).
Serum specimens
Sequential serum specimens for anti-equine antibody analysis were collected immediately before transplantation and weekly for at least 1 month. The samples were frozen and maintained at − 20C until assayed.
Human anti-equine antibody determination
Patient sera were diluted (1 : 100) in sample diluent that consisted of 0.5% casein and 0.5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). The 1 : 100 initial dilution was selected to avoid non–specific interactions yielding false positives. This is the same condition used in previous studies with this assay. It is possible that anti-equine antibody of titers less than 1 : 100 were not detected. Human-anti-equine antibody was detected using an enzyme-linked immunosorbent assay (ELISA). Equine antithymocyte globulin was bound to the plate and peroxidase-conjugated goat anti-human IgG (heavy/light chain reactive) was used as the secondary detect-ing reagent. The methodology previously described by Kimball et al. was followed (15). If the mean absorbance of quadruplicate wells was ≥50% of the positive control sera, which was included in each assay run, the serum specimens were considered positive. Serum specimens positive at 1 : 100 were further diluted and retested to define titers.
ELISA validation in presence of study drugs
The ELISA was tested for possible interference by MMF, AZA, or sirolimus, as previously described by Kimball et al. (15). No evidence of interference with the ELISA was detected (data not shown).
Statistical analysis
Patients were considered sensitized if they had at least one serum sample that was reactive in the ELISA assay. Patients were considered nonsensitized if they had no positive serum specimens and at least 2 negative serum specimens both obtained between days 10–30 post transplant. The percentage of anti-equine antithymocyte globulin antibody formation was compared utilizing a chi-square test with a Bonferroni correction for multiple comparisons.
Results
Sera from 42 patients who received AZA (n = 15), MMF (n = 12), or sirolimus (n = 15) were studied. Patients who had received MMF were slightly older than those receiving AZA or sirolimus (Table 1). Patients who had received sirolimus were treated with slightly fewer mean doses of equine antithymocyte globulin (Table 1). The cyclosporine (CyA) concentrations were similar in the groups at both 2 and 4 weeks post transplant.
| Sirolimus | MMF | AZA | |
|---|---|---|---|
| (n=15) | (n=12) | (n=15) | |
| Age (mean±SD) | 44±8 | 56±13 | 43±11 |
| Doses of ATGAM | 6±3 | 8±3 | 8±3 |
| CyA 2weeks | 259±111 | 305±105 | 255±114 |
| CyA 4weeks | 247±94 | 305±62 | 258±117 |
- ATGAM, equine antithymocyte globulin; AZA, azathioprine; MMF, mycophenolate mofetil; SD, standard deviation. CyA is mean±S.D ng/mL.
No patient had detectable anti-equine antibodies at baseline. Anti-equine antibody formation was significantly different among the three treatment groups (Figure 1). Fewer patients receiving MMF (17%, p = 0.007) or sirolimus (13%, p = 0.003) were sensitized to equine antithymocyte globulin compared with patients receiving AZA (66%). There was no significant difference (p = 0.81) in the rate of sensitization to equine antithymocyte globulin for the patients receiving MMF compared to those receiving sirolimus. In the sensitized patients, high anti-equine antibody titers (≥1 : 500) were more common in those receiving AZA (n = 3) than MMF (n = 0) or sirolimus (n = 1). There were no clinical consequences noted in those patients who developed anti-equine Ig responses.
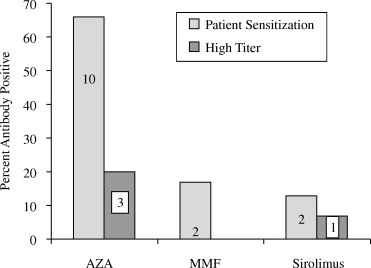
Incidence of sensitization to equine antithymocyte globulin and high IgG anti-equine antithymocyte globulin titers ( ≥1 : 500).
Discussion
This is the first report of the influence of sirolimus on the in vivo humoral response in humans. Sirolimus, when added to cyclosporine A, significantly reduced anti-equine antibody formation. Fewer patients receiving sirolimus or MMF were sensitized to equine antithymocyte globulin than those receiving AZA. The rate of antibody formation in the AZA group was similar to an earlier study from our group, implying that the lower rate in the sirolimus group was not due to an aberrantly high response in the AZA group. Kimball et al., in collaboration with our group, first reported that MMF reduced the anti-equine response. With regard to the AZA and MMF groups in the current report, there was significant overlap in the patients studied, such that the current results add little to those findings (15). The new findings resulting from the present study are that sirolimus, when given with cyclosporine A, also significantly decreases the incidence of sensitization to equine antithymocyte globulin compared with AZA and at a rate comparable to MMF.
It is unlikely that the small demographic differences among the treatment groups influenced the results. Age has been inversely related to antibody formation, with older patients less likely to form antibodies, but this is most pronounced in the pediatric age group (9). Since the patients treated with MMF tended to be older, the rate of antibody formation may have been lower in that group secondary to their age or other factors. This concern is not relevant to the sirolimus vs. AZA comparison, since the ages are similar. Although the patients receiving sirolimus were treated with fewer equine antithymocyte globulin doses, duration of therapy did not influence the incidence of antibody formation in other studies (9,15). It is not possible from these data to exclude that the inhibition seen with sirolimus compared to AZA is solely due to sirolimus. It is possible that an interaction between sirolimus and cyclosporine leading to enhanced immunosuppressive activity, which does not occur with AZA and cyclosporine, is the immediate cause. This could only be tested in patients who receive sirolimus and corticosteroids without cyclosporine, which was not done in these studies. A similar argument can also be made regarding the inhibition seen with MMF compared to AZA.
Fewer patients treated with sirolimus or MMF tended to exhibit high anti-equine antithymocyte globulin titers (≥1 : 500). Patients with high antibody titers (≥1 : 500) are reported to experience inferior allograft survival (9). Since antibody formation has been associated with a 3-fold increase in the probability of allograft failure (9), the clinical relevance of sirolimus attenuating anti-equine antithymocyte globulin antibody formation may be significant.
Serum sickness is a well-described consequence of sensitization to polyclonal antibodies (4,5,7,8). Another significant outcome due to sensitization to equine antithymocyte globulin may include immune-complex mediated graft injury (4). Additionally, reduced immunosuppressive efficacy, resulting from immune complex formation and accelerated clearance of equine antithymocyte globulin from circulation, may predispose sensitized patients to lower allograft survival rates (9). These potential consequences, however, were not seen in this study.




