Interaction between metabolism of atmospheric H2S in the shoot and sulfate uptake by the roots of curly kale (Brassica oleracea)
Abstract
Exposure of curly kale (Brassica oleracea L.) to gaseous H2S resulted in a decreased sulfate uptake by the roots. At 0.2 μl l−1 H2S, a level sufficient to meet the sulfur need of plants for growth, the sulfate uptake was maximally decreased by 50% after 3 or 4 days of exposure. Higher levels up to 0.8 μl l−1 H2S did not further affect the sulfate uptake. The nitrate uptake was not affected upon exposure to 0.2–0.8 μl l−1 H2S. H2S exposure did not affect the sulfate content of the plants, but it resulted in an increased content of thiols and cysteine in the shoots, whereas that in the roots was hardly affected. Plants grown under sulfate-deprived conditions had a decreased biomass production, very low content of sulfate and decreased content of thiols in both shoot and roots. Sulfate-deprived plants had a two-fold higher sulfate uptake after transfer to a sulfate-containing solution, while nitrate uptake was decreased by 50%. When sulfate-deprived plants were exposed to 0.25 μl l−1 H2S, plant biomass production and nitrate uptake were restored but the sulfate uptake after transfer to a sulfate-containing solution remained high. Also here, H2S exposure resulted in an increase in the thiol and cysteine content of both shoot and roots, whereas the content of sulfate remained low. The presented results clearly demonstrate a direct interaction between the regulation of sulfate uptake by the roots and the metabolism of gaseous H2S by the shoot. Curly kale is able to use both sulfate and H2S as a sulfur source for growth, and matching the supply of sulfur in the form of pedospheric or atmospheric sulfur to the sulfur needed for growth appears to be regulated nicely. However, the significance of thiols as signal in the shoot/root coordination of sulfate uptake appears to be limited. From the data it is evident that there is no direct mutual regulation between the uptake of sulfate and nitrate by the roots.
Abbreviations
-
- DTNB
-
- 5,5′-dithiobis(2-nitrobenzoic acid)
-
- RGR
-
- relative growth rate
Introduction
Generally plants utilize sulfate taken up by the roots as the sulfur source for growth. The major proportion of the sulfate taken up is reduced to sulfide, which is subsequently incorporated into various organic sulfur compounds ( Brunold 1990; Cram 1990; Clarkson et al. 1993). The predominant proportion of the reduced sulfur (up to 90%) is present in the protein fraction as cysteine and methionine ( Giovanelli 1990; Stulen and De Kok 1993). Plant roots contain all enzymes of the assimilatory sulfate reduction pathway, however, the contribution of the roots to the plant's reduced sulfur need is still obscure ( Brunold 1993). The main proportion of the sulfate taken up by the roots is loaded into the xylem vessels and transported to the shoot by the transpiration stream; in the shoot it is reduced in the chloroplasts and subsequently further metabolized ( Brunold 1990; Clarkson et al. 1993). The uptake of sulfate by the roots is mediated by a sulfate transporter protein ( Cram 1990; Clarkson et al. 1993; Hawkesford and Smith 1997). The regulation and expression of the sulfate transporter is controlled by the sulfur nutritional status of the plant. Sulfate itself, or a metabolic product of sulfate assimilation, may be involved as a signal in the regulatory control of the uptake and transport of sulfate ( Cram 1990; Clarkson et al. 1993; Smith et al. 1995; Hawkesford and Smith 1997). It has been proposed that shoot to root allocation of glutathione by phloem transport may fulfill a signal function in the regulation of the uptake of sulfate by the roots and its loading into the xylem ( Herschbach and Rennenberg 1991, 1994; Herschbach et al. 1995a,b; Lappartient and Touraine 1996). However, the specificity of glutathione is questionable since glutathione analogues and various amino acids were also able to decrease sulfate uptake by tobacco roots ( Gunz et al. 1993).
It has been suggested that a regulatory linkage between the uptake of sulfate and nitrate may exist in plants ( Cram 1990; Vidmar et al. 1999). Nitrate uptake appears to be closely linked to the relative growth rate and its regulation is thought to be mediated by reduced nitrogen compounds or the nitrate concentration at the cytoplasmic site ( Rodger and Barneix 1988; Zhang and MacKown 1993; Muller et al. 1995; Ter Steege et al. 1999).
Chronically high levels of sulfurous air pollutants may be phytotoxic ( De Kok 1990; De Kok et al. 1998). On the other hand, H2S and SO2 may be beneficial and utilized as the sulfur source for plant growth, especially when the sulfur supply to the roots is limited ( Brunold and Erismann 1974, 1976; De Kok 1990). Plants are able to actively take up gaseous H2S by their shoots, and H2S may completely replace pedospheric sulfate as the sulfur source ( De Kok 1990; De Kok et al. 1991, 1997, 1998). H2S is assimilated with high affinity into cysteine and subsequently, into other sulfur metabolites ( De Kok 1989, 1990; De Kok et al. 1998). H2S exposure generally results in a slight overload of the plant's sulfur supply, thereby increasing the size and altering the composition of the thiol pool. It generally results in strongly enhanced glutathione levels in both shoots and roots ( De Kok 1990; Buwalda et al. 1993, 1994; Poortinga and De Kok 1995). H2S exposure may reduce sulfate uptake by the roots and its loading into the xylem ( Brunold and Erismann 1974; Herschbach et al. 1995a,b; De Kok et al. 1997, 1998).
In order to get more insight into the signals involved in the regulation of sulfate uptake by the roots in relation to the sulfur demand for growth, the interaction between the uptake of H2S by the shoot and uptake of sulfate by the roots was investigated in Brassica oleracea L. (curly kale). Nitrate uptake was determined simultaneously in order to get more insight into the possible interaction between sulfate and nitrate uptake. Furthermore, the significance of thiols as a regulatory signal in the coordination between shoot and roots was evaluated.
Materials and methods
Plant material
Seeds of curly kale (Brassica oleracea L. cv. Bornick F1; Nickerson-Zwaan, The Netherlands) were germinated in vermiculite in a climate-controlled room. Day and night temperatures were 22 and 18°C, respectively, the relative humidity was 60–70% and the photoperiod was 12 h at a photon fluence rate of 250 μmol m−2 s−1 (PAR 400–700 nm, measured with a quantum sensor, SKP215, Skye, Llandrindod Wells, UK), supplied by Osram TL 31 and 21 in a ratio of 2:1.
Exposure to H2S
Plants were exposed to H2S in 150-l cylindrical stainless steel cabinets (diameter 0.6 m) with a polycarbonate top, as described by Stuiver et al. (1992). Day and night temperatures were 20 and 16°C (±1°C), respectively, relative humidity was 55±5% and the photoperiod was 14 h at a photon fluence rate of 150–180 μmol m−2 s−1 (PAR 400–700 nm), with a Philips HPI-T (400 W) light source or at a photon fluence rate of 250–300 μmol m−2 s−1 (PAR 400–700 nm range), with a Philips HPL(R)N (400 W) light source. The air temperature was controlled by adjusting the cabinet wall temperature, the air exchange was 40 l min−1 and the air inside the cabinets was stirred continuously by a ventilator. Pressurized H2S diluted with N2 (1 ml l−1) was injected into the incoming air stream and adjusted to the desired level by ASM electronic mass flow controllers (Bilthoven, The Netherlands). The H2S level in the cabinets was controlled with an SO2 analyzer (model 9850) equipped with a H2S converter (model 8770, Monitor Labs, Measurement Controls Corporation, Englewood, CO, USA).
For studies on the impact of H2S on sulfate uptake with time, 12-day-old seedlings were grown in a climate-controlled room (see above) in a 25% Hoagland nutrient solution (30-l tanks, 60 plants per tank) as described by Herschbach et al. (1995b). After 2 weeks, plants were transferred to 12-l stainless steel containers (19.5×15.0×45.0 cm) filled with freshly prepared 25% Hoagland nutrient solution and placed in the cabinets for 7 days. For sulfate uptake measurements, plants were transferred to vessels with freshly prepared 25% Hoagland nutrient solution containing 0.5 mM sulfate (3 plants per 1 l) and exposed to 0 or 0.2 μl l−1 H2S at a photon fluence rate of 250–300 μmol m−2 s−1 (PAR 400–700 nm). Sulfate uptake was measured by removing an aliquot from the nutrient solution after adjustment to the original volume, after 1, 2, 3, 5 and 7 days of the exposure period over a 24-h period.
For studies on the uptake of sulfate at various H2S levels, 12-day-old seedlings were grown in a climate-controlled room (see above) on 25% Hoagland nutrient solution (30-l tanks, 60 plants per tank) for 2 weeks. The plants were acclimated for 1 day in the cabinets at a photon fluence rate of 250–300 μmol m−2 s−1 (PAR 400–700 nm) after transfer to 12-l stainless steel containers (19.5×15.0×45.0 cm) filled with 25% Hoagland nutrient solution. Subsequently, the plants were transferred to vessels with fresh 25% Hoagland nutrient solution (3 plants per 1 l) in the cabinets and exposed to 0, 0.2, 0.4 or 0.8 μl l−1 H2S at a photon fluence rate of 250–300 μmol m−2 s−1 (PAR 400–700 nm) for 3 days. Sulfate uptake was measured at the various H2S levels over a 72-h period. The level of nutrient solution was adjusted with distilled water when needed.
For studies on the impact of sulfate nutrition and H2S exposure on the sulfate uptake, 10- to 13-day-old seedlings were transferred to a 25% Hoagland nutrient solution at 0 or 0.5 mM sulfate (30-l tanks, 60 plants per tank) and grown in a climate-controlled room (see above) for 1 week. In the Hoagland solution without sulfate, MgSO4 was replaced with MgCl2. Subsequently, plants were transferred to 12-l stainless steel containers (19.5×15.0×45.0 cm) filled with 25% Hoagland nutrient solution at 0 or 0.5 mM sulfate and placed in the cabinets and exposed to 0 or 0.25 μl l−1 H2S at a photon fluence rate of 150–180 μmol m−2 s−1 (PAR 400–700 nm) for 1 week. Sulfate uptake was measured in the cabinets over a 24-h period after transfer to vessels with fresh aerated 25% Hoagland nutrient solution at 0.5 mM sulfate (3 plants per 1.1 l).
Thiol and cysteine content
Leaf tissue was homogenized at 0°C with an Ultra Turrax (Polytron pt 3000, Kinematica AG, Littau, Switzerland) for 15 s in a mixture of 80 mM sulfosalicylic acid, 1 mM EDTA and 0.15% (w/v) ascorbic acid; (1 g fresh weight in 10 ml solution). Oxygen from the solution had been previously removed by N2 bubbling. The homogenate was filtered through one layer of Miracloth and the filtrate was centrifuged at 30 000 g for 15 min (0°C). The determination of cysteine was based on the reactivity of its sulfhydryl group with methylglyoxal according to De Kok et al. (1988), and was determined by adding 1 ml of the 30 000 g deproteinized supernatant extract to 0.1 ml 0.1 mM methylglyoxal. After a 10-min incubation at room temperature, 0.1 ml 10 mM DTNB (pH 7.0) and 2 ml 0.4 M Tris-HCl, pH 8.0, were added and the yellow color developed was directly measured at 415 nm ( De Kok et al. 1988).
Anion content
The sulfate and nitrate contents in the nutrient solution and in plant tissue were determined according to the method of Maas et al. (1986). Leaf tissue was homogenized in demineralized water at 0°C with an Ultra Turrax (Polytron pt 3000, Kinematica AG) for 15 s (1 g in 10 ml). The homogenate was incubated in tubes in a waterbath at 100°C for 10 min, filtered through one layer of Miracloth and the filtrate was centrifuged at 30 000 g for 15 min (0°C). The anions were separated on a IonoSpher A anion exchange column (250×4.6 mm; Chrompack, Middelburg, The Netherlands) and 25 mM potassium biphthalate (pH 4.3), containing 0.02% (w/v) Na azide, was used as a mobile phase. The flow rate was 1 ml min−1; detector temperature was kept at 25°C by a waterbath. A Knauer differential refractometer (model 98.00, Bad Homburg, Germany) was used as detector.
Sulfate and nitrate uptake
For measurements on the sulfate and nitrate uptake 3 plants were transferred to vessels containing exactly 1 or 1.1 l, depending on the experiment, of freshly prepared 25% Hoagland nutrient solution at 0.5 mM sulfate and 3.75 mM nitrate. At the start of the uptake measurements and after 24 or 72 h, after adjusting the nutrient solution to the original volume, an aliquot was taken from the nutrient solution and the sulfate and nitrate contents were analyzed as described above. The average degree of sulfate and nitrate depletion was 0.15 and 1.5 mM, respectively. The uptake of sulfate and nitrate was calculated as the difference in ion amount (μmol) between samples taken at the start and after 24 or 72 h, divided by the total root or plant fresh weight (g) after 24 and 72 h, and expressed as μmol g−1 fresh weight [24 h]−1 or μmol g−1 fresh weight [72 h]−1. In sulfate-deprived plants, the sulfate uptake capacity was determined after transferring the plants to a sulfate-containing nutrient solution.
Data analysis
All experiments were carried out at least twice and gave similar results. Data of typical experiments are shown. All statistical analyses were performed with the GraphPad Prism Package (GraphPad Software, Inc. 1994–1996, San Diego, CA, USA). Differences found between the treatments for sulfate and nitrate uptake, thiol and cysteine content and the sulfate content were tested using Student's t-test.
Results
The impact of H2S exposure on sulfate uptake with time
When curly kale was exposed to 0.2 μl l−1 H2S, a level sufficient to meet the plants sulfur demand for growth ( De Kok et al. 1998), it resulted in a decreased sulfate uptake by the roots. On the second day of exposure to 0.2 μl l−1 H2S, the sulfate uptake, calculated on both root and plant fresh weight basis, was significantly decreased by 30% ( Fig. 1a,b). The sulfate uptake, expressed on both root and plant fresh weight basis, was maximally decreased by 50% at the third day of exposure. The nitrate uptake remained unaffected during the whole exposure period ( Fig. 1c,d). The decrease in sulfate uptake was not caused by a decrease in growth, since the RGR and the shoot/root ratio of the plants remained unaffected upon H2S exposure (see legend to Fig. 1).
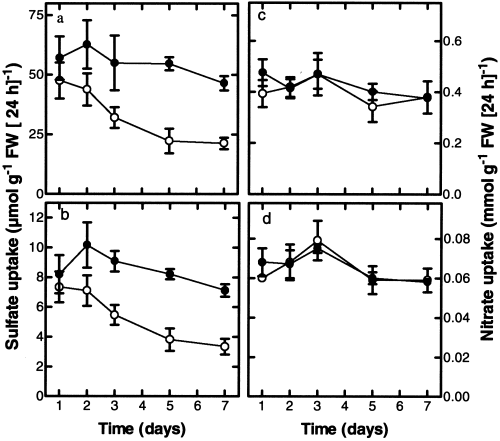
The impact of H2S exposure on the sulfate and nitrate uptake with time. Two-week-old plants were exposed for 7 days to ambient air (●) or 0.2 μl l−1 H2S (○). The uptake of sulfate calculated on a root (a) and a plant (b) FW basis, and the uptake of nitrate calculated on a root (c) and a plant (d) FW basis was measured over a 24-h period. The RGR calculated on a FW basis and measured over a 7-day interval of plants exposed to 0 and 0.2 μl l−1 was 20.6 and 20.7% day−1, respectively. The shoot/root ratio on day 7 of plants exposed to 0 and 0.2 μl l−1 was 5.5±0.2 and 5.4±0.9, respectively. Data on day 1, 2, and 3 represent the mean of 12 measurements and on day 5 and 7 the mean of 6 measurements with 3 plants in each ±sd.
The effect of various levels of H2S on sulfate uptake
To investigate to what extent the observed decrease in sulfate uptake was dependent on the level of H2S, plants were exposed for 3 days to levels ranging from 0.2 to 0.8 μl l−1. Sulfate uptake was measured over a 72-h period. Sulfate uptake, expressed on a root fresh weight basis, was decreased by 12, 32 and 37%, at 0.2, 0.4 and 0.8 μl l−1 H2S, respectively ( Fig. 2a). When expressed on a plant fresh weight basis sulfate uptake was decreased by 20, 50 and 54%, at 0.2, 0.4 and 0.8 μl l−1 H2S, respectively ( Fig. 2b). At all H2S levels, nitrate uptake was not significantly affected ( Fig. 2c,d). The fresh weight and shoot/root ratio were not significantly affected upon H2S exposure (see legend to Fig. 2). The thiol and cysteine content of the shoot increased linearly up to 0.4 μl l−1 H2S ( Fig. 3a,b). In the roots, exposure to 0.2 μl l−1 H2S did not cause an increase in the level of thiols, but exposure to 0.4 and 0.8 μl l−1 H2S caused a significantly increased thiol content. The cysteine content in the roots remained unaffected by H2S exposure. The sulfate content in the shoot and roots was slightly decreased upon exposure to 0.4 and 0.8 μl l−1 H2S ( Fig. 3c).
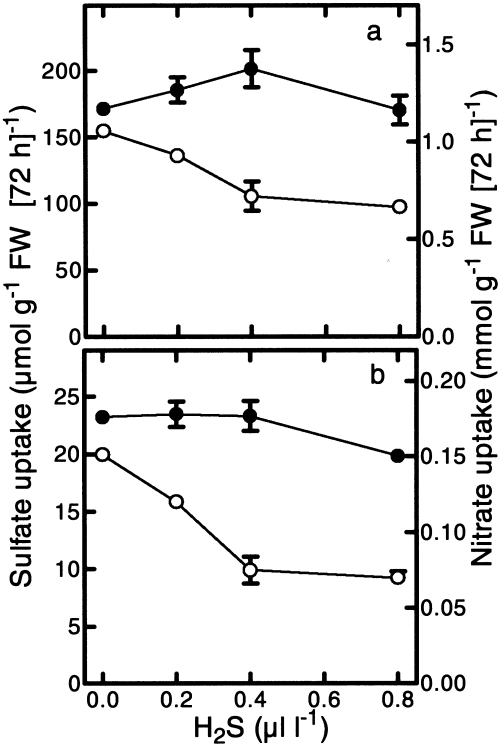
The impact of various H2S levels on the uptake of sulfate and nitrate. Plants were exposed to 0, 0.2, 0.4 and 0.8 μl l−1 H2S for 72 h. The uptake of sulfate (○) and nitrate (●), calculated on a root (a) and plant (b) FW basis, was measured over a 72-h period. The initial plant FW was 2.33±0.38 g and the average shoot/root ratio was 6.3±0.5 g. Data represent the mean of 6 measurements with 3 plants in each ±sd.
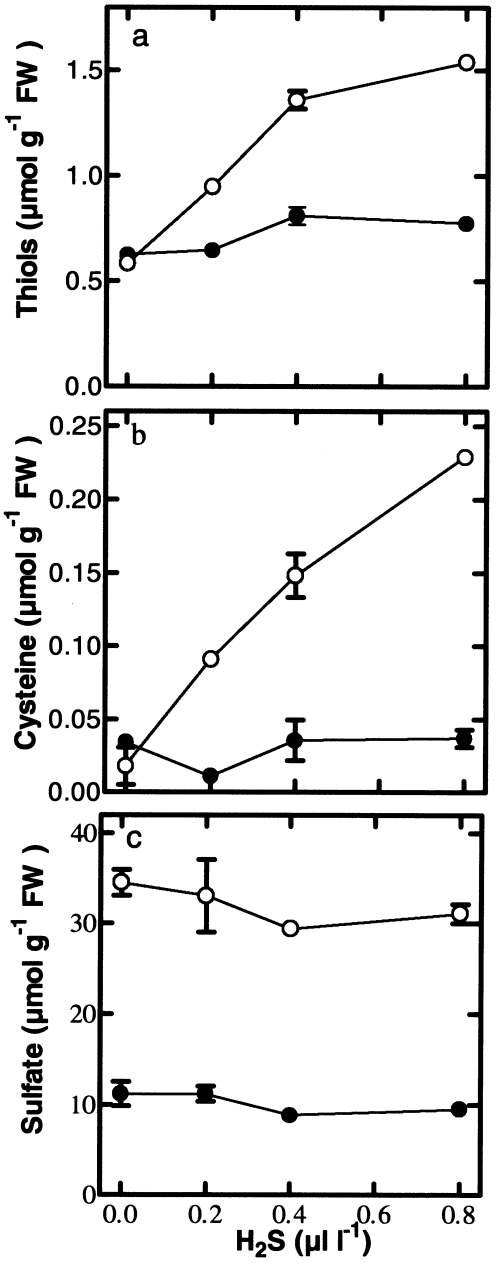
The impact of various H2S levels on the sulfate, thiol and cysteine content. Plants were exposed to 0, 0.2, 0.4 and 0.8 μl l−1 H2S for 72 h. The thiol (a), cysteine (b) and the sulfate (c) content of the shoot (○) and the roots (●) is shown. Data represent the mean of 6 measurements with 3 plants each ±sd.
The effect of sulfate nutrition and H2S exposure on the sulfate uptake
If plants were grown under sulfate-deprived conditions for 2 weeks, it resulted in a decreased biomass production, as compared to control plants grown at ample sulfate supplied to roots ( Table 1). This decrease in biomass production was more pronounced in the shoot than in the roots. Furthermore, sulfate-deprived plants were characterized by a very low content of sulfate and thiols in both shoot and roots. The content of nitrate in the shoot of the sulfate-deprived plants was significantly enhanced, whereas that in the roots was similar to that in control plants. The sulfate uptake capacity of sulfate-deprived plants was two-fold higher than that of control plants grown at ample sulfate supplied to the roots. The nitrate uptake of sulfate-deprived plants was 50% lower than that of the control plants.
| Parameter | −Sulfate | +Sulfate | ||
|---|---|---|---|---|
| −H2S | +H2S | −H2S | +H2S | |
| Shoot | ||||
| FW (g) | 0.73±0.07a | 1.20±0.11b | 1.06±0.12b | 1.04±0.11b |
| Thiols (μmol g−1 FW) | 0.349±0.008a | 0.889±0.030b | 0.484±0.016c | 0.965±0.019d |
| Sulfate (μmol g−1 FW) | 0.2±0.1a | 1.6±0.2b | 42.2±0.7c | 36.0±2.4d |
| Nitrate (μmol g−1 FW) | 115.1±6.7a | 85.5±3.3b | 87.8±2.7b | 95.0±6.0c |
| Roots | ||||
| FW (g) | 0.184±0.016a | 0.279±0.046b | 0.216±0.028b | 0.205±0.027b |
| Thiols (μmol g−1 FW) | 0.136±0.021a | 0.404±0.020b | 0.697±0.039c | 0.715±0.021c |
| Sulfate (μmol g−1 FW) | 0.76±0.01a | 1.65±0.13b | 15.6±0.80c | 14.3±0.70c |
| Nitrate (μmol g−1 FW) | 42.3±2.6a | 43.5±3.4a | 45.2±2.1a | 54.2±6.1b |
| Ion uptake | ||||
| On a root FW basis | ||||
| Sulfate (μmol g−1 FW [24 h]−1) | 74.3±18a | 75.5±14a | 43.2±8.9b | 24.4±11c |
| Nitrate (μmol g−1 FW [24 h]−1) | 145±51a | 305±55b | 354±60b | 365±70b |
| On a plant FW basis | ||||
| Sulfate (μmol g−1 FW [24 h]−1) | 13.4±2.4a | 13.6±1.2a | 6.6±0.9b | 3.0±1.0c |
| Nitrate (μmol g−1 FW [24 h]−1) | 26.4±11.1a | 57.1±9.7b | 56.4±7.2b | 53.3±8.3b |
When sulfate-deprived plants were exposed to 0.25 μl l−1 H2S, the biomass production was comparable to that of the control plants grown with ample sulfate supply without H2S ( Table 1). The thiol content of both the shoot and roots was significantly increased by H2S exposure in sulfate-deprived plants. The sulfate content in both the shoot and roots was slightly increased by H2S exposure, but remained very low. The nitrate content of the shoot returned to levels similar to those of the control plants, while that in the roots remained unaffected. The high sulfate uptake capacity found upon sulfate-deprivation was not affected by exposure to 0.25 μl l−1 H2S. The uptake of nitrate increased to a value similar to that observed in control plants.
The biomass production of the plants grown with an ample amount of sulfate in the nutrient solution and simultaneously exposed to H2S was not affected ( Table 1). The thiol content was not increased in the roots compared to the controls, but the thiol content of the shoot was strongly increased. The nitrate content remained unaffected upon H2S exposure. The sulfate content was slightly decreased in the shoot and remained unaffected in the roots upon H2S exposure. The sulfate uptake of the plants grown at an ample supply of sulfate in the root environment and which were simultaneously exposed to 0.25 μl l−1 H2S decreased by 40%, whereas the nitrate uptake was unaffected.
Discussion
H2S exposure and regulatory aspects of sulfate uptake
The sulfate uptake by the roots can be regulated at the level of gene expression and/or post-translational regulation of the transporter protein by the in situ concentrations of sulfate and/or thiol compounds such as cysteine or glutathione ( Cram 1990; Clarkson et al. 1993; Hawkesford and Smith 1997). Furthermore the transport of sulfate from the roots to the shoot, viz. the xylem-loading, is under direct metabolic control as well ( Herschbach and Rennenberg 1991; Clarkson et al. 1993; Herschbach et al. 1995a,b). The rate of sulfate uptake by the roots and its transport to the shoot responds to changes in the sulfur nutritional status. When plants were grown without an external sulfate supply the expression of the sulfate transporter proteins was already increased within 1 day ( Hawkesford and Smith 1997). After re-supplying sulfate to the roots, the mRNA pool-size decreased and the uptake capacity decreased rapidly within a few hours to levels comparable to those of the control plants. Exposure of plants to sulfurous air pollutants resulted in a reduced xylem-loading of sulfate ( Herschbach et al. 1995a,b).
The present study showed that the increased sulfate uptake capacity of the sulfate-deprived plants was not affected by H2S exposure. Exposure to H2S did result in an increase of the thiol content in the roots to values found in the plants grown with ample sulfate supplied to the roots. However, H2S exposure resulted in a decrease in the sulfate uptake capacity of plants that were grown with ample sulfate. The thiol content of the roots of these plants was unaffected by H2S ( Table 1). These results suggest that the thiol content of the root tissue was not directly involved in the regulation of the sulfate uptake. However, the possibility remains open that the sulfate uptake at the xylem-loading site is very sensitive to even a very small increase in the level of thiols, which are transported from the shoot to the roots via the phloem. The absence of a reduction of the sulfate uptake of the plants grown without an external sulfate supply upon exposure to H2S could possibly be explained by the extremely low sulfate content in the roots at sulfur-deprived conditions. The low content of sulfate might overrule the signal coming from the shoot and in this way prevent a reduction in sulfate uptake similar to that which occurred upon H2S exposure of plants grown with an ample amount of sulfate in the nutrient solution. O-acetyl- l-serine is thought to play a regulatory role in the interaction between nitrogen and sulfur assimilatory pathways, and under conditions of sulfur starvation may be involved in the stimulation of sulfate uptake capacity ( Clarkson et al. 1999; Leustek and Saito 1999). It is not the content of glutathione or cysteine alone, but possibly a combination of factors that is involved in the regulation of the uptake of sulfate in plants. The nature of the signal remains unclear and needs to be further investigated, e.g. with the use of labeled H2S.
Previous experiments have demonstrated that exposure of curly kale for 3 weeks to a H2S level sufficient to meet the sulfur demand for growth resulted in a 38% reduction in the net sulfate uptake by the roots ( De Kok et al. 1997, 2000). This reduction was achieved after 1 week of exposure to 0.25 μl l−1 H2S ( De Kok et al. 1997). Upon a 3-day exposure to 0.2 μl l−1 H2S, the sulfate uptake was decreased by a maximum of 40–50%, even though the thiol and cysteine content of the roots were not increased, whereas that of the shoot was increased ( 2, 3). Higher H2S levels did cause an increase of the thiol content in both shoot and roots, but did not further affect the sulfate uptake ( Fig. 2). These results suggest that the thiol content of the root tissue was not directly involved in the regulation of the sulfate uptake. It seems to be rather conflicting that the uptake of sulfate is not completely downregulated even though 0.2 μl l−1 H2S supply should be sufficient to cover the sulfur need of curly kale for growth. De Kok et al. (2000) showed that the organic sulfur content of H2S-exposed curly kale plants, which were grown without any sulfate in the root environment, was 40% of that in the plants grown at ample sulfate supplied. The total organic sulfur content of the latter plants was not affected by H2S exposure, not even at levels up to 0.8 μl l−1. Apparently the proportion of H2S taken up by the shoot was sufficient to cover the organic sulfur need for growth, and the proportion of the sulfate taken up by the roots that ended up in the organic sulfur pool appeared to be replaced by sulfur absorbed from the atmosphere. Evidently, curly kale is able to switch from sulfate to H2S as the source for its reduced sulfur and the matching of the supply from the pedosphere or the atmosphere to the need for growth appears to be well regulated.
H2S exposure and interaction between sulfate and nitrate uptake
Sulfate uptake measured over a 24-h period, was doubled and the uptake of nitrate was decreased by 50%, when plants were grown without any sulfate in the nutrient solution for 2 weeks ( Table 1). In previous experiments it has also been demonstrated that, in curly kale suffering from severe sulfur deficiency, the net nitrate uptake was strongly decreased ( Stuiver et al. 1997). The latter was related to the reduction in growth occurring upon sulfur depletion. In higher plants in general the net nitrate uptake rate appears to be closely linked to the relative growth rate ( Rodger and Barneix 1988; Muller et al. 1995; Ter Steege et al. 1999). In the present study, the plants that were not exposed to H2S and were grown without sulfate in the root environment had a decreased biomass production compared to the plants grown with an ample supply of sulfate ( Table 1). When plants that were grown without sulfate were exposed to 0.25 μl l−1 H2S, the biomass production was restored and nitrate uptake was recovered. However, in contrast, sulfate uptake did not return to the levels measured in control plants grown with an ample sulfate amount supplied to the roots. Sulfate uptake remained enhanced to the same extent as in the sulfur-deprived plants. When plants were grown under conditions with an ample supply of sulfate to the roots and a simultaneous exposure to H2S, the biomass production of the plants was not changed. Sulfate uptake was decreased by 50% but the nitrate uptake remained unaffected ( Fig. 2). Apparently, the impact of H2S exposure on the uptake of plants grown with an ample sulfate supply to the roots was specific for sulfate. These results indicate that the sulfate and nitrate uptake mechanisms are regulated in a different way, since upon exposure to H2S no direct linkage is present between the uptake of these anions. The consequences of the exposure to H2S for the balance between total N and S remains to be investigated.




