Clinical outcomes for nasopharyngeal cancer with intracranial extension after taxane-based induction chemotherapy and concurrent chemo-radiotherapy in the modern era
Abstract
Objective
To evaluate the survival outcomes for a cohort of nasopharyngeal cancer with intracranial extension (ICE) treated with induction chemotherapy (ICT) followed by chemo-intensity-modulated radiotherapy (CTRT) at a tertiary cancer center.
Methods
We retrospectively analyzed 45 patients with histologically proven, non-metastatic NPC with ICE treated at our institute between October 2008 and October 2016. Patients were classified as minor ICE or major ICE, based on the extent of ICE. All the patients received 2–3 cycles of a taxane-based ICT regimen followed by CTRT. Radiotherapy was delivered with “risk-adapted” intensity-modulated radiotherapy (IMRT) technique in all patients.
Results
After a median follow up of 45 months (range: 8–113 months), the estimated 5-year DFS, LRFS, DMFS, and OS of the entire cohort was 58%, 82%, 67% and 74% respectively. On multivariate analysis, histological subtype was an independent predictor of LRFS, and age was an independent predictor of DFS. The extent of ICE showed only a trend towards worse DFS (P = 0.06). None of the factors significantly predicted for DMFS or OS. Gender, N-stage, and response to ICT did not significantly affect any of the outcomes. Grade 2 or worse subcutaneous fibrosis was seen in 22% of patients and grade 2 or worse xerostomia was seen in 24% of patients at last follow up. Thirty-three percent of the patients developed clinical hypothyroidism at last follow up. None of the patients experienced any neurological or vascular complications.
Conclusions
Taxane-based induction chemotherapy followed by chemo-intensity modulated radiotherapy resulted in excellent locoregional control and survival with acceptable toxicities in patients of nasopharyngeal cancer with intracranial extension. Distant metastasis continues to be the predominant problem in these patients.
Background
Nasopharyngeal cancer (NPC) is a chemo/radiosensitive malignancy and chemo-radiotherapy (CTRT) is considered the standard of care for the management of locally-advanced nasopharyngeal cancer (LA-NPC) after the results of various phase 3 trials.1-5 However, patients with intracranial extension (ICE) typically did poorly even after CTRT owing to the limitations of conventional radiotherapy (2DRT). The proximity of the tumor to the critical neural structures (brain stem, optic nerves and temporal lobes) and inability to shape the dose distributions around these structures resulted in lower 5-year survival ranging between 20% and 30% in these patients.6-8
A major breakthrough for these patients came in the form of intensity-modulated radiotherapy (IMRT), with highly conformal dose distributions and steep dose gradients, IMRT was able to achieve a higher dose to the tumor with a better organ at risk sparing compared to conventional radiotherapy techniques.9,10 Encouraging results with IMRT have been reported across various studies with an associated survival benefit, which was mostly due to an improvement in outcomes of locally advanced disease.11,12 The 5-year survival for patients with ICE ranges between 50% and 60% across various IMRT series with most patients failing at distant sites.13,14
Recently, a large phase III trial of induction chemotherapy (ICT) followed by CTRT versus CTRT alone in LA-NPC showed a significant disease-free survival (DFS) and overall survival (OS) benefit in the ICT arm due to a reduction in distant metastases rate.15 ICT also provides other advantages like reduction of the primary tumor to give a wider margin for irradiation, which would result in a reduction of radiotherapy (RT) portals, allowing delivery of optimal radiation doses as well as improved dose conformity, better ability to spare the adjacent critical structures and improved tumor control probability.16,17
In the current study we aim to report the outcomes of NPC patients with ICE treated with ICT followed by Chemo-IMRT.
Materials and methods
Patient and tumor characteristics
We retrospectively analyzed 45 patients of NPC with ICE treated with ICT followed by CTRT at our institute during the period October 2008 to October 2016. All patients had a biopsy-proven, non-metastatic and magnetic resonance imaging (MRI) staged NPC with an ICE. Pre-treatment workup included a complete physical examination, nasopharyngoscopy, a biopsy from the primary/lymph node, MRI scan of the head and neck region and a metastatic work up with a PET-CT in all patients. Patients were restaged according to the 2008 American Joint Committee on Cancer (AJCC) TNM staging system.
Most patients underwent an audiometry, ophthalmologic evaluation and regular TFT at baseline and at follow-up.
ICE was further subcategorized as minor involvement defined as tumor extension limited to parasellar region/cavernous sinuses, and major involvement defined as tumor extending above and beyond the cavernous sinuses or into the orbit and the ethmoid sinus anteriorly, or to the prepontine region and the posterior cranial fossa (Fig. 1).
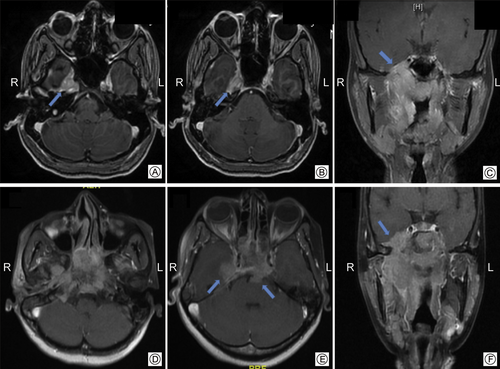
Axial and coronal MRI images demonstrating minor intracranial extension (arrows) limited to the cavernous sinuses or parasellar regions (A, B, C) and major intracranial involvement with extension beyond the cavernous sinus regions (arrows), into the prepontine regions (arrows) (D, E, F).
Table 1 highlights the relevant patient and tumor-related characteristics. Median age was 36 years (range: 18–61 years) and the majority (62%) were males. The most common histological subtype was undifferentiated carcinoma in 89% of patients and majority (69%) had an advanced (N2–N3) nodal stage.
| Characteristics | Numbers | Percentages (%) | |
|---|---|---|---|
| Gender | Male | 28 | 62 |
| Female | 17 | 37 | |
| Age group | <35 yr | 21 | 47 |
| >35 yr | 24 | 53 | |
| Histology | KSCC | 3 | 7 |
| NKSCC | 2 | 4 | |
| UD | 40 | 89 | |
| Nodal stage (N) | N0 | 06 | 13 |
| N1 | 08 | 18 | |
| N2 | 22 | 49 | |
| N3 | 09 | 20 | |
| Intracranial extension | Minor | 21 | 47 |
| Major | 24 | 53 | |
- KSCC: keratinizing squamous cell carcinoma; NKSCC: non-keratinizing squamous cell carcinoma; UD: undifferentiated carcinoma.
Treatment details and characteristics
All patients received a taxane-based ICT regimen repeated every three weeks for 2–3 cycles, this was followed by intensity modulated radiotherapy with concurrent weekly Cisplatin.
Chemotherapy details
Induction chemotherapy consisted of either a TIP regimen: Paclitaxel IV 175 mg/m2 (Day 1) + Ifosfamide IV 1200 mg/m2 (Day 1–5) + Cisplatin IV 15 mg/m2 (Day 2–6), the ICT protocol between 2008 and 2012 period, or a TPF regimen: Docetaxel IV 75 mg/m2 (Day 1) + Cisplatin IV 75 mg/m2 (Day 1) + 5-Fluorouracil IV 750 mg/m2 (Day 1–5), the protocol during later period (2012–2015) with appropriate concomitant medications. G-CSF prophylaxis was administered to all patients on the TPF regimen. Elderly patients or patients considered, not suitable for three drug regimen were treated with a two-drug regimen of Paclitaxel + Carboplatin (PC, during the 2008–2012 period) or Docetaxel + Cisplatin (DC, during the 2012–2016 period). Concurrent chemotherapy consisted of weekly Cisplatin at a dose of 30 mg/m2 with adequate hydration. Prior to commencement of CTRT, tumor response to induction therapy was evaluated by physical examination and MRI scan.
Radiotherapy details
After immobilization in a thermoplastic mould, all patients underwent a CECT scan of 2.5 mm slice thickness for target volume delineation. The gross tumor volume (GTV) which included the nasopharyngeal primary (GTVp) and the positive nodes (GTVn), was delineated using the information from the MRI and PET-CT scans.
Clinical target volume (CTV) consisted of a high-risk CTV region (HR-CTV), intracranial CTV region (CTV-IC) and a low-risk CTV region (LR-CTV). HR-CTV included the pre-chemotherapy GTV (excluding the intracranial part) with 5 mm margins, as well as the entire nasopharynx, skull base, sphenoid sinus, parapharyngeal space, medial pterygoid fossae, posterior parts of the nasal cavity, retropharyngeal nodal regions and the involved nodal levels. CTV-IC consisted of the intracranial part of the GTV with margins ranging between 3 and 5 mm, depending on the proximity to critical organs at risk (Fig. 2). Uninvolved nodal regions constituted the LR-CTV. The three CTVs were expanded by 5 mm to generate the respective planning tumor volumes (HR-PTV, PTV-IC, and LR-PTV). While HR-PTV was treated to a dose equivalent of 70 Gy, PTV-IC was prescribed a dose equivalent of 60–64 Gy and the parts of PTV-IC overlapping with critical structures received a further reduced dose of 54–60 Gy (Fig. 2). This was done to respect the tolerance of the neurological structures while balancing the dose needed for optimal tumor control. Inpatients where the dose constraints to the critical organ at risk (OAR) could not be achieved without a significant underdosing of the tumor, a higher dose (maximum dose > 60 Gy to optic nerves and optic chiasm) was accepted after a discussion with the patient and after obtaining a high-risk consent from the patients. This was required in 5 (10%) of the patients. Two different dose-fractionations were utilized over the period of the study. Table 2 summarizes the target volumes and the various dose prescriptions that were practiced.
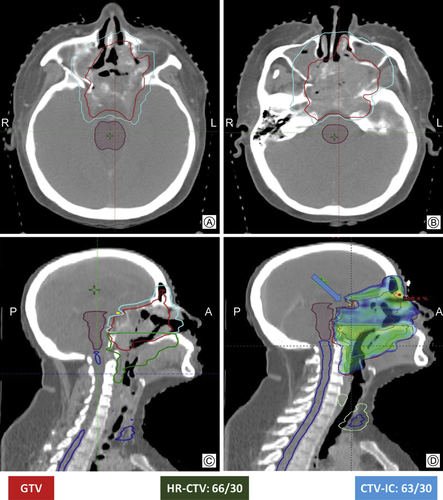
Axial and sagittal CT images demonstrating the delineation of various CTVs (A, B, C) as per the departmental protocol and conformal IMRT plan (D) with sparing of the organs at risk (sparing of optic chiasma – shown with arrow).
| Target volumes | Dose protocol 1 | Dose protocol 2 |
|---|---|---|
| HR-CTV (nasopharyngeal primary with margins + adjacent areas at risk for microscopic extension + involved nodal levels) | 66 Gy/30#/6 weeks | 70 Gy/35#/7 weeks |
| LR-CTV (uninvolved neck nodal regions) | 54 Gy/30#/6 weeks | 56 Gy/35#/7 weeks |
| CTV-IC (intracranial part of GTV with margins of 0–5 mm depending on proximity to critical OARs) | 63 Gy/30#/6 weeks | 63 Gy/35#/7 weeks |
- HR-CTV: high-risk clinical target volume; LR-CTV: low-risk clinical target volume; CTV-IC: intracranial CTV region; OAR: organ at risk.
All patients were treated with 7–9 field LA-based IMRT or tomotherapy based IMRT with concurrent weekly Inj. Cisplatin at 30 mg/m2. Electronic portal imaging (EPID) or cone beam CT (CBCT) for treatment verification was done at least twice weekly or more frequently as deemed necessary. Monitoring of weight, acute toxicities and compliance was carried out every week during the period of chemoradiation. Acute toxicities were scored according to the Radiation Therapy Oncology Group (RTOG) acute toxicity criteria.
Follow up
Response assessment following chemoradiation was performed at 8–12 weeks after treatment and included a detailed history, physical examination, a nasopharyngoscopy, and a PET-CT scan. Tumor response was classified according to the Response Evaluation Criteria in Solid Tumours (version 1.1). Patients were then followed up every 3 months in the first 2 years, every 6 months in the third and fourth year, and yearly thereafter. Each of the visits included a history and physical examination, nasopharyngoscopy and documentation of late radiation toxicity of the skin, subcutaneous tissue, and salivary gland using the late radiation morbidity scoring criteria of the RTOG. A PET-CT was performed annually or in cases of suspected recurrence. A recurrence was then confirmed with a biopsy/FNAC, whenever feasible.
Treatment characteristics
The majority (84%) were treated with 2 cycles of induction chemotherapy. ICT resulted in a complete response in 22% of the patients and a partial response in 69%. Remaining 9% had stable disease with no patient having disease progression on ICT. Most common RT dose prescription was 66 Gy over 30 fractions in 6 weeks (64%) delivered as a Simultaneous Integrated Boost (SIB). Overall 89% of patients completed the prescribed radiotherapy dose and 75% received at least 6 cycles of concurrent Cisplatin. There was no difference in treatment compliance between patients with minor and major ICE (90% vs 88%, P = 0.75). Complete response after CTRT was seen in 89% of patients. Table 3 highlights the relevant treatment-related characteristics.
| Characteristics | Numbers | Percentages (%) | ||
|---|---|---|---|---|
| ICT regimen | TIP | 18 | 40 | |
| PC | 6 | 14 | ||
| DCF | 10 | 22 | ||
| DC | 11 | 24 | ||
| RT dose prescription and compliance | 66 Gy/30 fr | Complete | 27 | 60 |
| Incomplete (range: 59.4 Gy/27 to 61.6 Gy/28 fr) | 2 | 4 | ||
| 70 Gy/35 fr | Complete | 13 | 29 | |
| Incomplete (range: 60 Gy/30 to 66 Gy/33 fr) | 3 | 7 | ||
| Critical OAR doses | OAR | Average of max dose to 0.3 cc volume (range) | ||
| Brainstem | 52.2 Gy (37.2–60.3 Gy) | |||
| Spinal cord | 43.4 Gy (28.2–50.7 Gy) | |||
| Optic nerve | 53.8 Gy (19.5–68.3 Gy) | |||
| Optic chiasm | 54.6 Gy (18.0–69.3 Gy) | |||
- ICT: induction chemotherapy; TIP: Paclitaxel + Ifosfamide + Cisplatin; PC: Paclitaxel + Carboplatin; DCF: Docetaxel + Cisplatin + 5-Fluorouracil; DC: Docetaxel + Cisplatin; RT: radiotherapy; fr: fractions; OAR: organ at risk.
Statistical analysis
All survival endpoints were calculated from the date of the diagnosis. DFS was the primary endpoint. Locoregional relapse-free survival (LRFS), distant metastasis-free survival (DMFS) and overall survival were the secondary endpoints. DFS was defined by progression or death which ever occurred first and OS was defined by death from any cause. Kaplan–Meier method was used for the survival analysis and the log-rank test was used to test the difference between groups. A multivariate Cox proportional hazards model was used to identify prognostic factors and hazard ratios were reported with a 95% confidence interval. All statistical analysis was performed using SPSS, version 21.0.0 (SPSS Inc., Chicago, IL).
Results
Patterns of failure
A total of 18 (40%) patients experienced failures (Table 4) and distant metastases was the most common (n = 13, 72%) mode of failure. Amongst distant failures, the bone was the commonest site (70%), followed by liver and lung. Locoregional failures were seen in 7 (15%) patients, 3 patients experienced isolated local failure and rest experienced a combination of local + nodal failures (n = 2) or distant + regional failures (n = 2), there was no isolated regional failure. All of the local failures (n = 5) occurred in the high dose region (66/30 PTV region), this precluded any aggressive salvage options in these patients. At last follow-up, only 1 out of 18 patients (6%) was alive without any disease, a patient with oligometastatic recurrence in the bone who had been treated with palliative chemotherapy and high-dose radiotherapy. The overall failure rate was higher in patients with major ICE (12/24) compared to patients with minor ICE (6/21), however this difference was not statistically significant (P = 0.143).
| Failure patterns | Minor ICE | Major ICE | Total (%) | Last FU status |
|---|---|---|---|---|
| No failures | 15 | 12 | 27 (60) | ANED-27 |
| Persistent primary | 0 | 3 | 3 (7) | AwD-2, DdD-1 |
| Persistent primary + node | 0 | 1 | 1 (2) | DdD-1 |
| Prim and nodal recurrence | 1 | 0 | 1 (2) | DdD-1 |
| Distant metastases | 3 | 8 | 11 (24) | ANED-1, AwD-5, DdD-5 |
| Nodal recurrence with distant metastases | 2 | 0 | 2 (5) | AwD-1, DdD-1 |
| Total | 21 | 24 | 45 (100) | ANED-28, AwD-8, DdD-9 |
- ICE: intracranial extension; ANED: alive with no evidence of disease; AwD: alive with disease; DdD: died due to disease.
DFS, LRFS, OS, and DMFS
Median follow up of the surviving patients was 45 months (range: 8–113 months). At last follow up 9 patients had died (all due to disease) and 36 patients were alive, of which 8 patients were alive with disease. The estimated 5-year DFS, LRFS, DMFS, and OS for the entire cohort was 58%, 82%, 70% and 74% respectively (Fig. 3).
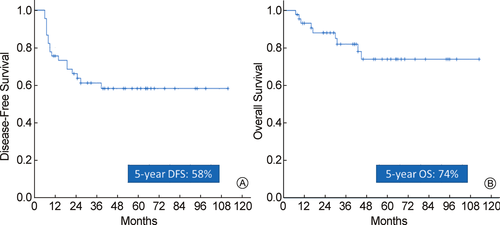
Kaplan–Meier curves for disease-free survival (A) and overall survival (B) respectively.
On univariate analysis (Table 5), age was the only significant predictor of DFS, with age > 35 years predicting a worse outcome (P = 0.05). A trend towards a worst DFS was also seen in patients with major ICE. Histological subtype was the only significant predictor of LRFS, with subtype other than undifferentiated carcinoma predicting a worse locoregional control. None of the factors were significant predictors of DMFS or OS. Gender, ethnicity, nodal stage and response to ICT did not impact any of the outcomes significantly. RT dose was not included in the univariate analysis as only a very small percentage of patients did not complete the prescribed dose.
| Prognostic factor | Subtype (number) | 5-year DFS % (P value) | 5-year LRFS % (P value) | 5-year DMFS % (P value) | 5-year OS % (P value) |
|---|---|---|---|---|---|
| Age | <35 yrs (21) | 76 (0.05) | 90 (0.27) | 80 (0.12) | 83 (0.18) |
| >35 yrs (24) | 41 | 73 | 51 | 63 | |
| Gender | Male (28) | 50 (0.36) | 82 (0.76) | 57 (0.24) | 69 (0.81) |
| Female (17) | 71 | 82 | 81 | 82 | |
| Ethnicity | Endemic (5) | 80 (0.30) | 100 (0.29) | 80 (0.49) | 50 (0.89) |
| Non-endemic (40) | 56 | 80 | 60 | 75 | |
| Histology | Undifferentiated (40) | 61 (0.30) | 89 (<0.05) | 67 (0.83) | 77 (0.17) |
| Rest (5) | 40 | 40 | 67 | 53 | |
| ICE | Minor (21) | 71 (0.06) | 85 (0.60) | 76 (0.20) | 83 (0.17) |
| Major (24) | 45 | 82 | 55 | 66 | |
| Nodal stage | N0–N1 (14) | 71 (0.30) | 77 (0.61) | 83 (0.16) | 91 (0.13) |
| N2–N3 (31) | 53 | 86 | 60 | 66 | |
| ICT response | CR (10) | 70 (0.32) | 100 (0.11) | 70 (0.71) | 88 (0.25) |
| <CR (35) | 55 | 77 | 66 | 70 |
- DFS: disease-free survival; LRFS: locoregional relapse-free survival; DMFS: distant metastasis-free survival; OS: overall survival; ICE: intracranial extension; ICT: induction chemotherapy; CR: complete response.
Multivariate analysis was performed using the Cox proportional hazards model for DFS and LRFS as depicted in Table 6. Age and extent of ICE retained their prognostic significance for DFS; increasing age and major ICE predicting a worse outcome. Histological subtype retained its prognostic significance for LRFS.
| Variables | DFS | LRFS | ||||
|---|---|---|---|---|---|---|
| P value | HR | CI 95% | P value | HR | CI 95% | |
| Age: >35 years | 0.050 | 2.79 | 0.98–7.93 | 0.390 | 2.18 | 0.35–13.4 |
| Histology: rest | 0.176 | 2.52 | 0.66–9.61 | 0.018 | 11.21 | 1.50–83.6 |
| ICE: major | 0.067 | 2.52 | 0.94–6.81 | 0.261 | 2.59 | 0.49–13.6 |
| N stage: N2–N3 | 0.142 | 2.71 | 0.71–10.27 | 0.547 | 1.87 | 0.24–14.3 |
| Post ICT response: PR | 0.560 | 1.51 | 0.37–6.07 | – | – | – |
- DFS: disease-free survival; LRFS: locoregional relapse-free survival; HR: hazard ratio; CI: confidence interval; ICE: intracranial extension; ICT: induction chemotherapy; PR: partial response.
Toxicity
During induction chemotherapy, 10 (22%) patients developed grade 3 or worse hematological toxicities; grade 3 or worse leukopenia occurred in 9 (20%) patients, followed by neutropenia (7 (16%)), thrombocytopenia (2 (4%)), and anemia (2 (4%)). Grade 3 or worse non-hematological toxicities that included stomatitis, nausea, vomiting, and electrolyte disturbances were seen in 19 (42%) patients.
The acute toxicities during CTRT were primarily related to skin and mucosa. Grade 2 or worse acute skin and mucosal toxicity were seen in 74% and 89% of patients during CTRT, of which grade 3 constituted, 15% and 5% respectively. Grade 3 or worse hematological toxicities were seen in 9% of patients (leukopenia (9%) and neutropenia (4%)) during CTRT. Three (5%) patients required hospital admissions for supportive care due to acute toxicities and five (11%) patients could not complete the intended dose of radiotherapy.
Most common late toxicities (grade 2 or worse) were subcutaneous fibrosis and xerostomia seen in 22%, and 24% patients respectively. Fifteen patients (33%) developed a clinically overt hypothyroidism requiring thyroxine supplementation at last follow-up. There was no difference in the incidence of hypothyroidism when patients were classified according to the extent of ICE (major ICE vs minor ICE, P = 0.526). None of the patients experienced any neurological, visual or vascular complications.
Discussion
Chemoradiotherapy is considered as the standard of care for locally advanced nasopharyngeal cancer and is associated with excellent survival for these patients, especially in the IMRT era.1-3,5,11,12,18-20 However, the treatment of ‘T4’ nasopharyngeal cancer with intracranial extension has always posed a challenge; technical difficulties in delivering adequate doses to the tumor, owing to the proximity to critical neural structures, combined with a high metastatic rate have historically resulted in poor survival rates for this disease.7,8,21 Conventional radiotherapy was severely limited in its ability to deliver high doses of radiation, especially to the intracranial part of the tumor, necessary for local control and thus resulted in poorer survival ranging between 20% and 30%, even with the use of concurrent chemotherapy.6-8 Hu et al6 reported 84 patients with ICE treated in the 2D era, 61% had an N2–N3 nodal stage and only 37% received concomitant chemotherapy. Distant metastasis rate at 5 years was 30% and 70% patients died at last follow-up. The 5-year LRFS and OS in patients with limited disease was 65.7% and 42.9% respectively vs 31.6% and 3.4% in patients with extensive disease. The extent of intracranial extension and nodal stage were important predictors of survival.
With the advent of IMRT complex dose distribution and steep dose gradients, delivery of high doses to the tumor was possible, which saw an improvement in survival with a reduction in toxicities for LA-NPC compared to conventional radiotherapy. The survival benefit was higher in patients with T3–T4 stages compared to T1–T2 stages.11,12 Cao et al13,14,22 reported 137 patients of NPC with ICE treated with IMRT, 59% had an N2–N3 nodal stage, 66% patients had limited ICE, 70% patients received concurrent chemotherapy and 12% patients received neo-adjuvant chemotherapy. Distant metastasis rate was 20%. The 5-year LRFS, DMFS and OS was 74.8%, 71.6% and 51.6% respectively. There was no significant difference in terms of OS, LRFS or DMFS between patients with limited and extensive ICE, which they attributed to the use of IMRT. The distant metastasis rate in this study was surprisingly low even though 18% of patients did not receive any form of chemotherapy and this could be due to a lower percentage of N2–N3 stages. The reported distant metastasis rates in literature for T4 with N2–N3 stages are as high as 32%.23
Following the improvement in local control rates with IMRT, distant metastasis became the predominant problem in LA-NPC, necessitating intensification of systemic treatment. Owing to excellent compliance and acceptable toxicity profile in various phase II studies induction chemotherapy was revisited as the method for systemic intensification.21,24 Recently a larger phase III trial of ICT plus CTRT versus CTRT alone in locoregionally advanced NPC by Sun et al,15 showed a significant benefit with ICT in distant failure-free survival, failure-free survival, and overall survival. The 3-year failure-free survival, locoregional failure-free survival, distant failure-free survival, and overall survival in the ICT plus CTRT arm was 80%, 92%, 90% and 92% respectively, compared with 72%, 89%, 83% and 86% respectively, in the CTRT arm. ICT also provides other advantages like reduction of the primary tumor to give a wider margin for irradiation, which would result in a reduction of RT portals as well as improved dose conformity and improved tumor control probability.16,17 Niu et al17 evaluated 32 patients with intracranial invasion NPC treated with neoadjuvant chemotherapy and replanning IMRT with concurrent chemotherapy in which the doses to the brain stem, optic nerve, optic chiasm, and temporal lobe were reduced and the 2-year local control rates and DFFS were 88.2% and 89.6%, respectively. Thus the combination of ICT, to tackle the problem of distant metastases and IMRT, to improve locoregional control seems to be the optimum strategy in the current era for LA-NPC.
To evaluate this premise in NPC with ICE, we retrospectively analyzed 45 ICE-NPC patients treated with ICT followed by chemo-IMRT. Up to 70% of our patients had advanced (N2–N3) nodal stages compared to 60% in the previous series. We utilized a “risk-adapted” approach for our IMRT. Intracranial part of the pre-chemotherapy GTV was delineated separately as CTV-IC with 3–5 mm margin depending on proximity to the OARs and was prescribed a slightly lower dose of 60–64 Gy equivalent (Table 2 and Fig. 2). The portion of the PTV-IC overlapping the critical neural structures was underdosed to meet their tolerance (Fig. 2). This was done to maintain an optimum balance between tumor control and complication rate. The estimated 5-year DFS, LRFS, DMFS, and OS in our study was 58%, 82%, 70% and 74% respectively. The OS rates in the current study appear to be much higher than the series by Cao et al22 and could be explained by improved LRFS in our series. Improvement in LRFS in our series probably relates to the use of ICT which resulted in a CR in 22% and a partial response in additional 69% of patients allowing radiotherapy to consolidate this effect and improving LRFS. The distant metastases rate is still very high and could be related to the higher percentage of patients with the advanced nodal burden, lower cumulative dose of concurrent Cisplatin and only 2 cycles of ICT in the majority (84%) of the patients.
Multivariate analysis demonstrated that histology was the most important predictor of locoregional control and age was the most important predictor of DFS. The extent of ICE showed only a trend towards worse DFS. None of the factors predicted for DMFS or OS owing to a small number of events in each group that was tested. Toxicity rates in the current study appear comparable to our previously published data of ICT in nasopharyngeal cancer and ICT doesn't seem to increase the rates of toxicities from IMRT.15,25,26
The strength of our study is that all patients were optimally staged using MRI and PET-CT and uniformly treated with ICT followed by chemo-IMRT, which could be described as an optimal treatment in the current era. Limitations of our study include its retrospective nature and lack of dose volume correlation with the GTV and OAR.
Distant metastases continue to remain high and require further intensification of our systemic treatments, starting with increasing the number of ICT cycles in our patients. Studies for stratifying the patients into risk groups that may benefit the most with treatment intensification are needed.
Conclusions
Taxane-based induction chemotherapy followed by chemo-intensity modulated radiotherapy resulted in excellent locoregional control and survival with minimal toxicities in patients of nasopharyngeal cancer with intracranial extension. Distant metastasis continues to be the predominant problem in these patients and requires further treatment intensification.
Funding
No funding received from any funding agencies either public or commercial.
Declaration of Competing Interest
The authors have no conflicts of interest to disclose.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.wjorl.2020.02.003.




