Dose-Dependent Effects of AbobotulinumtoxinA (Dysport) on Spasticity and Active Movements in Adults With Upper Limb Spasticity: Secondary Analysis of a Phase 3 Study
Abstract
Background
AbobotulinumtoxinA has beneficial effects on spasticity and active movements in hemiparetic adults with upper limb spasticity (ULS). However, evidence-based information on optimal dosing for clinical use is limited.
Objective
To describe joint-specific dose effects of abobotulinumtoxinA in adults with ULS.
Design
Secondary analysis of a phase 3 study (NCT01313299).
Setting
Multicenter, international, double-blind, placebo-controlled clinical trial.
Participants
A total of 243 adults with ULS >6 months after stroke or traumatic brain injury, aged 52.8 (13.5) years and 64.3% male, randomized 1:1:1 to receive a single-injection cycle of placebo or abobotulinumtoxinA 500 U or 1000 U (total dose).
Methods
The overall effects of injected doses were assessed in the primary analysis, which showed improvement of angles of catch in finger, wrist, and elbow flexors and of active range of motion against these muscle groups. This secondary analysis was performed at each of the possible doses received by finger, wrist, and elbow flexors to establish possible dose effects.
Main Outcome Measures
Angle of arrest (XV1) and angle of catch (XV3) were assessed with the Tardieu Scale, and active range of motion (XA).
Results
At each muscle group level (finger, wrist, and elbow flexors) improvements in all outcome measures assessed (XV1, XV3, XA) were observed. In each muscle group, increases in abobotulinumtoxinA dose were associated with greater improvements in XV3 and XA, suggesting a dose-dependent effect.
Conclusions
Previous clinical trials have established the clinical efficacy of abobotulinumtoxinA by total dose only. The wide range of abobotulinumtoxinA doses per muscle groups used in this study allowed observation of dose-dependent improvements in spasticity and active movement. This information provides a basis for future abobotulinumtoxinA dosing recommendations for health care professionals based on treatment objectives and quantitative assessment of spasticity and active range of motion at individual joints.
Level of Evidence
I
Introduction
Upper limb spasticity (ULS) is a common symptom after stroke and traumatic brain injury (TBI) and is associated with impaired self-care and additional burden of care 1-5. Among several treatment strategies, guidelines recommend intramuscular botulinum toxin injections as a first-line treatment for adults with ULS 6-11.
Botulinum toxin type A (BoNT-A) injections may target upper extremity muscle groups from the shoulder, to decrease adductor and internal rotation tone, to the elbow, wrist, fingers, and thumb, to decrease flexor tone 12, 13. Specific muscle selection is based on the pattern of muscle overactivity, functional deficits, and patient goals 6. These goals include increased passive and active range of motion, improved function (feeding and dressing), easier care (palmar and axillary hygiene), and reduction of pain 13.
Evidence-based information on optimal dosing for clinical use is relatively sparse. Dosing is not interchangeable between different BoNT-A products; therefore, improving our understanding of product-specific dosing will minimize confusion among injectors and improve the quality of patient care 13.
Among BoNT-A formulations, abobotulinumtoxinA (Dysport; Ipsen Biopharm, Wrexham, United Kingdom) has been shown to decrease muscle tone (as measured by the Modified Ashworth Scale [MAS]) 13-17 and pain 18 and to facilitate goal attainment 19 in adults with ULS. A recent systematic review 13 of 12 randomized controlled trials (RCTs) in ULS concluded that abobotulinumtoxinA (total dose range, 500-1500 U) was generally well-tolerated, with “strong evidence” to support reduced muscle tone.
This paper presents the results of a secondary analysis from a recently published large international clinical trial, demonstrating improved active range of motion following abobotulinumtoxinA treatment in adults with hemiparesis and ULS >6 months after stroke or TBI 20. This phase 3, randomized, double-blind, placebo-controlled study demonstrated that a total dose of either 500 U or 1000 U abobotulinumtoxinA injected in the upper extremity also resulted in decreased muscle tone and improvements in global physician-assessed clinical benefit compared with placebo.
Apart from a systematic measurement of active range of motion (XA) against finger, wrist, and elbow flexors, another unique aspect of the trial was the assessment of spasticity at the finger, wrist, and elbow flexor groups with the Tardieu Scale (TS) 21, 22. The TS is a standardized evaluation used to assess the angle of arrest at slow speed (ie, passive range of motion, XV1) and the angle of catch at fast speed (XV3). The trial demonstrated improvements for finger, wrist, and elbow joints at week 4 in XV3 at both abobotulinumtoxinA doses and in XA at 1000 U; for the 500-U dose, improvements in XA were seen in the finger flexors. Both doses were associated with a favorable safety profile 20. This analysis aims to provide a detailed description of improvements in spasticity and the active range of motion for individual muscle groups by dose and to provide information on muscle-specific dosing, which can be used in future recommendations for injectors.
Methods
Trial Design
The clinical trial (NCT01313299) was conducted between August 4, 2011, and September 4, 2013, and involved 34 sites across 9 countries (Belgium, Czech Republic, France, Hungary, Italy, Poland, Russian Federation, Slovakia, USA). The trial was a phase 3, prospective, randomized, placebo-controlled study involving one double-blinded treatment cycle with at least 12 weeks' follow-up. Subjects were randomized to 1 of 3 treatment groups (1:1:1) receiving a total upper extremity dose of 500 U abobotulinumtoxinA, 1000 U abobotulinumtoxinA, or placebo, attributed in a blinded manner. A full description of the main study design and primary results has been published previously 20.
Inclusion/Exclusion Criteria
- • finger flexors (flexor digitorum profundus and flexor digitorum superficialis [FDS]);
- • wrist flexors (flexor carpi ulnaris and flexor carpi radialis [FCR]); and
- • elbow flexors (brachialis with optional addition of brachioradialis).
Of note, a greater MAS threshold was required to designate the PTMG if the subject had received BoNT previously (MAS ≥3) as opposed those who were BoNT-naïve (MAS ≥2) in the affected limb. Additional criteria included a substantial subjective perception of disability (score ≥2 on the Disability Assessment Scale in the domain selected as the principal target of treatment), spasticity angle ≥10° on the TS for the most spastic muscle group, and a mean Modified Frenchay Scale score of 1-8 (average of all items, range 0-10). Pertinent exclusion criteria included major limitations in the angle of arrest (XV1); history of BoNT nonresponse; BoNT treatment within 4 months before enrollment or the need for lower extremity treatment during the study; previous treatment with surgery, alcohol, or phenol in the affected limb; medical conditions increasing the risk of BoNT-A-related adverse events; and major neurologic impairment (other than hemiparesis) influencing functional performance.
Study Treatment
Once identified, muscles of the PTMG received a fixed, predetermined injection volume containing a lower or higher abobotulinumtoxinA dose or placebo, depending on the total dose attributed by the blinded randomization (Table 1). In addition to the PTMG, the investigators were required to inject at least 2 other upper limb muscles. The volume/dose for these additional muscles was not fixed but rather selected by the investigator within a prespecified recommended range (Table 1).
| All Muscles Injected | Volume, mL∗ | Dose Range | ||
|---|---|---|---|---|
| Non-PTMG | PTMG | aboBoNT-A 500 U | aboBoNT-A 1000 U | |
| Finger and thumb muscles | 2 | |||
| Flexor digitorum profundus† | 0-1 | 1‡ | 0-100 | 0-200 |
| Flexor digitorum superficialis† | 0-1 | 1‡ | 0-100 | 0-200 |
| Flexor pollicis longus | 0-1 | – | 0-100 | 0-200 |
| Adductor pollicis | 0-0.25 | – | 0-25 | 0-50 |
| Wrist muscles | 2 | |||
| Flexor carpi ulnaris† | 0-1 | 1‡ | 0-100 | 0-200 |
| Flexor carpi radialis† | 0-1 | 1‡ | 0-100 | 0-200 |
| Elbow muscles | 2-3 | |||
| Brachialis† | 0-2 | 2‡ | 0-200 | 0-400 |
| Brachioradialis† | 0-1 | 1§ | 0-100 | 0-200 |
| Biceps brachii | 0-2 | – | 0-200 | 0-400 |
| Pronator teres | 0-1 | – | 0-100 | 0-200 |
| Shoulder muscles | – | |||
| Triceps brachii (long head) | 0-1.5 | – | 0-150 | 0-300 |
| Pectoralis major | 0-1.5 | – | 0-150 | 0-300 |
| Subscapularis | 0-1.5 | – | 0-150 | 0-300 |
| Latissimus dorsi | 0-1.5 | – | 0-50 | 0-300 |
- a PTMG = primary target muscle group; aboBoNT-A = abobotulinumtoxinA.
- ∗ The total volume was fixed at 5 mL for all upper extremity muscles injected.
- † Muscles included in the PTMG.
- ‡ Mandatory injection volume if designated a PTMG.
- § Optional injection volume, if elbow muscles were selected as PTMG.
Initial data review revealed that, for a given muscle, investigators consistently chose to inject lower abobotulinumtoxinA volumes/doses into non-PTMGs compared with the required volume/dose for the PTMG at each of the 3 selected joints. This allowed the designation of 4 mean dose groups corresponding to the 4 following situations (lowest to highest dose): 500 U/non-PTMG, 500 U/PTMG, 1000 U/non-PTMG, and 1000 U/PTMG for muscles eligible to be PTMGs: finger, wrist, and elbow flexors. For the other muscles, 2 dose levels were identified corresponding to the 2 possible randomized total doses (Table 2).
| Muscle Group | aboBoNT-A, 500 U (n = 81), nMean Dose (SD) [range] | aboBoNT-A, 1000 U (n = 81), nMean Dose (SD) [range] | aboBoNT-A, All Doses (n = 162)Prevalence, n (%) |
|---|---|---|---|
| Finger and thumb muscles | |||
| Flexor digitorum profundus | 5493.5 (17.0) [50-100] | 65195.5 (25.9) [100-300] | 119 (73.5) |
| PTMG | 44100.0 (0) [100-100] | 49200 (20.4) [100-300] | |
| Non-PTMG | 1065.0 (24.1) [50-100] | 16181.9 (35.4) [100-200] | |
| Flexor digitorum superficialis | 6395.4 (14.3) [50-100] | 73196.8 (28.4) [100-300] | 136 (84.0) |
| PTMG | 44100.0 (0) [100-100] | 49202.0 (14.3) [200-300] | |
| Non-PTMG | 1984.7 (23.2) [50-100] | 24186.3 (43.9) [100-300] | |
| Flexor pollicis longus | 2464.4 (25.6) [20-100] | 32139.7 (46.1) [50-200] | 56 (34.6) |
| Adductor pollicis | 1030.0 (10.5) [25-50] | 1450.7 (14.9) [40-100] | 24 (14.8) |
| Wrist muscles | |||
| Flexor carpi radialis | 5792.2 (18.1) [25-100] | 57178.1 (45.5) [80-300] | 114 (70.4) |
| PTMG | 11100 (0) [100-100] | 12195.8 (14.4) [150-200] | |
| Non-PTMG | 4690.3 (19.8) [25-100] | 45173.3 (49.7) [80-300] | |
| Flexor carpi ulnaris | 4789.9 (25.7) [25-180] | 49171.2 (45.2) [80-200] | 96 (59.3) |
| PTMG | 11100 (0) [100-100] | 12195.8 (14.4) [150-200] | |
| Non-PTMG | 3686.8 (28.7) [25-180] | 37163.2 (48.9) [80-200] | |
| Elbow muscles | |||
| Brachioradialis | 4288.3 (28.5) [50-200] | 28172.1 (44.8) [50-200] | 70 (43.2) |
| PTMG | 20100 (0) [100-100] | 12200 (0) [200-200] | |
| Non-PTMG | 2277.7 (36.6) [50-200] | 16151.3 (50.2) [50-200] | |
| Brachialis | 60148.5 (60.2) [50-200] | 43321.4 (103.2) [100-400] | 103 (63.6) |
| PTMG | 26194.2 (29.4) [50-200] | 20400 (0) [400-400] | |
| Non-PTMG | 34113.5 (54.1) [50-200] | 23253.0 (99.2) [100-400] | |
| Biceps brachii | 28106.4 (35.6) [50-200] | 19207.4 (62.6) [100-400] | 47 (29.0) |
| Pronator teres | 1481.8 (42.4) [45-200] | 30157.3 (51.9) [80-200] | 44 (27.2) |
| Shoulder muscles | |||
| Triceps brachii (long head) | 2100.0 (0.0) [100-100] | 1200.0 (N/A) [200-200] | 3 (1.9) |
| Pectoralis major | 4100.0 (0.0) [100-100] | 6200.0 (63.2) [100-300] | 10 (6.2) |
| Subscapularis | 2100.0 (0.0) [100-100] | 0N/A | 2 (1.2) |
| Latissimus dorsi | 3100.0 (0.0) [100-100] | 4175.0 (50.0) [100-200] | 7 (4.3) |
- a AboBoNT-A = abobotulinumtoxinA; SD = standard deviation; PTMG = primary target muscle group; N/A = not applicable.
Outcomes
For this analysis, outcomes considered were XV1 and XV3, assessed with the TS, and the XA for finger, wrist, and elbow flexors, measured at week 4 postinjection.
Statistical Analyses
Descriptive statistics (mean, standard deviation [SD], range, percentages) were used to characterize demographics, abobotulinumtoxinA dosage and efficacy variables. The mean change in XV1, XV3, and XA, measured in degrees, between baseline and the follow-up assessment at week 4 postinjection was calculated. Results were plotted by mean abobotulinumtoxinA dose injected in each treatment group as PTMG and non-PTMG (and placebo) and muscle group (finger, wrist, or elbow flexors).
Results
Sample Demographics
As described in the primary publication 20, 243 subjects were randomized, with 81 subjects in each treatment group (placebo, 500 U abobotulinumtoxinA, 1000 U abobotulinumtoxinA). Baseline characteristics were similar across all treatment groups, with a mean (SD) age of 52.8 (13.5) years and 64.3% male subjects. Etiology of spasticity was stroke in 90.3% of subjects, and 45.4% subjects were BoNT-naïve in the affected limb.
Finger Muscles: Dosage and Outcomes
Mean abobotulinumtoxinA doses by individual finger muscles are outlined in Table 2. Mean (SD) abobotulinumtoxinA doses, regardless of PTMG, were 93.5 (17.0) U and 195.5 (25.9) U for flexor digitorum profundus and 95.4 (14.3) U and 196.8 (28.4) U for the FDS in the 500 U and 1000 U groups, respectively. When these muscles were injected as non-PTMG, the doses were 8%-35% lower than in the PTMG situation. Thumb muscles (injected in 12%-40% of subjects, Table 2) were not eligible for PTMG designation and, therefore, the dosing entirely reflects the clinical choice of investigators within the limits outlined in Table 1.
As shown in Figure 1A, placebo had little or no effect on XV1, XV3, and XA. For abobotulinumtoxinA, a progressive improvement in XV1 was observed from 103 to 402 U, with a lesser improvement at 284 U. XV3 improved regularly with increasing mean abobotulinumtoxinA doses. A maximum effect was observed for XA, at a mean dose of 200 U, and slightly reduced improvements were observed at greater doses. Additional details of these changes are shown in Table 3.
| Variable | Change From Baseline at Week 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-PTMG | PTMG | Overall (Irrespective of PTMG) | |||||||
| Mean Dose | n | Mean Change (SD) | Mean Dose | n | Mean Change (SD) | Mean Dose | n | Mean Change (SD) | |
| Extrinsic finger flexors | |||||||||
| XV1 (°) | |||||||||
| Placebo | – | 30 | 2.3 (31.0) | – | 41 | 1.6 (25.6) | – | 70 | 1.9 (28.0) |
| AbobotulinumtoxinA 500 U | 103 U | 22 | 4.8 (23.6) | 200 U | 44 | 15.7 (30.5) | 167 U | 66 | 12.0 (28.7) |
| AbobotulinumtoxinA 1000 U | 284 U | 25 | 7.0 (23.2) | 402 U | 48 | 24.0 (38.6) | 361 U | 73 | 18.2 (34.9) |
| XV3 (°) | |||||||||
| Placebo | – | 30 | 11.7 (53.7) | – | 41 | 8.0 (32.9) | – | 70 | 9.8 (42.8) |
| AbobotulinumtoxinA 500 U | 103 U | 22 | 33.2 (54.3) | 200 U | 44 | 42.4 (50.1) | 167 U | 66 | 39.3 (51.3) |
| AbobotulinumtoxinA 1000 U | 284 U | 25 | 47.2 (39.3) | 402 U | 48 | 48.0 (51.8) | 361 U | 73 | 47.7 (47.7) |
| XA (°)∗ | |||||||||
| Placebo | – | 28 | 3.0 (32.4) | – | 41 | -6.2 (33.8) | – | 69 | -2.4 (33.3) |
| AbobotulinumtoxinA 500 U | 103 U | 18 | 12.2 (46.8) | 200 U | 44 | 23.9 (27.6) | 167 U | 62 | 20.5 (34.3) |
| AbobotulinumtoxinA 1000 U | 284 U | 26 | 19.8 (42.3) | 402 U | 48 | 17.6 (30.9) | 361 U | 74 | 18.4 (35.0) |
| Wrist flexors | |||||||||
| XV1 (°) | |||||||||
| Placebo | – | 41 | 3.2 (25.0) | – | 15 | 7.5 (12.7) | – | 55 | 4.4 (22.5) |
| AbobotulinumtoxinA 500 U | 149 U | 48 | 10.1 (15.1) | 200 U | 11 | 13.6 (19.2) | 158 U | 59 | 10.8 (15.8) |
| AbobotulinumtoxinA 1000 U | 288 U | 43 | 14.3 (17.7) | 392 U | 12 | 5.0 (12.6) | 309 U | 55 | 12.2 (17.1) |
| XV3 (°) | |||||||||
| Placebo | – | 41 | 2.6 (20.8) | – | 15 | 2.1 (16.7) | – | 55 | 2.5 (19.8) |
| AbobotulinumtoxinA 500 U | 149 U | 48 | 26.0 (28.9) | 200 U | 11 | 31.8 (37.6) | 158 U | 59 | 27.1 (30.4) |
| AbobotulinumtoxinA 1000 U | 288 U | 43 | 35.0 (28.0) | 392 U | 12 | 33.7 (23.7) | 309 U | 55 | 34.7 (26.9) |
| XA (°)∗ | |||||||||
| Placebo | – | 36 | -0.9 (23.1) | – | 15 | 3.5 (29.1) | – | 51 | 0.4 (24.8) |
| AbobotulinumtoxinA 500 U | 149 U | 43 | 6.3 (20.2) | 200 U | 11 | 15.7 (19.8) | 158 U | 54 | 8.2 (20.3) |
| AbobotulinumtoxinA 1000 U | 288 U | 42 | 9.6 (23.3) | 392 U | 12 | 26.4 (26.7) | 309 U | 54 | 13.4 (24.8) |
| Elbow flexors | |||||||||
| XV1 (°) | |||||||||
| Placebo | – | 32 | -2.2 (13.9) | – | 23 | 2.0 (4.3) | – | 55 | -0.5 (11.1) |
| AbobotulinumtoxinA 500 U | 139 U | 40 | 1.2 (4.8) | 271 U | 25 | 3.5 (6.3) | 191 U | 65 | 2.1 (5.5) |
| AbobotulinumtoxinA 1000 U | 275 U | 29 | 1.1 (6.8) | 520 U | 19 | 0.7 (7.1) | 373 U | 48 | 1.0 (6.9) |
| XV3 (°) | |||||||||
| Placebo | – | 32 | 3.4 (14.8) | – | 23 | 6.1 (13.9) | – | 55 | 4.5 (14.3) |
| AbobotulinumtoxinA 500 U | 139 U | 40 | 14.3 (23.6) | 271 U | 25 | 28.0 (26.7) | 191 U | 65 | 19.6 (25.5) |
| AbobotulinumtoxinA 1000 U | 275 U | 29 | 19.5 (28.8) | 520 U | 19 | 35.1 (27.6) | 373 U | 48 | 25.7 (29.2) |
| XA (°)∗ | |||||||||
| Placebo | – | 24 | 7.3 (24.1) | – | 23 | 2.7 (14.9) | – | 47 | 5.0 (20.0) |
| AbobotulinumtoxinA 500 U | 139 U | 30 | 4.5 (21.2) | 271 U | 25 | 12.6 (21.8) | 191 U | 55 | 8.1 (21.7) |
| AbobotulinumtoxinA 1000 U | 275 U | 21 | 13.4 (20.1) | 520 U | 19 | 15.8 (22.4) | 373 U | 40 | 14.6 (21.0) |
- a XV1 = passive range of motion; XV3 = angle of catch at fast speed; XA = active range of motion; PTMG = primary targeted muscle group; SD = standard deviation.
- ∗ Number of subjects in the non-PTMG group for XA are lower as this parameter was only compulsory in PTMGs.
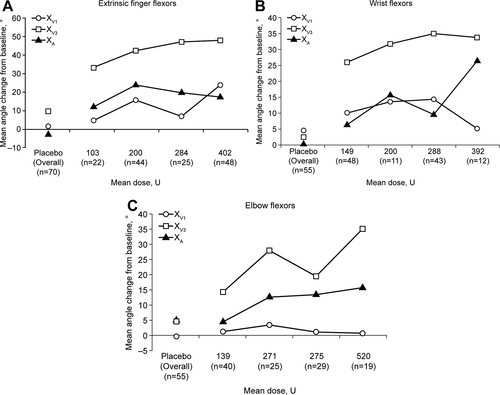
Change from baseline of Tardieu Scale parameters and of active range of motion week 4 postinjection in (A) extrinsic finger flexors, (B) wrist flexors, and (C) elbow flexors. Dose groups were as follows (lowest to highest dose): 500 U/non-PTMG, 500 U/PTMG, 1000 U/non-PTMG, and 1000 U/PTMG. Standard deviations and mean change from baseline values are detailed in Table 3. PTMG = primary targeted muscle group; XV1 = passive range of motion; XV3 = angle of catch at fast speed; XA = active range of motion.
Wrist Muscles: Dosage and Outcomes
Mean abobotulinumtoxinA doses for the wrist muscles are outlined in Table 2. Mean (SD) abobotulinumtoxinA doses, regardless of PTMG, were 92.2 (18.1) U and 178.1 (45.5) U for FCR and 89.9 (25.7) and 171.2 (45.2) U for FCU, for the 500 U and 1000 U groups, respectively. When these same muscles were injected as a non-PTMG, the doses were approximately 10%-12% lower.
As with the finger flexors, placebo had little or no effect on XV1, XV3, and XA in the wrist muscles (Figure 1B and Table 3). However, abobotulinumtoxinA was again associated with improvements in all 3 outcome measures. For XV1, abobotulinumtoxinA was associated with improvements from 149 to 288 U, although the greater dose of 392 U showed a reduced improvement. XV3 increased with mean abobotulinumtoxinA doses of 149 U through to 288U and stabilized at the highest dose of 392 U. For XA, progressive improvements were observed across all doses, with less improvement at 288 U.
Elbow Muscles: Dosage and Outcomes
Mean abobotulinumtoxinA doses by individual elbow muscles are outlined in Table 2. Mean (SD) abobotulinumtoxinA doses, regardless of PTMG, were as follows: 148.5 (60.2) U and 321.4 (103.2) U for brachialis and 88.3 (28.5) U and 172.1 (44.8) U for brachioradialis in the 500 U and 1000 U abobotulinumtoxinA groups, respectively. When these same muscles were injected as a non-PTMG, the doses were approximately 35%-40% lower for the brachialis and 25% lower for the brachioradialis (Table 2). Investigators often chose to inject the brachialis without injecting the brachioradialis. Similar to the thumb muscles, the biceps brachii and pronator teres were not eligible for PTMG designation; therefore, doses represent clinical acumen rather than required protocol dosing.
As with the other muscle groups, minimal changes in the elbow muscles were observed with placebo (Figure 1C). For abobotulinumtoxinA, there were very limited changes in the elbow XV1 for any of the doses. XV3 increased with mean abobotulinumtoxinA doses of 139 U through to 520 U, with a lessor change at 275 U, whereas XA showed regular improvements across all doses. Details of all changes (XV1, XV3, and XA) are shown in Table 3.
Safety Evaluation
Safety assessments, including the recording of adverse events and vital signs, electrocardiogram, and laboratory variables monitored throughout the study, were reported previously 20. Both 500 U and 1000 U total abobotulinumtoxinA doses in the upper limb showed favorable safety data and were consistent with previous literature on abobotulinumtoxinA and other types of BoNT-A. No dose-dependent effect on type or severity of adverse events was reported.
Discussion
Using data from a recently published international, phase 3, double-blind, randomized clinical trial 20, we outline in these analyses the characteristics of abobotulinumtoxinA doses at individual upper extremity muscles associated with favorable clinical outcomes among 162 subjects with hemiparesis. All doses administered were within the approved dose limits for abobotulinumtoxinA 6. Improvements in XV1, XV3, and XA were demonstrated in those subjects receiving abobotulinumtoxinA at specific upper extremity joints compared with subjects receiving placebo. Moreover, a greater dose of abobotulinumtoxinA generally was associated with greater improvements in XV1, XV3, and XA.
A major strength of this study was that outcomes were collected by clinicians with extensive initial and follow-up training, who were competency tested and blinded to randomization assignment 20. The favorable outcomes and trends observed over a wide range of abobotulinumtoxinA doses (2-fold with regard to total abobotulinumtoxinA dose, and 2.5- to 4-fold from lowest to highest dose), with few adverse events, suggest robust clinical decision-making in selecting abobotulinumtoxinA doses for a given muscle 20.
Comparing these findings with those of previous studies is challenging as a result of the wide variety of methodologies used: blinded RCTs 13, 16, 17 versus open-label trials 23; BoNT injection at varying time points postneurologic injury 17; the absence of investigator training 19, 24 versus extensive training 20; and a wide array of muscle-injection schemes and outcome measures 3, 8, 13, 25, 26. Yet, the overall dosing characteristics within the setting of this highly regimented, international clinical trial (Table 2), are generally in line with previous research studies. In a recent systematic review, Dashtipour et al 13 reported a wide range of abobotulinumtoxinA dosing, with total doses ranging from 500 to 1500 U in upper extremity muscles in the 12 studies identified. Understandably, larger dose variations were observed in larger muscles (biceps brachii: 100-600 U compared with FCR: 50-225 U) 13. Two previous studies have reported abobotulinumtoxinA dosing in nonresearch settings. In a survey of 275 European injectors, Hubble et al 27 found ULS doses ranging from 508 to 773 U abobotulinumtoxinA, giving a maximum upper extremity dose slightly lower than the one presented here. Nonetheless, the individual muscle doses of the present article are similar to those reported across 5 countries 27, except for FDS at 218 U (range 100-1000) in the United Kingdom, which was somewhat higher than the highest mean dose of 196.8 U in the present study. Turner-Stokes et al 24 identified the same trend of larger dose variations in larger muscles among injectors in a real-life setting (biceps brachii: 50-750 U versus FCR: 20-350 U). No mean upper limb abobotulinumtoxinA dose was reported 24. None of these studies provided joint-specific outcomes associated with abobotulinumtoxinA dose. Despite our standardized protocol 20, our data likely reflect real-world injections in that numerous different muscle and dose injection combinations were used, as in past studies 8, 13, 14, 17, 19.
Two aspects of the clinical trial reported here allowed for a unique consideration of abobotulinumtoxinA dose-response by joint. First, the inclusion of XV1, XV3, and XA as outcome measures provides unique, joint-specific data, including active (XA) and passive (XV1) range of motion and XV3. XV1, XV3, and XA measures appear to be less subject to placebo effects than MAS 20. The TS (including XV1 and XV3 reported here) has not yet been used widely as an outcome assessment for spasticity. Turner-Stokes et al 24 reported that <20% of 456 adults were assessed with the TS in their worldwide, observational study. Among those subjects with TS data, about 90% improved after BoNT treatment, and the scale had some correlation with goal attainment scaling results (r = 0.43, P < 0.001) 24.
Second, in this study, a given muscle fell under 2 scenarios for finger, wrist, and elbow flexors in the abobotulinumtoxinA dose groups—a mandatory, predetermined dose for PTMGs and a flexible dosing option within a predetermined range for non-PTMGs. This allowed for the evaluation of 4 dosing groups by total upper extremity dose (500 U or 1000 U) and PTMG or non-PTMG. Investigators administered lower abobotulinumtoxinA doses to non-PTMG muscles, which is not unexpected, given that the most hypertonic muscles were part of the PTMG. This also may reflect a relative uniformity in assessment and decision-making among injectors from 34 sites around the world. In addition, injectors could not exceed the total volume/dose; thus, once the PTMG was injected, the available volume/dose was reduced. Injectors also may be conservative and inclined to select a lower, rather than higher, dose when presented with a potential range of options, at least for the first injection.
In this study, an abobotulinumtoxinA dose-dependent effect was most evident for XV3, a direct measure of velocity-dependent stretch responses. The dose–response trend for the XA also was generally consistent, especially for the wrist and elbow flexors. By contrast, for finger flexors there was a reduced improvement in the XA at the higher doses, which might have been due to concomitant higher dose injections into wrist flexors, which would have reduced the tenodesis effect and therefore hampered improvement in finger extension. It is important to note that the trends in XA reflect a net gain in active range compared with placebo for every dose at every joint. Moreover, the improved active range of motion alongside a reduction in muscle tone and spasticity improvement is an important observation, as this indicates the patient can improve movement in their joints without over-weakening the muscles.
The wide range of abobotulinumtoxinA doses per muscle group used in the present study allowed for flexibility of the doses injected according to the investigators assessment of individual patient impairment and treatment objectives. Quantitative measurements of spasticity (XV1 and XV3 using the TS) and XA, in conjunction with treatment objectives, are clinically important to assess and follow the patient evolution throughout treatment and should therefore be used to guide dosing at individual joints. In particular, although the objective of injecting abobotulinumtoxinA is to improve spasticity, it should not negatively impact the active range of motion, which may be seen when exceeding the recommended range of doses.
There are a limited number of published studies with multiple abobotulinumtoxinA dosing groups to which these results might be compared. Smith et al 28 reported a small RCT (N = 21) with abobotulinumtoxinA doses of 500, 1000, and 1500 U (individual muscle dosing was not standardized). A dose–response relationship was reported for spasticity at the elbow (dose range 322-980 U) and wrist (dose range 127-280 U), and for range of motion at the elbow, up to 1000 U. No relationship was noted at the fingers (dose range 113-238 U).
Limitations of the Study
There are limitations to the present analysis that should be noted. On average, the subjects involved had suffered initial neurologic injury 5-10 years before this study, suggesting a highly motivated and engaged group of individuals at high risk of severe muscle shortening; however, this may not reflect general clinical practice. It is important to note that although the total dose administered to the upper limb was determined by randomization, the PTMG was not, and for each muscle group there were some muscles not injected at all. The 4 dose groups likely differed in characteristics other than the mean abobotulinumtoxinA dose (eg, baseline spasticity, voluntary movement, contracture, naïve or previous exposure, etc) and this may have contributed to some of the variability in XV1, XV3, and XA parameters among doses. These groups also had variable subject numbers reflecting the greater proportion of subjects in whom finger flexors were designated the PTMG over wrist or elbow flexors. It should be kept in mind that the relationship between BoNT dose at a given muscle and outcomes at a given joint are complicated. The biomechanical and neurophysiological interaction among other treated or untreated muscles is complex and may impact findings at the joint. For example, the tethering effect of the finger flexors as they cross the wrist 29.
Conclusion
This analysis presents novel data relating abobotulinumtoxinA dosing at specific muscles to outcomes at specific joints in the upper extremity. Previous clinical trials have established the clinical efficacy of abobotulinumtoxinA by total dose but did not fully characterize the effective mean doses of abobotulinumtoxinA by muscle. All abobotulinumtoxinA doses yielded important improvements in both spasticity and in subjects' active range of motion. The dose–response generally is consistent, with positive clinical outcomes at specific upper extremity muscle groups over a wide range of doses. This information thus provides a basis for future dosing recommendations for abobotulinumtoxinA for injectors based on treatment objectives and quantitative assessment of spasticity and active range of motion at the individual joints.
Acknowledgments
Medical writing and submission support, under the direction of the authors, were provided by Jacqueline Harte and Germanicus Hansa-Wilkinson of Watermeadow Medical, an Ashfield Company, funded by Ipsen.
CME Question
- a. Elbow flexors.
- b. Finger flexors.
- c. Wrist flexors.
- d. Both (A) and (C).
Answer online at me.aapmr.org




