EphA2 modulates radiosensitive of hepatocellular carcinoma cells via p38/mitogen-activated protein kinase-mediated signal pathways
Abstract
This experiment was conducted to investigate the role of EPH receptor A2 (EphA2) in the modulation of radiosensitivity of hepatic cellular cancer (HCC) cells and to determine whether p38/mitogen-activated protein kinase (p38MAPK) signaling mediated EphA2 function in this respect. The protein expressions of EphA2 and phosphorylated p38MAPK were tested in HCC and normal hepatic tissues. In HCC 97H cells, EphA2 was overexpressed and knocked out by transfection with EphA2 expression vector and EphA2-ShRNA, respectively, prior to cell exposure to low-dose irradiation. Significantly upregulated EphA2 and phosphorylated p38MAPK were observed in HCC tissues, compared with those in normal hepatic tissues. Low-dose irradiation (1 Gy) only caused minor damage to HCC 97H cells, as assessed by alterations in cell viability, apoptosis rate, and cell healing capacity (p = 0.072, p = 0.078, and p = 0.069 respectively). However, EphA2 knock-out in HCC 97H cells induced significant reduction in cell viability and cell healing capacity after these cells were subjected to low-dose irradiation. Apoptosis rate underwent dramatic increase (p < 0.01). By contrast, EphA2 overexpression in HCC 97H cells reversed these effects and enhanced cell colony formation rate, thus displaying remarkable attenuation of radiosensitivity of HCC 97H cells. Further, SB203580, a specific inhibitor of p38MAPK, was added to HCC 97H cells over-expressing EphA2. The effect of EphA2 overexpression on the radiosensitivity of HCC 97H cells was abrogated. Thus, the present study indicates that EphA2 have the ability to negatively regulate the radiosensitivity of HCC 97H cells, which mainly depends on 38MAPK-mediated signal pathways.
Introduction
Hepatocellular carcinoma (HCC) is a serious health problem. More than 600,000 new cases are diagnosed every year worldwide, yet a safe and effective therapy remains to be identified [1]. Conventional radiotherapy, for example, has played a limited role in HCC treatment during the past few decades, because of the low tolerance of nontumoral liver parenchyma to strong radiation as well as the high risk from radiation-induced deterioration of pre-existing diseases such as liver cirrhosis (the occurrence of HCC is commonly observed with the presence of cirrhosis and other relevant conditions) [2], [3]. Low-dose radiotherapy is a recent advance in the field of radiotherapy that comes with a set of adjuvant therapies, including the key strategy of sensitizing cancer cells to irradiation to achieve therapeutic effects similar to those observed with traditional high-dose radiotherapy, but with less toxicity to neighboring innocent organs and tissues. However, at present, poor understanding of the precise mechanisms involved in regulating the radiosensitivity of HCC cells impedes extensive application of low-dose radiotherapy to clinical settings.
EPH receptor A2 (EphA2), an important member of the Eph receptor family, has been observed to be highly expressed in diverse kinds of cancers such as breast cancer, prostate cancer, lung cancer, malignant glioma, and gastric cancer; this triggers extensive interest in its potential role in cancer [4]. Previous literature shows overexpression of EphA2 correlates with cancer progression and lymphogenous metastasis in gastric cancer [5]. Moreover, an analysis of EPHA2-knock-out mice indicated their deficiency in the ability to support the invasion and metastasis of implanted tumors [6]. This evidence indicates that upregulation of EphA2 confers properties such as increased cancer motility, invasiveness, and metastasis. Furthermore, emerging evidence shows that EphA2 blockade enhances the antiendothelial effect of radiation and inhibits irradiated tumor cell-induced migration of endothelial cells, implying that EphA2 probably gives cancer cells an opportunity to escape radiation stress [7].
P38/mitogen-activated protein kinase (p38MAPK), belonging to the MAPK family, has been implicated in the regulation of cell proliferation and apoptosis after exposure to irradiation. P38MAPK inhibition after treatment with a specific chemical inhibitor completely attenuated the mitochondrial translocation of Bax in response to radiation, suggesting that p38MAPK is involved in radiation-induced mitochondrial apoptotic cell death, whereas there is evidence that p38MAPK restores homeostasis in UV-stressed cells and facilitates cell survive via cell cycle arrest and DNA repair [8], [9]. It is believed that the ability of p38MAPK to determine cell fate, between cell survival and apoptotic cell death, correlates with the extent of radiation stress. Under overwhelming stress conditions, p38MAPK is more likely to activate p53 and other signal pathways to induce apoptosis. However, if the level of radiation is low or not too high, p38MAPK probably chooses other pathways to help maintain cell survival [9]. Thus, p38MAPK is proposed to be an important therapeutic target in low-dose radiotherapy.
In this study, the protein expressions of EphA2 and phosphorylated p38MAPK (p-p38MAPK) were tested in HCC tissues (these tissues were obtained from patients with poorly differentiated HCC) to compare with those in normal hepatic tissues. Next, we conducted our experiment in HCC 97H cell line, which was established from high malignant HCC cells and exhibits strong proliferation, migration, and invasion abilities [10], [11]. EphA2 in HCC 97H cell line was artificially up- or downregulated via the transfection with EphA2 expression vector or EphA2-small hairpin RNA (EphA2-shRNA) to provide an insight on the role of EphA2 in the modulation of HCC radiosensitivity. It was found that HCC radiosensitivity is negatively correlated with EphA2 expression. The specific inhibitor of p38MAPK signal, SB203580, was added to the EphA2-overexpressing group to explore further whether p38MAPK mediated the function of EphA2, which is serving as the radioresistance promoter. The aim of this study was to lay a theoretical foundation for the modulation of the radiosensitivity of HCC cells during low-dose radiotherapy.
Methods
Ethics statement
This study was approved by the Ethics Committee of Xiangya Medical School (Changsha, China). All patients provided written informed consent in compliance with the code of ethics of the World Medical Association (Declaration of Helsinki; Ferney-Voltaire, France).
Tissue treatment and cell culture
Twenty tissue specimens from HCC tissues (n = 10) and normal hepatic tissues (n = 10) were obtained from the Xiangya Medical School. Western blotting was used to detect the protein expressions of EphA2 and p-p38MAPK in both tissue types. Human HCC 97H cell line was purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco's modified Eagle's medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco-BRL) in a 37°C incubator with 5% CO2.
Western blotting
Cell extracts were prepared by using the lysis buffer containing 1% NP-40, 0.1% sodium dodecyl sulfate, 50mM dithiothreitol, 2 μg/mL aprotinin, 2 μg/mL leupeptin, and 1mM phenylmethane sulfonylfluoride. Total protein concentration was measured using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA) to ensure that equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins separated on electrophoresis gels were transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA) and these membranes were blocked in phosphate-buffered saline containing 0.1% Tween 20 and 5% powdered milk and probed with primary antibody directed against Epha2, p38MAPK, p-p38MAPK (Tyr 182), and β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Thereafter, the membranes were washed and incubated with peroxidase-conjugated goat anti-rabbit or anti-mouse immunoglobulin G secondary antibody (Santa Cruz Biotechnology, Inc.) at a dilution of 1:20,000.
Transfection and drug treatment
An open reading frame clone of homo EphA2 was subcloned into enhanced green fluorescent protein plasmid-C1 vector to construct EphA2 expression vector (Invitrogen Life Technologies, Carlsbad, CA, USA). Small hairpin RNA targeting EphA2 was synthesized by GenePharma Co., Ltd (pGPU6/GFP/Neo-EPHA2-homo-310; Shanghai, China) as follows: GGGACCTGATGCAGAACATCA. ShRNA duplexes containing nonspecific sequences (pGPU6/GFP/Neo-shNC) were used as a negative control: GTTCTCCGAACGTGTCACGT. EphA2 expression vector and EphA2-shRNA were added to the culture medium and transfected into HCC 97H cells using Lipofectamine 2000 (Invitrogen Life Technologies), according to the manufacturer's instructions. One group of HCC 97H cells transfected with EphA2 expression vector was treated with SB203580 (p38MAPK signal-specific inhibitor) (Calbiochem, Darmstadt, Germany). SB203580 solution was prepared by dissolving it in the culture medium to a final concentration of 5μM.
Irradiation
In our pilot experiment, HCC 97H cells were exposed to different doses of ionizing radiation (0 Gy, 1 Gy, 2 Gy, 4 Gy, or 8 Gy). Briefly, HCC 97H cells were seeded into T-25 flasks. After 48 hours' incubation, the exponentially growing cells were cultured in the media without fetal bovine and irradiated with 6 MeV photons/20 cm focus–surface distance at a dose rate of 2 Gy/min at room temperature. The irradiation range was restricted in 20 cm × 20 cm. The total amount of irradiation that HCC 97H cells suffered from was set up as 0 Gy, 1 Gy, 2 Gy, 4 Gy, or 8 Gy separately. After the irradiation, the cells were cultivated for 72 hours, which allowed sufficient time for the generation of irradiation-induced cytotoxicity. Relative tests showed that 1 Gy irradiation only induced minor decrease in cell viability and cell wound healing capacity (Figures 1A and 2A), though the cell colony formation rate underwent dramatic reduction (Figure 3A). This means that irradiation at this level is still insufficient to pose effective damage to HCC 97H cells. With the elevation of irradiation, however, damage inflicted by radiation was gradually enhanced. Finally, 1 Gy irradiation was selected and used in our formal experiment.
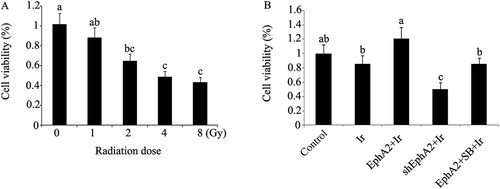
Viability of HCC 97H cells after exposure to different treatments. (A) Viability of HCC 97H cells after exposure to different doses of irradiation. (B) EphA2 expression vector and EphA2-ShRNA were transfected into HCC 97H cells. One group of HCC 97H cells transfected with EphA2 expression vector was treated with SB203580, a specific inhibitor of p38MAPK. Next, HCC 97H cells subjected to irradiation (dose = 1 Gy), followed by the test to viability. Bars not sharing a common letter differ (p < 0.05). EphA2 = EphA2 expression vector; Ir = irradiation; SB = SB203580; ShEphA2 = EphA2-small hairpin RNA.
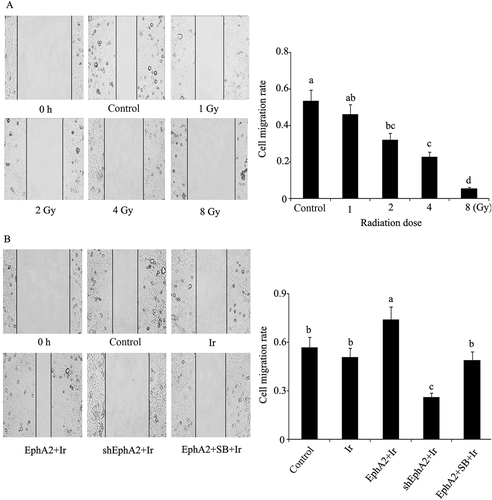
Migration rate of HCC 97H cells after exposure to different treatments. (A) Migration rate of HCC 97H cells after exposure to different doses of irradiation was obtained by wound healing test. (B) EphA2 expression vector and EphA2-ShRNA were transfected into HCC 97H cells. One group of HCC 97H cells transfected with EphA2 expression vector was treated with SB203580, a specific inhibitor of p38MAPK. Next, HCC 97H cells subjected to irradiation (dose = 1 Gy), followed by wound healing test. Bars not sharing a common letter differ (p < 0.05). EphA2 = EphA2 expression vector; Ir = irradiation; SB = SB203580; ShEphA2 = EphA2-small hairpin RNA.
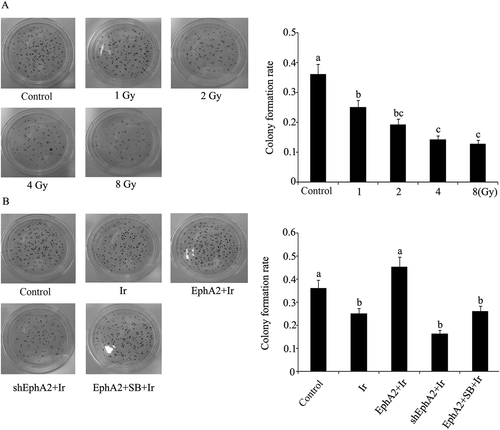
Colony formation rate of HCC 97H cells after exposure to different treatments. Colony formation rate of HCC 97H cells after exposure to different doses of irradiation. (B) EphA2 expression vector and EphA2-ShRNA were transfected into HCC 97H cells. One group of HCC 97H cells transfected with EphA2 expression vector was treated with SB203580, a specific inhibitor of p38MAPK. Next, HCC 97H cells subjected to irradiation (dose = 1 Gy), followed by the test to colony formation rate. Bars not sharing a common letter differ (p < 0.05). EphA2 = EphA2 expression vector; Ir = irradiation; SB = SB203580; ShEphA2 = EphA2-small hairpin RNA.
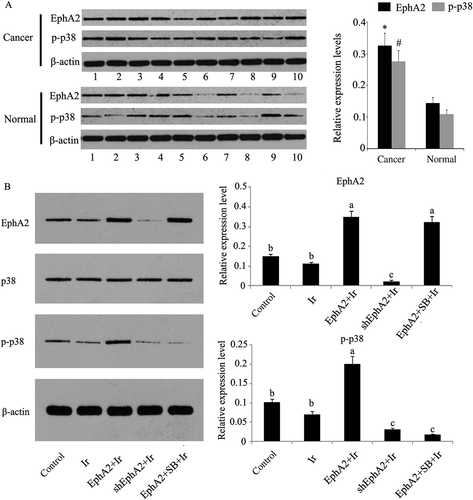
Relative protein expression levels of indicated proteins in HCC and normal hepatic tissues and in HCC 97H cells that received different treatments. (A) Relative protein expression levels of EphA2 and p-p38MAPK in HCC tissues (n = 10) compared those in normal hepatic tissues (n = 10). Bars with marks * and # have significant difference from normal groups (p < 0.05). (B) EphA2 expression vector and EphA2-ShRNA were transfected into HCC 97H cells. One group of HCC 97H cells transfected with EphA2 expression vector was treated with SB203580, a specific inhibitor of p38MAPK. Next, HCC 97H cells were subjected to irradiation (dose = 1 Gy). As there was no significant difference in the relative protein expression level of p38MAPK across groups receiving different treatments, this result was excluded. Bars not sharing a common letter differ (p < 0.05). Cancer = HCC tissues; EphA2 = EphA2 expression vector; Ir = irradiation; normal = normal hepatic tissues; p38 = p38MAPK; p-p38 = p-p38MAPK; SB = SB203580; ShEphA2 = EphA2-small hairpin RNA.
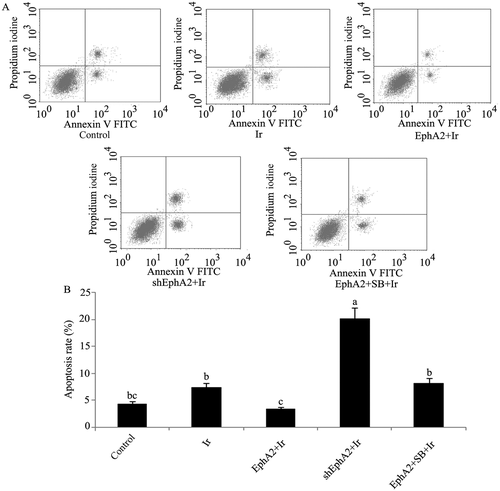
Apoptosis rate of HCC 97H cells after exposure to different treatments. EphA2 expression vector and EphA2-ShRNA were transfected into HCC 97H cells. One group of HCC 97H cells transfected with EphA2 expression vector was treated with SB203580, a specific inhibitor of p38MAPK. Next, HCC 97H cells subjected to irradiation (dose = 1 Gy). (A) Flow cytometry images of HCC 97H cells exposed to different treatments. (B) Apoptosis rate of HCC 97H cells exposed to different treatments. Bars not sharing a common letter differ (p < 0.05). EphA2 = EphA2 expression vector; Ir = irradiation; SB = SB203580; ShEphA2 = EphA2-small hairpin RNA.
Cell viability assay
The cells seeded at a density of 1 × 105 cells/well into 96-well plates were cultured with 100 μL of culture medium per well for 24 hours and with 90 μL of culture medium plus 10 μL CCK-8 reagents per well for 1 hour. The optical density at 490 nm (OD490 nm) for each well was determined by an enzyme immunoassay analyzer.
Annexin V-FITC/propidium iodide double-staining
After irradiation, the cells were double-stained with Annexin V-FITC and propidium iodide, according to the manufacturer's instruction (Solarbio, Bei Jin, China), and then analyzed with a dual laser flow cytometer (Becton Dickinson, San Jose, CA, USA). The extent of apoptosis was estimated using ModFit LT software (Verity Software House, Topsham, ME, USA).
Wound healing assay
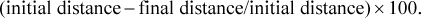 (1)
(1)Colony formation assay
After irradiation, cells were plated on soft agar and incubated for 1 week to allow colony growth. Colonies were stained with crystal violet and images of the stained plates were captured. Colonies > 75 μm in diameter or containing > 50 cells were counted as one positive colony. Colony formation rate was calculated using the following equation: positive colony number/cells that originally seeded in the plate.
Statistical analysis
Data from these experiments were analyzed by SPSS version 12.0 software (IBM, Armonk, NY, USA). The results of the expressions of indicated proteins in HCC tissues and normal hepatic tissues are presented as the mean ± standard error of the mean (n = 10) after evaluation by unpaired Student t test; other results are presented as the mean ± standard error of the mean of three independent experiments, and one-way analysis of variance (ANOVA) with post hoc testing was used for multiple comparisons between each group. Significant differences were established at p < 0.05.
Results
Protein expression levels of EphA2, p38MAPK, and p-p38MAPK
According to results of western blotting (Figure 4A), significantly upregulated EphA2 and p-p38MAPK were observed in HCC tissues, compared with those in normal hepatic tissues (p < 0.05). In Figure 4B, HCC 97H cells exposed to low-dose irradiation (1 Gy) showed minor effects on relative expression levels of EphA2, p38MAPK, and p-p38MAPK compared to the control (p = 0.068, p = 0.073, and p = 0.071). Cells transfected with EphA2 expression vector or EphA2-shRNA before irradiation significantly upregulated or downregulated the relative expression of EphA2 and p-p38MAPK. Adding the specific inhibitor of p38MAPK, SB203580, into cells transfected with EphA2 expression vector failed to alter EphA2 overexpression, but it remarkably inhibited the relative expression of p-p38MAPK (p < 0.05). Our trials showed no marked difference in p38MAPK expression between each group.
Cell viability and apoptosis rate
Postirradiation cell viability and apoptosis rate are shown in 1, 5, respectively. As can be noted from our data, HCC 97H cells exposed to irradiation at a dose of 1 Gy only did not significantly affect cell viability and apoptosis rate. Postirradiation cell viability was much higher in HCC 97H cells transfected with EphA2 expression vector, compared to the nontreated cells (p < 0.05), but much lower in HCC 97H cells transfected with EphA2-shRNA (p < 0.05). SB203580 treatment abolished the beneficial effect of overexpressed EphA2 on cell viability. With respect to postirradiation apoptosis rate, HCC 97H cells transfected with the EphA2 expression vector exhibited dramatic reduction, compared to the nontreated cells; this decrease was not observed after treatment with SB203580. What needs special mention is that expressional inhibition of EphA2 greatly augmented the apoptosis rate (p < 0.01).
Wound healing capability and cell colony formation rate
After irradiation, the cells transfected with EphA2 expression vector had higher migration rate compared to nontransfected cells (p < 0.05), whereas SB203580-treated cells showed a rate similar to that of nontransfected cells (Figure 2). EphA2-shRNA-transfected cells showed a considerable decrease in migration rate. In Figure 3B, 1 Gy irradiation triggered significant decrease in cell colony formation rate, but this inhibitory effect was abolished by the transfection with EphA2 expression vector. EphA2 knock-out only marginally enhanced the detrimental effect of 1 Gy irradiation in terms of cell colony formation.
Discussion
In the present study, EphA2 that was shown to be upregulated in HCC tissues plays a significant role in the modulation of HCC 97H cells radiosensitivity. In HCC 97H cells, overexpressing EphA2 via the transfection of EphA2 expression vector (without SB203580 treatment) simultaneously potentiated cell viability and attenuated apoptosis rate after exposure to low-dose irradiation. Conversely, EphA2 knockdown by transfection of EphA2-ShRNA rendered HCC vulnerable to irradiation, resulting in lower cell viability and extremely high apoptosis rate. These data indicate that EphA2 contributes to the development of resistance to the cytotoxicity of radiation in HCC.
Furthermore, in the wound healing test where cells were subjected to artificial injury and allowed to recover and proliferate in the next round of cultivation in order to reflect the healing capacity of cells, HCC migration rate was found to be positively correlated with the expression level of EphA2. Given the fact that the higher the cell migration rate, the stronger the healing capacity, EphA2 can promote cell healing capacity to attenuate the radiosensitivity of HCC. As an important index to reflect carcinogenesis [12], colony formation rate was significantly decreased following HCC 97H cell exposure to 1 Gy irradiation, but transfection with EphA2 expression vector restored cell colony formation rate to basal level. This means overexpressing EphA2 violates the inhibitory effect of low-dose irradiation (1 Gy) on carcinogenesis of HCC. EphA2 is a receptor tyrosine kinase that has been identified to induce pro-oncogenic effects, including enhancing tumorigenesis, tumor cell migration and invasion, angiogenesis, and metastasis [13], but few or no reports shows that EphA2 can diminish radiosensitivity. Our results herein broaden the view of EphA2 serving as a cancer supporter.
Radiotherapy represents an effective therapy for cancer, but it comes along with many side-effects; more importantly, tumor cells possess different mechanisms to escape cell death and defend against the cytotoxic effect of irradiation, especially at a low dose [4]. According to existing literature, the mechanisms underlying resistance to irradiation in cancer cells include, at the least, DNA repair, cell cycle arrest, antioxidant system enhancement and growth signal activation, and cell death signal inhibition [14]. Thus, the effect of EphA2 overexpression on resistance to irradiation in HCC cells suggests that some of these mechanisms are employed.
P38MAPK signals are known to be regulated by the Eph receptor family members. Upregulated EphA2 causes p38MAPK activation in endothelial progenitor cells [15]. In the current study, p-p38MAPK was shown to be dramatically upregulated in HCC tissues. To identify the role of p38MAPK in EphA2 function, a specific inhibitor of p38MAPK, namely SB203580, was added to EphA2-over-expressing cells. Activation of p38MAPK requires phosphorylation of Tyr182 [16]. Our results showed that the level of phosphorylation of p38MAPK at Tyr182 was considerably inhibited following the addition of SB203580, and more importantly, that EphA2-led enhancement of HCC radioresistance was blocked, suggesting that the functions exerted by EphA2 mainly relied on p38MAPK-mediated signal pathways.
P38MAPK can exert growth-promoting effects in different ways. P38MAPK provides an advantage for cell survival by regulating the cell cycle checkpoints, including G1/S and G2/M [9]. Irradiation causes the generation of substantial oxidative free radicals, which pose strong toxicity to DNA via the deterioration of its original molecular structures, while the cell cycle checkpoints allow time for DNA repair [17], [18]. Furthermore, p38MAPK has been associated with diverse kinds of key signal factors determining cell survival and death fates, such as STAT1, AP-1, cAMP-response element-binding protein, p53, nuclear factor-κB, and β-catenin [18]. It should be noted that, although p38MAPK is widely confirmed to have the ability to strengthen cell survival, the precise mechanisms mediated by p38MAPK involved in our experiment warrant further investigation. Besides, in our future study, the roles of EphA2 and p38MAPK in the modulation of radiosensitivity were detected in other types of HCC cell lines and HCC cell tumor xenografted nude mice to validate the mechanisms demonstrated by our experiment extensively exist in HCC cells.
In summary, significantly upregulated EphA2 and downregulated p-p38MAPK were observed in HCC tissues, compared with those in normal hepatic tissues. In HCC 97H cells, overexpressing EphA2 via the transfection of EphA2 expression vector attenuated radiosensitivity, with a remarkable increase in cell viability and healing capacity, and decreased apoptosis rate after low-dose irradiation of cell; these effects were abrogated by treatment with SB203580, a specific inhibitor of p38MAPK signals. The knock-out of EphA2 caused lower cell viability and healing capacity and higher apoptosis rate. Our findings collectively suggest that EphA2 is able to modulate HCC radiosensitivity adversely, mainly via its important downstream target, namely, p38MAPK. The molecular mechanism elucidated in this study may contribute to the design of future cancer therapies to bypass the adverse impacts of EphA2 on radiosensitivity.




