Oxygen uptake response to cycle ergometry in post-acute stroke patients with different severity of hemiparesis
Abstract
This study evaluated the impact of severity of hemiparesis on oxygen uptake 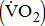 response in post-acute stroke patients. Sixty-four patients with a mean poststroke interval of 8.6 ± 3.8 days underwent a ramp cardiopulmonary exercise test on a cycling ergometer to volitional termination. Mean peak
response in post-acute stroke patients. Sixty-four patients with a mean poststroke interval of 8.6 ± 3.8 days underwent a ramp cardiopulmonary exercise test on a cycling ergometer to volitional termination. Mean peak  and work efficiency
and work efficiency 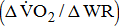 were measured by open-circuit spirometry during standard upright ergometer cycling. Severity of the hemiparetic lower limb was assessed by Brunnstrom's motor recovery stages lower extremity (BMRSL).
were measured by open-circuit spirometry during standard upright ergometer cycling. Severity of the hemiparetic lower limb was assessed by Brunnstrom's motor recovery stages lower extremity (BMRSL). 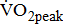 was 10% lower in hemiparetic leg with BMRSL V than in that with BMRSL VI, 20% lower in BMRSL IV, and 50% lower in BMRSL III.
was 10% lower in hemiparetic leg with BMRSL V than in that with BMRSL VI, 20% lower in BMRSL IV, and 50% lower in BMRSL III. 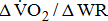 was higher for the group with increased BMRSL. The relations were consistent after adjustment for age, sex, body mass index, stroke type, hemiparetic side, modified Ashworth Scale, time poststroke, comorbidities, and medications. Our findings revealed that O2peak is dependent on the severity of hemiparesis in leg, and along with ΔO2/ΔWR closely related to the severity of hemiparesis in post-acute stroke patients, regardless of the types and locations of lesion after stroke, as well as the differences in comorbidities and medications.
was higher for the group with increased BMRSL. The relations were consistent after adjustment for age, sex, body mass index, stroke type, hemiparetic side, modified Ashworth Scale, time poststroke, comorbidities, and medications. Our findings revealed that O2peak is dependent on the severity of hemiparesis in leg, and along with ΔO2/ΔWR closely related to the severity of hemiparesis in post-acute stroke patients, regardless of the types and locations of lesion after stroke, as well as the differences in comorbidities and medications.
Introduction
Stroke is a leading cause of disability that results not only in persistent neurological deficits, but also in physical deconditioning that worsens disability and cardiovascular risk and increases risks for additional strokes [1]. Because the presence of cardiovascular disease can adversely affect the peak oxygen uptake (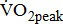 —the index of aerobic capacity attained during physical work to volitional exhaustion) and the ability to respond to physiologic stresses induced by prolonged physical effort [2], the limited
—the index of aerobic capacity attained during physical work to volitional exhaustion) and the ability to respond to physiologic stresses induced by prolonged physical effort [2], the limited 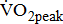 is likely to further disable stroke patients in their ability to carry out routine functional tasks and extended activities of daily living. Hence, cardiovascular and neuromuscular impairments, together with physical inactivity resulting from stroke, adversely affect
is likely to further disable stroke patients in their ability to carry out routine functional tasks and extended activities of daily living. Hence, cardiovascular and neuromuscular impairments, together with physical inactivity resulting from stroke, adversely affect 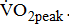
Although post-acute stroke patients often have poor 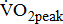 [3], fitness training has not been a standard component of stroke rehabilitation, in which strengthening, coordination, and self-care abilities have dominated immediately after the neurologic status becomes stable. Poststroke patients with low basal levels of
[3], fitness training has not been a standard component of stroke rehabilitation, in which strengthening, coordination, and self-care abilities have dominated immediately after the neurologic status becomes stable. Poststroke patients with low basal levels of 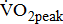 experience greatest benefits in terms of cardiovascular conditioning, even at relatively modest levels of physical exercise [4]. Even a small gain in aerobic power should improve not only functional capacity, but also survival prospects. Therefore, a more rational investigation of
experience greatest benefits in terms of cardiovascular conditioning, even at relatively modest levels of physical exercise [4]. Even a small gain in aerobic power should improve not only functional capacity, but also survival prospects. Therefore, a more rational investigation of 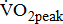 is critical for the design of aerobic exercise prescription for post-acute stroke patients. However, there are serious barriers to the assessment of limited
is critical for the design of aerobic exercise prescription for post-acute stroke patients. However, there are serious barriers to the assessment of limited 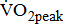 in post-acute stroke patients. Patients in the chronic poststroke period (>6 months) had abnormally low
in post-acute stroke patients. Patients in the chronic poststroke period (>6 months) had abnormally low 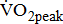 —approximately 25–45% below that of age- and gender-adjusted norms for sedentary individuals [5]-[8], and the mean
—approximately 25–45% below that of age- and gender-adjusted norms for sedentary individuals [5]-[8], and the mean 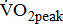 about 1 month poststroke was 60% of the normative values for sedentary healthy individuals [9]. This discrepancy in aerobic capacity assessment may be related to differences in patient selection; differences in stroke type, location of lesion, and timing of intervention after stroke; and differences in neuromuscular impairment.
about 1 month poststroke was 60% of the normative values for sedentary healthy individuals [9]. This discrepancy in aerobic capacity assessment may be related to differences in patient selection; differences in stroke type, location of lesion, and timing of intervention after stroke; and differences in neuromuscular impairment.
Treadmill exercise testing can be used to identify exercise-induced cardiac risks and develop an exercise prescription. Standard treadmill testing may especially be difficult in post-acute stroke patients because of impairments in the legs, trunk strength, and coordination. Cycle ergometry provides some advantages over the treadmill for post-acute stroke patients with impairments in walking and balance [3], [5]. In addition, cycle ergometry is preferred to the treadmill as it provides more precise estimation of the 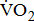 response, including work rate-
response, including work rate-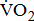 of work performance, and work efficiency (slope of the
of work performance, and work efficiency (slope of the 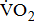 -WR relationship,
-WR relationship, 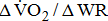 )—oxygen (O2) utilization by the muscle cells in relation to the quantity of external work performance [10]. For assessing
)—oxygen (O2) utilization by the muscle cells in relation to the quantity of external work performance [10]. For assessing 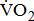 response during cycle ergometry in poststroke patients, it is important to distinguish between: the resting
response during cycle ergometry in poststroke patients, it is important to distinguish between: the resting 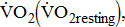 which shows the basal energy cost; the unloaded
which shows the basal energy cost; the unloaded 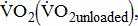 which reflects the energy required to move legs at a set frequency (i.e., internal work); and
which reflects the energy required to move legs at a set frequency (i.e., internal work); and 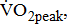 which represents the energy cost of performing the sum of internal and external work [11].
which represents the energy cost of performing the sum of internal and external work [11].
Leg movement accounts for approximately 60–70% of the energy cost in cycling [12]; however, little is known about the impact of severity of hemiparesis on 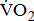 response during cycle ergometry in post-acute stroke patients. Therefore, the purpose of this study was to investigate the potential impact of severity of hemiparesis on
response during cycle ergometry in post-acute stroke patients. Therefore, the purpose of this study was to investigate the potential impact of severity of hemiparesis on 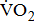 response in patients with post-acute stroke.
response in patients with post-acute stroke.
Materials and methods
Participants
All patients who had a primary diagnosis of first-time stroke, confirmed by both clinical and radiographic means, and who were admitted to the acute stroke service of Kaohsiung Medical University Medical Center were consecutively screened for eligibility. The inclusion criteria were as follows: (1) ≥18 years of age; (2) within 2 weeks poststroke [13]; (3) no evidence of dementia (as indicated by a score ≥24 of 30 on the mini-mental state examination [14]) or coronary artery disease (as indicated by the presence of at least one of the following: myocardial infarction by history or electrocardiogram, angina pectoris, percutaneous coronary intervention, or coronary artery bypass graft surgery [15]); (4) greater than Stage II of Brunnstrom's motor recovery stages lower extremity (BMRSL; where active voluntary movement is present without facilitation [16]); and (5) no contraindications to exercise testing outlined by the American College of Sports Medicine (ACSM) [17].
The clinical history and medical examination of each patient enrolled were recorded, noting their smoking habits and history of comorbidities such as hypertension, diabetes mellitus, and chronic obstructive pulmonary disease. The severity of hemiparetic lower limb was assessed by BMRSL. Spasticity of this limb was evaluated by the modified Ashworth Scale (MAS) [18]. None of the participants had any significant physical training prior to stroke, nor were they trained cyclists. The prospective individuals who met the criteria were informed of the procedures, known risks, and benefits. Informed consents (as approved by the Human Experiment and Ethical Committee, Kaohsiung Medical University Hospital) were obtained from all the participants. Remuneration was not offered.
Anthropometry
Body height and weight were measured following standard techniques with the participants in light clothes without shoes; the body height was determined to the nearest 0.5 cm and the body weight measured with a precision of ±0.1 kg. The body mass index (BMI) was calculated as body weight divided by height squared.
Exercise tests
All tests were performed on a calibrated, electromagnetically braked cycle ergometer (Corival V2; Lode BV, Groningen, The Netherlands). During the testing, the patients were seated in an upright position on the cycle with their hands in a relaxed position on the handlebars. The exercise testing on the ergometer was performed according to the ramp-incremental protocol for stroke patients and continued to either voluntary exhaustion or inability to maintain a pedaling rate of 50 rpm [19]. Following a 4-minute rest period, the patients began pedaling on the ergometer at 60 rpm at a work rate of zero load for 4 minutes. After the patients reached a steady rate at zero load, the testing advanced to the ramp protocol. Throughout all power outputs, they were required to maintain a constant pedaling rate of 60 rpm. The testing was terminated following the ACSM guidelines [17]. The expired gas was analyzed via open-circuit spirometry using a gas analyzer (MetaMax 3B; CORTEX Biophysik GmbH, Leipzig, Germany) to determine 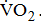 Volume calibration and calibration of gases, using standard gases, were carried out prior to each test. Each patient wore a mask and breathed room air through a two-way directional valve system that was attached.
Volume calibration and calibration of gases, using standard gases, were carried out prior to each test. Each patient wore a mask and breathed room air through a two-way directional valve system that was attached.
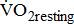 was determined during the 4th-minute rest period, and
was determined during the 4th-minute rest period, and 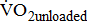 during the 4th-minute zero work load period.
during the 4th-minute zero work load period. 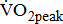 was the average of values recorded during the last 30 seconds of the test.
was the average of values recorded during the last 30 seconds of the test.
Statistical analysis
The calculated data were entered into a computer for statistical analysis, using the SAS statistical package (SAS Institute, version 9; Cary, NC, USA). A descriptive analysis was performed initially to evaluate the distribution of the variables. Means and their standard deviations (SDs) were obtained for values in participants. Frequencies and percentages were calculated for the categorical variables. For comparing between groups, either the Chi-square test or an analysis of covariance (ANOVA) was used, as appropriate. Multivariate adjustments were made to examine how far the effect of 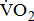 response might be explained by the severity of poststroke hemiparesis. Age (and sex in the combined model) was adjusted in model A; age, sex, and BMI were adjusted in model B; stroke type, hemiparetic side, MAS, and time poststroke were adjusted in model C, in addition to the adjustments in model B; and comorbidities and medications were adjusted in model D, in addition to the adjustments in model C. Adjusted means were computed from the ANOVA analysis of covariance models. The trend relationship between
response might be explained by the severity of poststroke hemiparesis. Age (and sex in the combined model) was adjusted in model A; age, sex, and BMI were adjusted in model B; stroke type, hemiparetic side, MAS, and time poststroke were adjusted in model C, in addition to the adjustments in model B; and comorbidities and medications were adjusted in model D, in addition to the adjustments in model C. Adjusted means were computed from the ANOVA analysis of covariance models. The trend relationship between 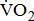 response and severity of poststroke hemiparesis was performed using Pearson's correlation analysis. Statistical significance was prospectively defined as p < 0.05.
response and severity of poststroke hemiparesis was performed using Pearson's correlation analysis. Statistical significance was prospectively defined as p < 0.05.
Results
Participant characteristics
Of the 69 patients enrolled in the study, one female patient of BMRSL III was excluded because she was unable to sit on the ergometer; in addition, one male and one female of BMRSL III and one male and one female of BMRSL IV did not cycle at a steady rate at zero load and were subsequently withdrawn from the study. Patients' demographics and clinical characteristics in different BMRSL are shown in Table 1. Study groups were similar in terms of age, gender, body weight, and BMI. Groups exhibited a similar distribution of medications and comorbidities, including hypertension, and current or past smoking; however, the groups with BMRSL III, IV, and V had a significantly higher prevalence of mellitus diabetes, and the groups with BMRSL IV and V had a significantly higher prevalence of hyperlipidemia.
| Clinical characteristics | Brunnstrom's motor recovery stages lower extremity | p | ||||
|---|---|---|---|---|---|---|
| All (n = 64) | III (n = 14) | IV (n = 16) | V (n = 22) | VI (n = 12) | ||
| Age (y) | 59.2 ± 10.7 | 60.1 ± 9.0 | 58.0 ± 11.7 | 61.2 ± 9.8 | 61.2 ± 9.6 | 0.4307 |
| Male | 51 (79.7) | 23 (79.3) | 14 (87.5) | 15 (68.2) | 10 (83.3) | 0.4210 |
| Height (cm) | 164.4 ± 8.1 | 166.0 ± 9.1 | 164.8 ± 8.4 | 162.5 ± 8.1 | 165.7 ± 7.0 | 0.5752 |
| Weight (kg) | 65.8 ± 10.9 | 69.4 ± 12.6 | 67.7 ± 10.4 | 62.4 ± 8.4 | 65.6 ± 12.9 | 0.2474 |
| BMI (kg/m2) | 24.3 ± 3.2 | 25.1 ± 2.9 | 24.9 ± 2.8 | 23.7 ± 3.1 | 23.8 ± 4.1 | 0.4949 |
| Medications | ||||||
| β-Blockers | 7 (10.9) | 0 (0) | 1 (6.3) | 4 (18.2) | 2 (16.7) | 0.2994 |
| Calcium-channel blockers | 30 (46.9) | 7 (50) | 9 (56.3) | 9 (40.9) | 5 (41.7) | 0.7856 |
| Angiotensin II receptor blockers | 25 (39.1) | 6 (42.9) | 9 (56.3) | 8 (36.4) | 2 (16.7) | 0.1979 |
| Angiotensin-converting enzyme inhibitors | 3 (4.7) | 2 (14.3) | 0 (0) | 1 (4.5) | 0 (0) | 0.2342 |
| Antiplatelets | 48 (75.0) | 7 (50.0) | 14 (87.5) | 17 (77.3) | 10 (83.3) | 0.0895 |
| Anticoagulants | 4 (6.3) | 1 (7.1) | 1 (6.3) | 1 (4.5) | 1 (8.3) | 0.9748 |
| Comorbidities | ||||||
| Diabetes mellitus | 29 (45.3) | 7 (50.0) | 8 (50.0) | 13 (59.1) | 1(8.3) | 0.0355 |
| Hypertension | 48 (75.0) | 11 (78.6) | 15 (93.8) | 16 (72.7) | 6 (50.0) | 0.0671 |
| Hyperlipidemia | 26 (40.6) | 2 (14.3) | 11 (68.8) | 10 (45.5) | 3 (25.0) | 0.0135 |
| Current smoking | 36 (56.3) | 9 (64.3) | 10 (62.5) | 7 (31.8) | 7 (58.3) | 0.1418 |
| Past smoking | 3 (4.7) | 0 (0) | 1 (6.3) | 1 (4.5) | 1 (8.3) | 0.7689 |
- Data are presented as n (%) or mean ± SD unless otherwise indicated. SD = standard deviation.
The clinical stroke features classified according to BMRSL are presented in Table 2. The type of stroke, hemiparetic side, and poststroke time were not significantly different between study groups. The MAS scores were higher in the group with BMRSL III; however, the differences were not statistically significant compared to the group with BMRSL IV.
| Clinical characteristics | Brunnstrom's motor recovery stages lower extremity | p | ||||
|---|---|---|---|---|---|---|
| All (n = 64) | III (n = 14) | IV (n = 16) | V (n = 22) | VI (n = 12) | ||
| Type of stroke | ||||||
| Ischemic | 52 (81.3) | 9 (64.3) | 15 (93.8) | 18 (81.8) | 10 (83.3) | 0.2285 |
| Hemorrhagic | 11 (17.2) | 5 (35.7) | 1 (6.3) | 4 (18.2) | 1 (8.3) | 0.1449 |
| Both | 1 (1.6) | 0 (0) | 0 (0) | 0 (0) | 1 (8.3) | 0.5340 |
| Hemiparetic side | ||||||
| Right | 30 (46.9) | 6 (42.9) | 9 (56.3) | 13 (59.1) | 2 (16.7) | 0.0949 |
| Left | 34 (53.1) | 8 (57.1) | 7 (43.8) | 9 (40.9) | 10 (83.3) | 0.0949 |
| MAS | 0.1 ± 0.4 | 0.4 ± 0.8 | 0.1 ± 0.3 | 0 | 0 | 0.0120 |
| Time poststroke (d) | 8.6 ± 3.8 | 9.9 ± 4.7 | 8.7 ± 4.0 | 8.5 ± 3.9 | 7.2 ± 1.3 | 0.3376 |
- Data are presented as n (%) or mean ± SD unless otherwise indicated. MAS = modified Ashworth Scale; SD = standard deviation.
Measures of 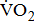 and
and 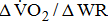
Measurements of 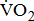 classified according to BMRSL are shown in Fig. 1. Measurements were pooled to determine the relations of
classified according to BMRSL are shown in Fig. 1. Measurements were pooled to determine the relations of 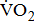 to group differences and workloads. Study groups were similar in terms of
to group differences and workloads. Study groups were similar in terms of 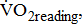 and
and 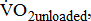 except for
except for 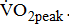 The
The 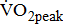 was significantly higher (p < 0.001) in the group with higher BMRSL. As displayed in Fig. 2, compared to the group with BMRSL VI (9.3 ± 1.4 mL/kg/min/W),
was significantly higher (p < 0.001) in the group with higher BMRSL. As displayed in Fig. 2, compared to the group with BMRSL VI (9.3 ± 1.4 mL/kg/min/W), 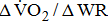 decreased in the group with BMRSL III (6.9 ± 3.2 mL/kg/min/W), and slightly decreased in the group with BMRSL IV (8.2 ± 1.6 mL/kg/min/W) and BMRSL V (8.8 ± 2.5 mL/kg/min/W). Although
decreased in the group with BMRSL III (6.9 ± 3.2 mL/kg/min/W), and slightly decreased in the group with BMRSL IV (8.2 ± 1.6 mL/kg/min/W) and BMRSL V (8.8 ± 2.5 mL/kg/min/W). Although 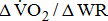 showed a trend of increase with increasing BMRSL, the differences were not statistically significant. All patients terminated testing of their own volition. The most common reasons for termination were leg fatigue and drop in pedaling rate (<50 rpm). These patients were closely monitored after test termination. No other symptoms, electrocardiographic abnormalities, or adverse effects were observed during or after these tests.
showed a trend of increase with increasing BMRSL, the differences were not statistically significant. All patients terminated testing of their own volition. The most common reasons for termination were leg fatigue and drop in pedaling rate (<50 rpm). These patients were closely monitored after test termination. No other symptoms, electrocardiographic abnormalities, or adverse effects were observed during or after these tests.
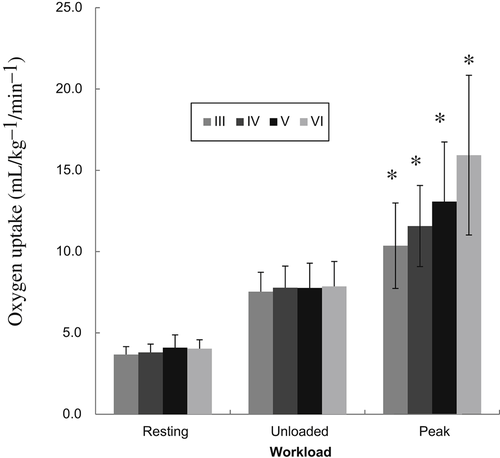
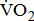 categorized by BMRSL III (
categorized by BMRSL III ( ), IV (
), IV ( ), V (■), and VI (
), V (■), and VI ( ) at three levels of cycling workloads: resting, unloaded, and peak. The higher BMRSL group shows higher
) at three levels of cycling workloads: resting, unloaded, and peak. The higher BMRSL group shows higher 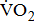 values for peak cycling workload (
values for peak cycling workload (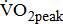 ). A significant increase of
). A significant increase of 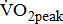 in BMRSL VI can be observed. BMRSL = Brunnstrom's motor recovery stages lower extremity;
in BMRSL VI can be observed. BMRSL = Brunnstrom's motor recovery stages lower extremity; 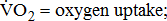
 * Significant difference between BMRSL, p < 0.001.
* Significant difference between BMRSL, p < 0.001.
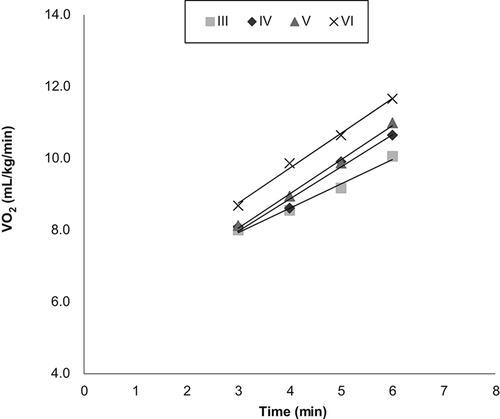
Slope of  response
response 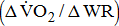 from the 3rd minute to the 6th minute after loaded cycling, categorized by BMRSL III (■), IV (◆), V (▲), and VI (X). The higher BMRSL group shows relatively steeper
from the 3rd minute to the 6th minute after loaded cycling, categorized by BMRSL III (■), IV (◆), V (▲), and VI (X). The higher BMRSL group shows relatively steeper 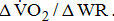 BMRSL = Brunnstrom's motor recovery stages lower extremity;
BMRSL = Brunnstrom's motor recovery stages lower extremity; 
Table 3 shows the 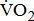 responses classified according to BMRSL in different multivariate adjusted models. The findings were consistent after adjustment for age, sex, BMI, stroke characteristics, history of comorbidities, and medications.
responses classified according to BMRSL in different multivariate adjusted models. The findings were consistent after adjustment for age, sex, BMI, stroke characteristics, history of comorbidities, and medications. 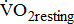 and
and 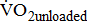 were not significantly different between the study groups. A significantly higher
were not significantly different between the study groups. A significantly higher 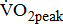 was associated with higher BMRSL. In addition,
was associated with higher BMRSL. In addition, 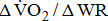 increased, but not significantly, with BMRSL. Table 4 shows the trend between oxygen uptake response and BMRSL in different multivariate adjusted logistic multiple regressions. No trends in
increased, but not significantly, with BMRSL. Table 4 shows the trend between oxygen uptake response and BMRSL in different multivariate adjusted logistic multiple regressions. No trends in 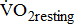 and
and 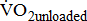 were significantly associated with BMRSL.
were significantly associated with BMRSL. 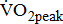 and
and 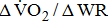 increased significantly in a linear trend with BMRSL. Furthermore, closer trends of
increased significantly in a linear trend with BMRSL. Furthermore, closer trends of 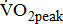 were found after adjustment for age and sex (models A, B, C, and D), as well as of
were found after adjustment for age and sex (models A, B, C, and D), as well as of 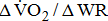 after adjustment for age, sex, and BMI (models B, C, and D).
after adjustment for age, sex, and BMI (models B, C, and D).
| Modela | Brunnstrom's motor recovery stages lower extremity | p | ||||
|---|---|---|---|---|---|---|
| III (n = 14) | IV (n = 16) | V (n = 22) | VI (n = 12) | |||
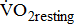 (mL/kg/min) (mL/kg/min) |
A | 3.7 ± 0.2 | 3.8 ± 0.2 | 4.1 ± 0.1 | 4.0 ± 0.2 | 0.2510 |
| B | 3.7 ± 0.2 | 3.9 ± 0.1 | 4.0 ± 0.1 | 4.0 ± 0.2 | 0.4017 | |
| C | 3.7 ± 0.2 | 3.9 ± 0.2 | 4.0 ± 0.1 | 4.0 ± 0.2 | 0.6197 | |
| D | 3.6 ± 0.2 | 3.8 ± 0.2 | 4.1 ± 0.1 | 4.1 ± 0.2 | 0.2655 | |
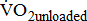 (mL/kg/min) (mL/kg/min) |
A | 7.6 ± 0.4 | 7.8 ± 0.3 | 7.7 ± 0.3 | 7.8 ± 0.4 | 0.9553 |
| B | 7.6 ± 0.3 | 7.9 ± 0.3 | 7.6 ± 0.3 | 7.8 ± 0.4 | 0.8478 | |
| C | 7.3 ± 0.4 | 8.2 ± 0.4 | 7.7 ± 0.3 | 7.6 ± 0.4 | 0.5109 | |
| D | 7.2 ± 0.5 | 7.9 ± 0.4 | 7.7 ± 0.3 | 8.0 ± 0.5 | 0.6637 | |
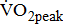 (mL/kg/min) (mL/kg/min) |
A | 10.1 ± 0.9 | 11.4 ± 0.9 | 13.3 ± 0.7 | 16.2 ± 1.0 | 0.0002* |
| B | 9.8 ± 0.9 | 11.6 ± 0.8 | 13.4 ± 0.7 | 16.2 ± 0.9 | <0.0001* | |
| C | 9.2 ± 1.0 | 12.4 ± 0.9 | 13.3 ± 0.7 | 16.1 ± 1.1 | 0.0005* | |
| D | 8.8 ± 1.2 | 11.9 ± 1.0 | 13.5 ± 0.8 | 16.8 ± 1.3 | 0.0024* | |
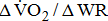 (mL/kg/min/W) (mL/kg/min/W) |
A | 7.0 ± 0.7 | 8.2 ± 0.7 | 8.8 ± 0.5 | 9.2 ± 0.7 | 0.1287 |
| B | 6.6 ± 0.7 | 7.9 ± 0.7 | 9.1 ± 0.5 | 9.4 ± 0.7 | 0.3008 | |
| C | 6.6 ± 0.9 | 8.1 ± 0.9 | 8.8 ± 0.6 | 9.8 ± 0.9 | 0.1048 | |
| D | 6.8 ± 1.0 | 8.7 ± 1.0 | 8.3 ± 0.7 | 9.9 ± 1.1 | 0.3332 | |
- Data are presented as mean ± SD unless otherwise indicated.
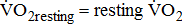 ;
; 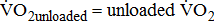 ;
; 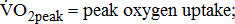
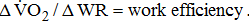
- a Model A: adjusted by age and sex; model B: additionally adjusted for BMI; model C: additionally adjusted for stroke type, hemiparetic side, MAS, and time poststroke; and model D: additionally adjusted for comorbidities and medications. * Significant difference between Brunnstrom's motor recovery stages lower extremity.
| Modela | R | p for trend | |
|---|---|---|---|
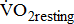 (mL/kg/min) (mL/kg/min) |
— | 0.24 | 0.0589 |
| A | 0.32 | 0.0635 | |
| B | 0.57 | 0.0973 | |
| C | 0.66 | 0.2131 | |
| D | 0.73 | 0.0705 | |
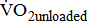 (mL/kg/min) (mL/kg/min) |
— | 0.10 | 0.6429 |
| A | 0.22 | 0.8119 | |
| B | 0.52 | 0.8962 | |
| C | 0.64 | 0.8814 | |
| D | 0.73 | 0.3767 | |
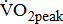 (mL/kg/min) (mL/kg/min) |
— | 0.49 | <0.0001* |
| A | 0.54 | <0.0001* | |
| B | 0.65 | <0.0001* | |
| C | 0.76 | <0.0001* | |
| D | 0.79 | 0.0002* | |
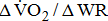 (mL/kg/min/W) (mL/kg/min/W) |
— | 0.33 | 0.0130* |
| A | 0.36 | 0.0201* | |
| B | 0.61 | 0.0042* | |
| C | 0.67 | 0.0128* | |
| D | 0.72 | 0.0169* |
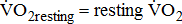 ;
; 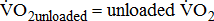 ;
; 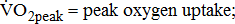
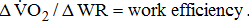
- a Model A: adjusted by age and sex; model B: additionally adjusted for BMI; model C: additionally adjusted for stroke type, hemiparetic side, MAS, and time poststroke; and model D: additionally adjusted for comorbidities and medications. * Significant difference between Brunnstrom's motor recovery stages lower extremity.
Discussion
The results of this study demonstrated that, in nontrained post-acute stroke patients cycling at 60 rpm, 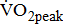 is dependent on and
is dependent on and 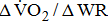 is closely related to the severity of hemiparesis in the legs, regardless of the types and locations of lesion after stroke, as well as of the differences in comorbidities and medications.
is closely related to the severity of hemiparesis in the legs, regardless of the types and locations of lesion after stroke, as well as of the differences in comorbidities and medications.
Impairments resulting from stroke, such as muscle weakness, pain, spasticity, and poor balance, can result in reduced tolerance to activity and poorer aerobic capacity. With mean 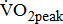 values of 12.7 mL/kg/min, aerobic capacity is significantly compromised in the post-acute period after stroke [3]. The published
values of 12.7 mL/kg/min, aerobic capacity is significantly compromised in the post-acute period after stroke [3]. The published 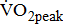 data for comparison are limited because previous studies involved patients with longer and wider poststroke intervals, and there were differences in the distribution of age and sexes, as well as the equipment used for exercise testing [5], [9], [20]-[24]. Moreover, none of the above-cited studies demonstrated varying degrees of poststroke motor impairments. This increased variation may be due to the study investigating a heterogeneous sample of individuals, with varying degrees of poststroke leg paresis that may affect
data for comparison are limited because previous studies involved patients with longer and wider poststroke intervals, and there were differences in the distribution of age and sexes, as well as the equipment used for exercise testing [5], [9], [20]-[24]. Moreover, none of the above-cited studies demonstrated varying degrees of poststroke motor impairments. This increased variation may be due to the study investigating a heterogeneous sample of individuals, with varying degrees of poststroke leg paresis that may affect 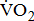 during resting and movement metabolism. Our results showed that
during resting and movement metabolism. Our results showed that 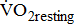 and
and 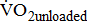 are similar for different severities of hemiparetic leg, and
are similar for different severities of hemiparetic leg, and 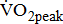 is 10% lower in the hemiparetic leg with BMRSL V than that with BMRSL VI, 20% lower in BMRSL IV, and 50% lower in BMRSL III. Although
is 10% lower in the hemiparetic leg with BMRSL V than that with BMRSL VI, 20% lower in BMRSL IV, and 50% lower in BMRSL III. Although 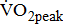 is related to age, gender, and anthropometric characteristics [25], we found that it is also related closely to stroke-related leg paresis, independent of comorbidities and stroke characteristics. There seemed to be an increased linear interpretation of the relationship between
is related to age, gender, and anthropometric characteristics [25], we found that it is also related closely to stroke-related leg paresis, independent of comorbidities and stroke characteristics. There seemed to be an increased linear interpretation of the relationship between 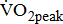 and severity of hemiparetic leg, irrespective of the probable biological mediating factors. This seems to show that difference in leg paresis may serve to explain the variation in poststroke aerobic capacity.
and severity of hemiparetic leg, irrespective of the probable biological mediating factors. This seems to show that difference in leg paresis may serve to explain the variation in poststroke aerobic capacity.
The finding of lower aerobic capacity with more severe poststroke leg paresis was, in part, supported by changes in the other indices of 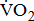 response. Poststroke patients showed a decrease in
response. Poststroke patients showed a decrease in 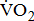 for a given external work rate
for a given external work rate 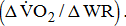 Although it was nonsignificant,
Although it was nonsignificant, 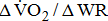 decreased with increases in the severity of stroke-related hemiparetic leg.
decreased with increases in the severity of stroke-related hemiparetic leg. 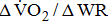 reflects the efficiency of the metabolic conversion of chemical potential energy to mechanical work and the mechanical efficiency of the musculoskeletal system.
reflects the efficiency of the metabolic conversion of chemical potential energy to mechanical work and the mechanical efficiency of the musculoskeletal system. 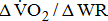 decreases, indicating lesser O2 utilization by the muscle cells in relation to the quantity of external work performed, i.e., the muscles were unable to extract the O2 required to perform the exercise [10]. Although
decreases, indicating lesser O2 utilization by the muscle cells in relation to the quantity of external work performed, i.e., the muscles were unable to extract the O2 required to perform the exercise [10]. Although 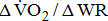 is independent of sex, age, or height, the less fit individuals present lower
is independent of sex, age, or height, the less fit individuals present lower 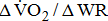 [25]. A reduction in the value may indicate inadequate O2 transport, as occurs in cases of disease of the heart, lungs, or circulation [26]. Additionally, the
[25]. A reduction in the value may indicate inadequate O2 transport, as occurs in cases of disease of the heart, lungs, or circulation [26]. Additionally, the 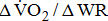 dependence on stroke-related paresis is consistent with the observed positive trend between
dependence on stroke-related paresis is consistent with the observed positive trend between 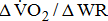 and the level of leg weakness (Table 4). These findings all suggested that the decrease in gross
and the level of leg weakness (Table 4). These findings all suggested that the decrease in gross 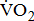 and
and 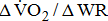 with increasing severity of hemiparesis at a given work rate during cycle ergometer exercise was due to the work performed in moving the legs. It was then mainly explained by a reduction in the number of motor units and a reduced ability to generate force and, consequently, workload in poststroke patients.
with increasing severity of hemiparesis at a given work rate during cycle ergometer exercise was due to the work performed in moving the legs. It was then mainly explained by a reduction in the number of motor units and a reduced ability to generate force and, consequently, workload in poststroke patients.
This study has several potential limitations. First, our study population was drawn from a single hospital in Kaohsiung, which may limit the generalizability of our findings. Second, results must be interpreted with caution because we used a small sample. These results need to be confirmed by another study with a larger sample. The severity of hemiparesis to impact 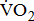 response evaluation after stroke should be tested rigorously in further studies using different progression strategies. The presentation and severity of impairments vary widely in poststroke patients, and, consequently, there is a need to develop more rationale interventions to monitor the levels of aerobic fitness. To take the potential impact of severity of hemiparesis into consideration, it is probable that we are precisely assessing
response evaluation after stroke should be tested rigorously in further studies using different progression strategies. The presentation and severity of impairments vary widely in poststroke patients, and, consequently, there is a need to develop more rationale interventions to monitor the levels of aerobic fitness. To take the potential impact of severity of hemiparesis into consideration, it is probable that we are precisely assessing 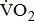 response in post-acute stroke patients. In summary, the results of this study demonstrated that post-acute stroke patients may have reduced
response in post-acute stroke patients. In summary, the results of this study demonstrated that post-acute stroke patients may have reduced 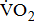 response that is significantly related to the severity of hemiparesis in their legs.
response that is significantly related to the severity of hemiparesis in their legs.
Acknowledgments
We thank Dr Yi-Hsin Yang for her assistance in the statistical analysis; the help from the Statistical Analysis Laboratory, Department of Clinical Research, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan is also acknowledged. This study was supported by a grant from the Kaohsiung Medical University Hospital (KMUH100-0M39).




