Comparison between laparoscopic and open partial nephrectomy: Surgical, oncologic, and functional outcomes
Abstract
The surgical, oncologic, and functional outcomes were retrospectively compared of laparoscopic partial nephrectomy (LPN) and open partial nephrectomy (OPN) for the treatment of renal masses. Between January 2006 and November 2011, 115 LPNs and 97 OPNs were performed. The patients' demographics were matched. Their intraoperative and postoperative data, oncologic and renal function outcomes were compared. Surgical time, renal arterial occlusion time, estimated blood loss, and postoperative hospitalization days were shorter in the LPN group (p < 0.01). The total complications were comparable; however, LPN had a higher intraoperative complication due to 12 subcutaneous emphysemas. The LPN group was followed up with a mean time of 29.3 ± 14.4 months and the OPN group with a mean time of 31.2 ± 12.6 months. All patients survived and no distant relapse or metastasis were observed. Kaplan–Meier estimates of 60-month local recurrence-free survival were comparable with 92.4% after LPN and 93.8% after OPN, respectively (p = 0.57). The reduction of glomerular filtration rate was more obvious after LPN at the 3-month follow-up (p < 0.01), but similar between the two groups at the 30.2-month follow-up. LPN provides similar results in oncologic and functional outcomes when compared to OPN. Long-term observations are still required to the oncologic and function outcomes.
Introduction
During the past 2 decades, partial nephrectomy (PN) has matured to an established approach for the treatment of small renal masses (≤4 cm) in the presence of a normal contralateral kidney [1]. With advances in laparoscopic and intracorporeal suturing techniques, and the availability of hemosealant substances, the laparoscopic approach has recently gained popularity for PN and become a well-defined method [2]. However, a disadvantage of this procedure is the long learning curve because tumor excision and wound anastomosis using laparoscopy is technically very challenging [3]. Another crucial point is renal warm ischemia, which may jeopardize renal function [4]. Short-term and long-term results of oncologic and functional outcomes remain to be defined.
Laparoscopic PN (LPN) and open PN (OPN) both are routinely performed surgical techniques at The First Affiliated Hospital, Medical College of Zhejiang University. In the current study, we summarized our nephron-sparing surgical experiences, and compared the surgical, oncologic, and renal functional outcomes between LPN and OPN.
Materials and methods
Between January 2006 and November 2011, 115 patients had undergone LPN and 97 patients had undergone OPN in our medical center. Preoperative work-up included renal sonography, chest X-ray, serum creatinine, and other routine tests. Computed tomography angiography was carried out in all patients to make clear the distribution of the renal artery. In cases not suitable for contrast medium application, magnetic resonance imaging studies were obtained.
Retroperitoneal OPN has been described previously, and was performed routinely [5]. Retroperitoneoscopic PN was performed with the patient in full-flank position. A retroperitoneal cavity was formed by blunt dissection from a small incision in the lumbar triangle along the midaxillary line and a 10-mm trocar introduced. The pneumoperitoneum was established and maintained at a pressure of 12–14 mmHg. Other trocars were placed under the endoscopic view routinely. The renal artery was blocked by renal artery clip once dissected. Circumcision of the tumor was performed by use of a scissors along with a margin of 0.5 cm in warm ischemia. If there was damage to the collection system, it was sutured with 3-0 absorbable suture. The surgical section was transfixion sutured using two or three needles. Finally, the renal parenchyma was sutured with running 2-0 absorbable suture and the end of suture was fixed by Hem-o-lok to maintain adequate suture tension. The specimen was retrieved with an endobag.
The patients were evaluated by renal ultrasound and serum creatinine at the 3rd month postoperatively and every 6 months thereafter for 2 years, then in yearly intervals. The chest X-rays were obtained in yearly intervals. The renal computed tomography was done only in some patients who had suspicion of tumor recurrence on renal ultrasound examination. If there was evidence of recurrence, radical nephrectomy was performed. Renal function was assessed by estimating glomerular filtration rate (GFR) using the abbreviated modification of diet in renal disease study equation [6]. Patient demographics, preoperative data, pathologic data, and oncologic and renal function outcomes were recorded for each patient. The study protocol was approved by the Institutional Review Board of The First Affiliated Hospital, Medical College of Zhejiang University. Each of the patients gave written informed consent for this study.
We retrospectively compared the outcomes of the two groups of patients who underwent either OPN or LPN. A two-tailed paired t test was carried out for preoperative and postoperative comparison of parameters, with results presented as means and standard error. The Chi-square test was used to compare categorical variables. Recurrence-free survival (RFS) rates for local and distant relapse were estimated using the Kaplan-Meier method with log-rank test statistics. All analyses were performed using SPSS version 13 (SPSS Inc., Chicago, IL, USA), with p < 0.05 considered statistically significant.
Results
The mean age in the laparoscopic group was 51.2 ± 9.8 years and in the open group was 53.1 ± 10.2 years (p = 0.87). The tumor location, including the laterality of the affected kidney and different renal segments, was comparable between the two groups (p = 0.7). The characteristics of these patients are shown in Table 1.
| LPN (n = 115) | OPN (n = 97) | p | |
|---|---|---|---|
| Age, y | 51.2 ± 9.8 (17–73) | 53.1 ± 10.2 (22–69) | 0.87 |
| Sex (male:female) | 72.0:43.0 | 59.0:38.0 | 0.79 |
| BMI | 23.26 ± 4.21 | 23.98 ± 5.36 | 0.68 |
| Laterality (right:left) | 60.0:55.0 | 48.0:49.0 | 0.70 |
| Tumor location | — | ||
| Upper | 35 (30.4) | 28 (28.9) | — |
| Middle | 50 (43.5) | 43 (44.3) | — |
| Lower | 30 (26.1) | 26 (26.8) | — |
| Surgical time, min | 94.3 ± 19.8 (41–168) | 117.6 ± 22.6 (41–168) | <0.01 |
| EBL, mL | 126.9 ± 41.8 (20–500) | 232.3 ± 86.7 (50–800) | <0.01 |
| Transfusion rate | 4.3% (5/115) | 3.1% (3/97) | 0.63 |
| Renal arterial occlusion time, min | <0.01 | ||
| LPN: WIT | 24.5 ± 8.6 (7–42) | 28.2 ± 7.5 (19–56) | |
| OPN: CIT | |||
| Postoperative hospitalization, d | 8.3 ± 2.3 (7–12) | 12.1 ± 5.2 (7–31) | <0.01 |
| Tumor size, cm | 3.23 ± 1.42 (1.2–7.5) | 3.09 ± 1.32 (1.0–5.8) | 0.34 |
| Pathologic outcomes | |||
| Benign tumor | 20 (17.4) | 24 (24.7) | 0.19 |
| Renal cell carcinoma | 95 (82.6) | 73 (75.3) | — |
| Clear cell | 86 (90.5) | 68 (93.2) | — |
| Others | 9 (9.5) | 5 (6.8) | — |
| Pathological grade (pT1a/pT1b) | 17/78 | 11/62 | — |
| PSM (n) | 1 | 0 | — |
- Data are presented as n (%) or mean ± SD (range), unless otherwise indicated. BMI = body mass index; CIT = cold ischemia time; EBL = estimated blood loss; LPN = laparoscopic nephrectomy; OPN = open partial nephrectomy; PSM = positive surgical margins; WIT = warm ischemia time.
The surgical and arterial occlusion times were shorter in the LPN group compared with the OPN group (p < 0.01). In patients undergoing LPN, mean surgical time was 94.3 ± 19.8 minutes and mean ischemia time was 24.5 ± 8.6 minutes. In patients undergoing OPN, the corresponding times were 117.6 ± 22.6 minutes and 28.2 ± 7.5 minutes. Mean estimated blood loss was less in the LPN group than the OPN group (p < 0.01). The LPN group had a shorter postoperative hospitalization than the OPN group (8.3 ± 2.3 days vs. 12.1 ± 5.2 days; p < 0.01). The transfusion rate was 4.3% (5/115) in the LPN group and 3.1% (3/97) in the OPN group (p = 0.63). The benign tumor rate was 17.4% (20/115) in the LPN group and 27.4% (24/97) in the OPN group (p = 0.19). The pathological results were comparable between the two groups. Only one patient in the laparoscopic group was diagnosed with positive surgical margin (PSM). The perioperative parameters are shown in Table 1.
In the LPN group, 17 patients had tumors > 4 cm in size. Compared with the remaining 98 patients in the group, these patients required longer surgical and arterial occlusion times (mean surgical time, 100.1 ± 24.2 minutes vs. 89.8 ± 19.2 minutes; mean warm ischemia time, 27.1 ± 11.3 minutes vs. 20.2 ± 7.3 minutes; p < 0.05). However, the estimated blood loss was equivalent. Table 2 presents the perioperative parameters of these two subgroups.
| Tumor size ≤ 4 cm (n = 98) | Tumor size > 4 cm (n = 17) | p | |
|---|---|---|---|
| Mean size, cm | 2.6 ± 1.1 (1.2–4) | 5.2 ± 1.8 (4.5–7.5) | |
| Surgical time, min | 89.8 ± 19.2 (41–145) | 100.1 ± 24.2 (65–168) | <0.05 |
| WIT, min | 20.2 ± 7.3 (7–38) | 27.1 ± 11.3 (9–42) | <0.05 |
| EBL, mL | 124.2 ± 45.1 (40–500) | 128.7 ± 52.6 (20–400) | 0.54 |
- Data are presented as mean ± SD (range). EBL = estimated blood loss; WIT = warm ischemia time.
The complications categorized as intraoperative and postoperative are presented in Table 3. The frequencies of postoperative and total complications were comparable between the two groups (p = 0.11; p = 0.43), but the LPN group had a higher intraoperative complication rate than the OPN group (p < 0.05). The LPN group developed 21 intraoperative complications, including 12 cases of subcutaneous emphysema. One patient in the LPN group presented with peritonitis after surgery, and descending colonic perforation was found on exploratory laparotomy. We repeatedly reviewed the surgical video and determined that the colon was injured by the trocar at insertion. Postoperative complications included wound infection, hematoma, deep venous thrombosis (DVT), and urine leakage.
| Complications | LPN group | OPN group | Overall | p |
|---|---|---|---|---|
| Intraoperative | 13 (11.3) | 3 (3.1) | 16 (7.5) | <0.05 |
| Pleural injury | 0 | 3 (3.1) | 3 (1.4) | — |
| Bowel injury | 1 (0.87) | 0 | 1 (0.47) | — |
| Subcutaneous emphysema | 12 (10.4) | 0 | 12 (5.7) | — |
| Postoperative | 3 (2.6) | 7 (7.2) | 10 (4.7) | 0.11 |
| Wound infection | 0 | 3 (3.1) | 3 (1.4) | — |
| Hematoma | 1 (0.87) | 1 (1.0) | 2 (0.94) | — |
| DVT | 0 | 1 (1.0) | 1 (0.47) | — |
| Urine leak | 2 (1.7) | 2 (2.1) | 4 (1.9) | — |
| Total | 16 (13.9) | 10 (10.3) | 26 (12.3) | 0.43 |
- Data are presented as n (%). DVT = deep venous thrombosis; LPN = laparoscopic nephrectomy; OPN = open partial nephrectomy.
In both groups, all malignant tumors were pT1 stage renal cell carcinoma (RCC). The mean follow-up for all the study participants was 30.2 ± 13.6 months: 29.3 ± 14.4 months (range, 1.6–68.6 months) in the LPN group and 31.2 ± 12.6 months (range, 3.4–64.5 months) in the OPN group (p = 0.31). All the patients were successfully followed up and were alive at the final follow-up. The preoperative GFR levels were comparable between the LPN group and the OPN group (p = 0.37), whereas the 3-month postoperative decline in the GFR was greater after LPN (20.2%) than after OPN (13.4%) (p < 0.01). However, after a mean follow-up of 30.2 months, the decline in mean GFR was comparable between the two groups (p = 0.14; Table 4). Local recurrences were found in both groups, 1.7% (2/115) in the LPN group and 1.0% (1/97) in the OPN group. No distant metastasis was found in either group. The Kaplan–Meier estimates for the 60-month probability of local RFS were 92.4% (95% confidence interval, 82.0–100%) and 93.8% (95% confidence interval, 81.8–100%) after LPN and OPN, respectively (p = 0.57; Fig. 1).
| Variable | LPN group | OPN group | p |
|---|---|---|---|
| GFR prior to operation, mL/min/1.73 m2 | 74.3 ± 11.2 (32.4–135.6) | 71.5 ± 12.3 (28.8–130.4) | 0.37 |
| GFR 3 mo after operation, mL/min/1.73 m2 | 58.8 ± 13.1 (24.7–129.4) | 64.6 ± 9.8 (26.2–126.8) | 0.09 |
| GFR at mean follow-up, mL/min/1.73 m2 | 62.1 ± 12.8 (28.2–132.2) | 65.1 ± 9.2 (26.8–125.9) | 0.62 |
| GFR decline from preoperative to 3 mo postoperative | 20.2 | 13.4 | <0.01 |
| GFR decline from preoperative to mean follow-up | 15.8 | 12.2 | 0.14 |
- Data are presented as % or mean ± SD (range). GFR = glomerular filtration rate; LPN = laparoscopic nephrectomy; OPN = open partial nephrectomy.
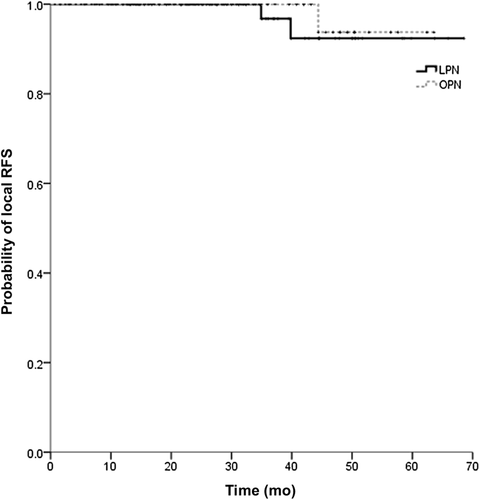
Kaplan–Meier estimates for local recurrence-free survival (RFS) after laparoscopic partial nephrectomy (LPN) and open partial nephrectomy. The 60-month local RFS was 92.4% (95% confidence interval: 82.0–100%) for laparoscopic partial nephrectomy and 93.8% (95% confidence interval: 81.8–100%) for open partial nephrectomy (p = 0.57).
Discussion
The widespread use of abdominal imaging has resulted in increased detection of renal masses, with 13–27% abdominal studies showing a renal lesion [7]. OPN is a safe procedure in appropriately selected patients with 5-year and 10-year cancer-specific survivals of 81% and 64%, respectively, and local recurrence-free survivals of 89% and 80%, respectively [8]. However, OPN may be associated with extensive muscle incisions, rib resection, and open flank incision [9]. The minimally invasive nephron-sparing surgical (including LPN and robotic-assisted LPN) is considered to be associated with less analgesia, shorter hospitalization, and quicker convalescence. In China, robotic-assisted LPN is still not widely used because of its associated costs, but it is gaining popularity.
Surgeons have varying opinions regarding the level of difficulty of LPN and the resultant necessity of prolonged operative times [10]-[12]. In our experience, LPN has shorter surgical and arterial occlusion times, shorter hospitalization, quicker convalescence, and equivalent complications compared with OPN. We ascribe these findings to the following: (1) laparoscopic skill due to cumulative experience and technical modifications; (2) laparoscopic magnification enabling fine dissection of the renal artery and suturing of the surgical section and damaged collection system; and (3) continuous suture, and application of Hem-o-lok to clamp the suture instead of traditional ligature, of the renal wound surface reducing surgical time. However, intraoperative complications in the LPN group were slightly higher because of a higher frequency of subcutaneous emphysema. However, no intraoperative complications leading to open conversion occurred in the LPN group. Bowel injury during LPN is one of the most feared complications. Bishoff et al. [13] reported that the bowel injury rate was 0.8% during LPN and the etiologies of the bowel injury were thermal (50%) and traumatic during access (32%). We experienced one case of bowel injury during trocar insertion. Unfortunately, the majority of bowel injuries are not recognized intraoperatively and manifest in the postoperative period—usually after an uneventful course—as peritonitis.
To prevent tumor recurrence after PN, many researchers have suggested that the tumor should be removed with margins of 0.5–1.0 cm in LPN. We used scissors to resect the tumor with a margin of 0.5 cm and sent three or four marginal specimens for immediate pathological examination. One LPN patient was found PSM because the tumor was located completely within the renal parenchyma. The intraparenchyma depth of the tumors can greatly influence the surgical feasibility. Tumors with a deep seated location commonly about the collecting system, have larger blood vessels encountered during deep resection. Compared with peripheral tumors, central masses require longer operative time, ischemia time, more frequent pelvicaliceal repair, and early postoperative complication rates [14], [15]. Therefore, the technical challenges inherent to laparoscopic performance make surgeons reluctant to approach these tumors by laparoscopy and we still consider it problematic.
Many studies have shown the technical feasibility and perioperative safety and efficacy of LPN for removal of renal tumors > 4 cm [16], [17]. In our LPN group, the surgical and arterial occlusion times were slightly higher in the patients with tumors > 4 cm in size, but no technical difficulties were encountered.
Tumor recurrence in the kidney can be due to incomplete tumor resection or the occurrence of de novo disease. Permpongkosol et al. [12] reported a group of 85 patients with T1N0M0 stage RCC that underwent LPN; the 5-year disease-free and actuarial survival rates were 91.4% and 93.8%, respectively. Marszalek et al. [18] reported a matched-pair comparison of 200 patients who underwent LPN or OPN; the 5-year RFS data were comparable—97% after LPN and 98% after OPN. The Cleveland Clinic Foundation reported excellent results, with 100% distant and 97.3% local RFS within 5.7 year of LPN [19]. In the current study, there were no significant differences between the LPN and OPN groups in the incidence of local 5-year RFS (92.4% vs. 93.8%, respectively; p = 0.568) and no evidence of distant recurrence or metastasis was found.
According to Rocca Rosetti [20], warm ischemia in open surgery can be classified as follows: (1) <10 minutes—harmless; (2) up to 30 minutes—generally reversible lesions; (3) >30 minutes—risk of irreversible parenchymal lesions increasing rapidly with the ischemic time; and (4) >60 minutes—irreversible lesions. During laparoscopy, the increase of retroperitoneal pressure due to the pneumoperitoneum generally causes oliguria. This situation can create an ischemic preconditioning that reduces tissue injury [21]. In our study, the mean warm ischemia time was 24.5 minutes and the decline in GFR was generally reversible. Although the decline in GFR was higher in the LPN group at the 3-month follow-up, there was no difference at the 30.2-month follow-up. Previous research also supported that the GFR might be significantly decreased at 3 months postoperatively despite the trend toward progressive recovery [4], [22].
We are aware that a nonrandomized comparative analysis of surgical techniques with different levels of development might have introduced bias. Furthermore, limited sample size might have reduced statistical power; a large series and longer follow-up is warranted to assess disease-free status of the treatment of small volume RCC by LPN compared with OPN. Further work will also be needed to compare renal function outcomes in the patients.
In conclusion, laparoscopic and open PN provide comparable results in surgical, oncologic, and renal function outcomes for T1N0M0 stage RCC. However, renal tumors that are difficult to access are still a major challenge for LPN. To minimize intraoperative complications as well as surgical and warm ischemia times, and their impact on renal function, LPN has to remain in the hands of experienced laparoscopic surgeons.




